Search by Drug Name or NDC
NDC 36800-0825-29 Topcare Miconazole 7 2 g/100g Details
Topcare Miconazole 7 2 g/100g
Topcare Miconazole 7 is a VAGINAL CREAM in the HUMAN OTC DRUG category. It is labeled and distributed by Topco Associates LLC. The primary component is MICONAZOLE NITRATE.
MedlinePlus Drug Summary
Vaginal miconazole is used to treat vaginal yeast infections in adults and children 12 years of age and older. Miconazole is in a class of antifungal medications called imidazoles. It works by stopping the growth of fungi that cause infection.
Related Packages: 36800-0825-29Last Updated: 11/30/2022
MedLinePlus Full Drug Details: Miconazole Vaginal
Product Information
| NDC | 36800-0825 |
|---|---|
| Product ID | 36800-825_887383d2-df39-4e9f-9b11-4fa83dc578ce |
| Associated GPIs | 55104050103710 |
| GCN Sequence Number | 007003 |
| GCN Sequence Number Description | miconazole nitrate CREAM/APPL 2 % VAGINAL |
| HIC3 | Q4F |
| HIC3 Description | VAGINAL ANTIFUNGALS |
| GCN | 28380 |
| HICL Sequence Number | 003031 |
| HICL Sequence Number Description | MICONAZOLE NITRATE |
| Brand/Generic | Generic |
| Proprietary Name | Topcare Miconazole 7 |
| Proprietary Name Suffix | n/a |
| Non-Proprietary Name | Miconazole nitrate |
| Product Type | HUMAN OTC DRUG |
| Dosage Form | CREAM |
| Route | VAGINAL |
| Active Ingredient Strength | 2 |
| Active Ingredient Units | g/100g |
| Substance Name | MICONAZOLE NITRATE |
| Labeler Name | Topco Associates LLC |
| Pharmaceutical Class | Azole Antifungal [EPC], Azoles [CS] |
| DEA Schedule | n/a |
| Marketing Category | ANDA |
| Application Number | ANDA074760 |
| Listing Certified Through | 2024-12-31 |
Package
Package Images

NDC 36800-0825-29 (36800082529)
| NDC Package Code | 36800-825-29 |
|---|---|
| Billing NDC | 36800082529 |
| Package | 1 TUBE, WITH APPLICATOR in 1 CARTON (36800-825-29) / 45 g in 1 TUBE, WITH APPLICATOR |
| Marketing Start Date | 2000-02-29 |
| NDC Exclude Flag | N |
| Pricing Information | N/A |
Standard Product Labeling (SPL)/Prescribing Information SPL 351905b1-f78c-4838-a036-23b2bfc775f2 Details
Uses
Warnings
For vaginal use only
Ask a doctor before use if you have
- •
- vaginal itching and discomfort for the first time
- •
- lower abdominal, back or shoulder pain, fever, chills, nausea, vomiting, or foul-smelling vaginal discharge. You may have a more serious condition.
- •
- vaginal yeast infections often (such as once a month or 3 in 6 months). You could be pregnant or have a serious underlying medical cause for your symptoms, including diabetes or a weakened immune system.
- •
- been exposed to the human immunodeficiency virus (HIV) that causes AIDS
Ask a doctor or pharmacist before use if you are
taking the prescription blood thinning medicine warfarin, because bleeding or bruising may occur.
When using this product
- •
- do not use tampons, douches, spermicides or other vaginal products. Condoms and diaphragms may be damaged and fail to prevent pregnancy or sexually transmitted diseases (STDs).
- •
- do not have vaginal intercourse
- •
- mild increase in vaginal burning, itching or irritation may occur
- •
- if you do not get complete relief ask a doctor before using another product
Directions
- •
- before using this product read the enclosed consumer information leaflet for complete directions and information
- •
- adults and children 12 years of age and over:
- •
- applicators: insert 1 applicatorful into the vagina at bedtime for 7 nights in a row. Throw applicator away after use.
- •
- use the same tube of cream if you have itching and irritation on the skin outside the vagina. Squeeze a small amount of cream onto your fingertip. Apply the cream onto the itchy, irritated skin outside the vagina. Use 2 times daily for up to 7 days, as needed.
- •
- children under 12 years of age: ask a doctor
Other information
Inactive ingredients
Consumer Information
7-Day Treatment
CONSUMER INFORMATION LEAFLET
Miconazole Nitrate Vaginal Cream (2%)
VAGINAL ANTIFUNGAL
Cures Most Vaginal Yeast Infections and Relieves Associated External Itching and Irritation
Why should I use this product?
This product contains a 2% miconazole nitrate cream that cures most vaginal yeast infections, and can be used for relief of itching and irritation on the skin outside the vagina (vulva) due to a yeast infection. Do not use this product if this is the first time you have vaginal discharge, itching, burning and discomfort. See your doctor or health professional first to find out the cause of your symptoms. If a doctor has told you in the past that you had a vaginal yeast infection and you have the same symptoms now (such as vaginal discharge, itching or burning), then this product may work for you.
What is a vaginal yeast infection?
A vaginal yeast infection is a common condition caused by an overgrowth of yeast (Candida) that may normally live in the vagina. Your doctor may call this infection “monilia” or “candidiasis.” Some women may have a yeast infection on the skin outside of the vagina (vulva) at the same time that they have a vaginal infection.
Who can get a vaginal yeast infection?
You can get a vaginal yeast infection at any age. It is most common during the childbearing years. Women who are pregnant or diabetic, taking antibiotics, birth control pills or steroids, or who have a weakened immune system are more likely to get repeated yeast infections that may not clear up easily with proper treatment.
Some medical conditions can weaken the body's normal ability to fight infection. One of the most serious of these conditions is infection with the human immunodeficiency virus (HIV - the virus that causes AIDS). The HIV virus causes the body to be more likely to get infections, including vaginal yeast infections that may not clear up easily with proper treatment. If you may have been exposed to HIV and get repeated vaginal yeast infections, you should see your doctor right away. For more information on HIV infection, please contact your doctor or the CDC National AIDS HOTLINE. The CDC phone numbers are:
1-800-232-4636 (English/Spanish), or
1-888-232-6348 (hearing impaired, TTY).
How can I tell if I have a vaginal yeast infection?
When you have a vaginal yeast infection, you may have one or more of the following symptoms:
- •
- vaginal itching
- •
- vaginal discharge that may be thick, white, and lumpy like cottage cheese
- •
- vaginal soreness, irritation, or burning
- •
- rash or redness on the skin outside the vagina (vulva)
- •
- burning on urination
- •
- painful vaginal intercourse (sex)
Note: Vaginal yeast infections do NOT cause fever, chills, lower abdominal, back or shoulder pain, foul-smelling vaginal discharge, or a missed period. These may be signs of a sexually transmitted disease (STD) or a tubal pregnancy. If you have these symptoms, call your doctor right away.
What are other causes of a vaginal discharge?
It is normal to have a small amount of vaginal discharge at certain times of the month. This normal discharge may be clear or slightly white and does not cause itching, pain or a foul odor.
The most common cause of an abnormal vaginal discharge is an infection. These infections include bacterial vaginosis (BV), trichomoniasis (Trich), gonorrhea (GC) and/or chlamydia. All of these may be transmitted sexually and are called sexually transmitted diseases (STDs). If you have more questions about sexually transmitted diseases (STDs) call the CDC Hotline at 1-800-232-4636.
Although many of the infections mentioned above can cause symptoms similar to a vaginal yeast infection (vaginal discharge, irritation and itching), their diagnosis must be made by a doctor so that proper treatment can be given.
If these infections are not properly treated or if proper treatment is delayed, serious problems, such as pelvic inflammatory disease (PID) may result, which may prevent you from having children in the future. If you are pregnant and do not get the proper treatment, the infection may be passed to your baby before or during delivery and may cause your baby to have permanent damage. If you have multiple sex partners or a new sex partner, you should also ask a doctor before use to make sure you do not have an STD.
Why do women get repeated vaginal yeast infections?
- •
- Women may get repeated vaginal yeast infections that may not clear up easily with proper treatment. Listed below are some of the causes of repeated yeast infections:
- •
- hormonal changes occurring a few days before the monthly period
- •
- use of antibiotics
- •
- use of some birth control pills
- •
- pregnancy
- •
- diabetes (“sugar” or “high blood sugar”)
- •
- clothing - wearing tight layers or moist clothing in the genital area
- •
- weakened immune system - some drugs (such as chemotherapy or steroids) and medical conditions can weaken the body's normal ability to fight infection. One of the most serious of these conditions is infection with the human immunodeficiency virus (HIV - the virus that causes AIDS). Infection with HIV causes the person to be more likely to get infections, including vaginal yeast infections.
If you get vaginal yeast infections often (such as once a month or 3 in 6 months), you should talk to a doctor.
Are vaginal yeast infections sexually transmitted?
Vaginal yeast infections are usually not spread by having intercourse (sex). However, if your partner has a rash, itching or discomfort in his genital area, he should contact a doctor to find out the cause of his symptoms and tell the doctor that you are treating your vaginal yeast infection with this product.
How can I prevent repeated vaginal yeast infections?
To lower your chances of getting another yeast infection:
- •
- Try to keep the genital area cool and dry. Yeast grow well in warm, moist areas. The following suggestions may be helpful:
- •
- Wear cotton underwear and loose-fitting clothes.
- •
- Change out of damp clothes or a wet bathing suit as soon as possible.
- •
- If you use minipads when you are not having a menstrual period, change the minipads often.
- •
- Talk with your doctor about any drugs you are now taking. You are more likely to get a vaginal yeast infection if you are taking certain drugs such as antibiotics, steroids, or birth control pills. Do not stop taking these drugs without first asking your doctor. A doctor may need to see you to make sure that you do not have other medical conditions such as diabetes or a weakened immune system.
Can I use this product during my menstrual period?
Yes, this product can be used during your menstrual period. In fact, many women get vaginal yeast infections just before their period because of hormonal changes. Using this product during your period will not affect how well this product works. If you have started treatment and your period occurs, you should complete the full course of treatment.
Do not use tampons while using this product, because tampons may remove some of the drug from the vagina. Use deodorant-free sanitary napkins or pads instead, and change them often.
Can I use other vaginal products with this product?
This drug should not be used with other vaginal products.
- •
- Douches and tampons may remove some of the cream from the vagina.
- •
- Spermicides may interfere with this product.
- •
- Condoms and diaphragms may be damaged by this product and fail to prevent pregnancy or sexually transmitted diseases (STDs).
How can I get the best results when treating my infection?
- •
- Use 1 applicator full of vaginal cream at bedtime, even during your menstrual period for 7 nights in a row.
- •
- Use the cream externally only while symptoms are present. If you have symptoms (such as itching and irritation) on the skin outside the vagina (vulva), apply the cream externally 2 times a day, up to a total of 7 days, as needed.
- •
- Dry the genital area thoroughly after a shower, bath or swim. Change out of a wet bathing suit or damp clothes as soon as possible. A dry area is less likely to lead to the overgrowth of yeast.
- •
- Wear cotton underwear and loose-fitting clothes.
- •
- Wipe from front to back after a bowel movement or after urination.
- •
- Do not douche, because douching may wash the drug out of the vagina.
- •
- Do not use tampons, because they remove some of the drug from the vagina. Use deodorant-free sanitary napkins or pads as needed.
- •
- Do not use spermicides, as they may interfere with this product.
- •
- Do not have vaginal intercourse while using this product.
- •
- Do not scratch the skin outside the vagina. Scratching can cause more irritation and can spread the infection.
- •
- Tell your doctor about any drugs you are now taking.Certain drugs such as antibiotics, steroids, and birth control pills, may make it more likely for you to get a vaginal yeast infection. If you are taking any of these drugs do not stop taking them without first asking a doctor.
- •
- If you have any other medical questions or concerns about vaginal yeast infections, call your doctor.
What warnings should I know about when using this product?
For vaginal use only.
Do not use if you have never had a vaginal yeast infection diagnosed by a doctor.
Ask a doctor before use if you have:
- •
- vaginal itching and discomfort for the first time. You may need a different treatment.
- •
- lower abdominal, back or shoulder pain, fever, chills, nausea, vomiting, or foul-smelling vaginal discharge. You could have a more serious condition.
- •
- vaginal yeast infections often (such as once a month or 3 in 6 months). You could be pregnant or have a serious underlying medical cause for your symptoms, including diabetes or a weakened immune system.
- •
- been exposed to the human immunodeficiency virus (HIV) that causes AIDS.
Ask a doctor or pharmacist before use if you are taking the prescription blood thinning medicine, warfarin (coumadin), because bleeding or bruising may occur.
When using this product:
- •
- do not use tampons, douches, spermicides, or other vaginal products. Condoms and diaphragms may be damaged and fail to prevent pregnancy or sexually transmitted diseases (STDs).
- •
- do not have vaginal intercourse
- •
- mild increase in vaginal burning, itching or irritation may occur
- •
- if you do not get complete relief ask a doctor before using another product
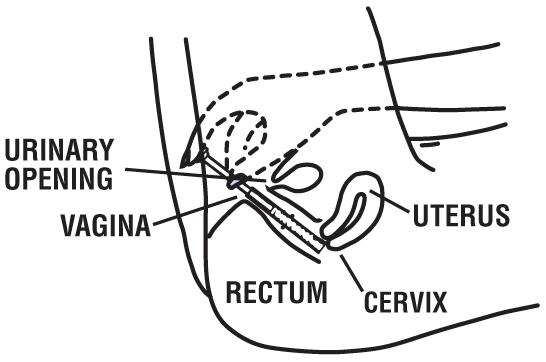
How Should I Use Miconazole Nitrate Vaginal Cream with Disposable Applicators?
This product is for adults and children 12 years of age and over. For children under 12 years, ask a doctor.
Directions for using the Vaginal Cream:
Begin treatment before going to bed:
1 Open the tube by unscrewing the cap. The first time the tube is opened, press the sharp point of the cap into the sealed end of the tube. Push down firmly until the seal is open.
2 Attach the applicator to the tube of cream by placing “A” end of the applicator firmly onto tube of cream. (See picture.)
3 Gently squeeze the cream into the applicator, continue squeezing until applicator is full. Separate the applicator from the tube. DO NOT release pressure on the tube until you have separated it from the filled applicator. After each use, replace the cap and roll up the tube from the bottom.
4 Hold the barrel as shown in the picture.
5 Gently insert the applicator into the vagina as far as it will go comfortably. This can be done while lying on your back with your knees bent (as shown in the picture) or while standing with your feet apart and knees bent.
6 With one hand holding the barrel, use the other hand to push the plunger all the way in to place the cream as far back in the vagina as possible. Then remove both parts of the applicator from the vagina.
7 Throw the applicator away after use. Do not flush in toilet.
8 Lie down as soon as possible after inserting the cream. This will reduce leakage.
9 Repeat steps 2 through 8 before bedtime for the next 6 days.
You may want to use deodorant-free pads or pantyshields to protect your clothing during the time that you are using this product. This is because the vaginal cream can leak or you may get some discharge. Do not use tampons, douches, spermicides, condoms or diaphragms until after you have completed the treatment and your symptoms are gone.
Directions for using the Vaginal Cream Externally
Use externally twice daily, for up to 7 days as needed.
1. Squeeze a small amount of cream onto your fingertip.
2. Apply the cream onto the skin outside the vagina (vulva) that itches and is irritated.
3. Screw the cap back on the tube.
4. Repeat steps 1-3 each morning and at bedtime for up to 7 days, as needed.
Stop use and ask your doctor if:
• symptoms do not get better in 3 days
• symptoms last more than 7 days
• you get a rash or hives, abdominal pain, fever, chills, nausea, vomiting, or foul-smelling vaginal discharge.
These may be signs that this product is not working, or you may have a more serious condition or an allergic reaction.
If pregnant or breast-feeding, ask a health professional before use.
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away (1-800-222-1222).
What side effects may occur with this product?
A mild increase in vaginal burning, itching, or irritation may occur when the applicator containing the cream is inserted. Abdominal cramping has also been reported.
Stop using this product and consult your doctor if you have abdominal pain, hives, skin rash, or if you have severe vaginal burning, itching, or irritation or swelling.
What should I do if I have questions about this product?
Questions of a medical nature should be taken up with your doctor. If you have any other questions or need more information on this product, call our toll-free number 1-800-719-9260
Other Information:
- •
- TAMPER-EVIDENT UNIT - do not use if tube seal has been punctured.
- •
- store at 20º-25ºC (68º-77ºF)
Active Ingredient: miconazole nitrate 2% (100 mg per applicator)
Inactive Ingredients: benzoic acid, butylated hydroxyanisole, glyceryl stearate, mineral oil, peglicol 5 oleate, pegoxol 7 stearate, purified water.
Distributed By
Perrigo®
Allegan, MI 49010
02/16
: 82500 00 J3
Principal Display Panel
INGREDIENTS AND APPEARANCE
| TOPCARE MICONAZOLE 7
miconazole nitrate cream |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Topco Associates LLC (006935977) |