Search by Drug Name or NDC
NDC 41250-0507-34 mucus and chest congestion 200 mg/10mL Details
mucus and chest congestion 200 mg/10mL
mucus and chest congestion is a ORAL SOLUTION in the HUMAN OTC DRUG category. It is labeled and distributed by Meijer Distribution Inc. The primary component is GUAIFENESIN.
MedlinePlus Drug Summary
Guaifenesin is used to relieve chest congestion. Guaifenesin may help control symptoms but does not treat the cause of symptoms or speed recovery. Guaifenesin is in a class of medications called expectorants. It works by thinning the mucus in the air passages to make it easier to cough up the mucus and clear the airways.
Related Packages: 41250-0507-34Last Updated: 11/30/2022
MedLinePlus Full Drug Details: Guaifenesin
Product Information
| NDC | 41250-0507 |
|---|---|
| Product ID | 41250-507_7c92afa0-dd2a-43c9-bf5a-9ea9db475420 |
| Associated GPIs | 43200010000910 |
| GCN Sequence Number | n/a |
| GCN Sequence Number Description | n/a |
| HIC3 | n/a |
| HIC3 Description | n/a |
| GCN | n/a |
| HICL Sequence Number | n/a |
| HICL Sequence Number Description | n/a |
| Brand/Generic | n/a |
| Proprietary Name | mucus and chest congestion |
| Proprietary Name Suffix | n/a |
| Non-Proprietary Name | Guaifenesin |
| Product Type | HUMAN OTC DRUG |
| Dosage Form | SOLUTION |
| Route | ORAL |
| Active Ingredient Strength | 200 |
| Active Ingredient Units | mg/10mL |
| Substance Name | GUAIFENESIN |
| Labeler Name | Meijer Distribution Inc |
| Pharmaceutical Class | n/a |
| DEA Schedule | n/a |
| Marketing Category | OTC MONOGRAPH FINAL |
| Application Number | part341 |
| Listing Certified Through | 2024-12-31 |
Package
Package Images
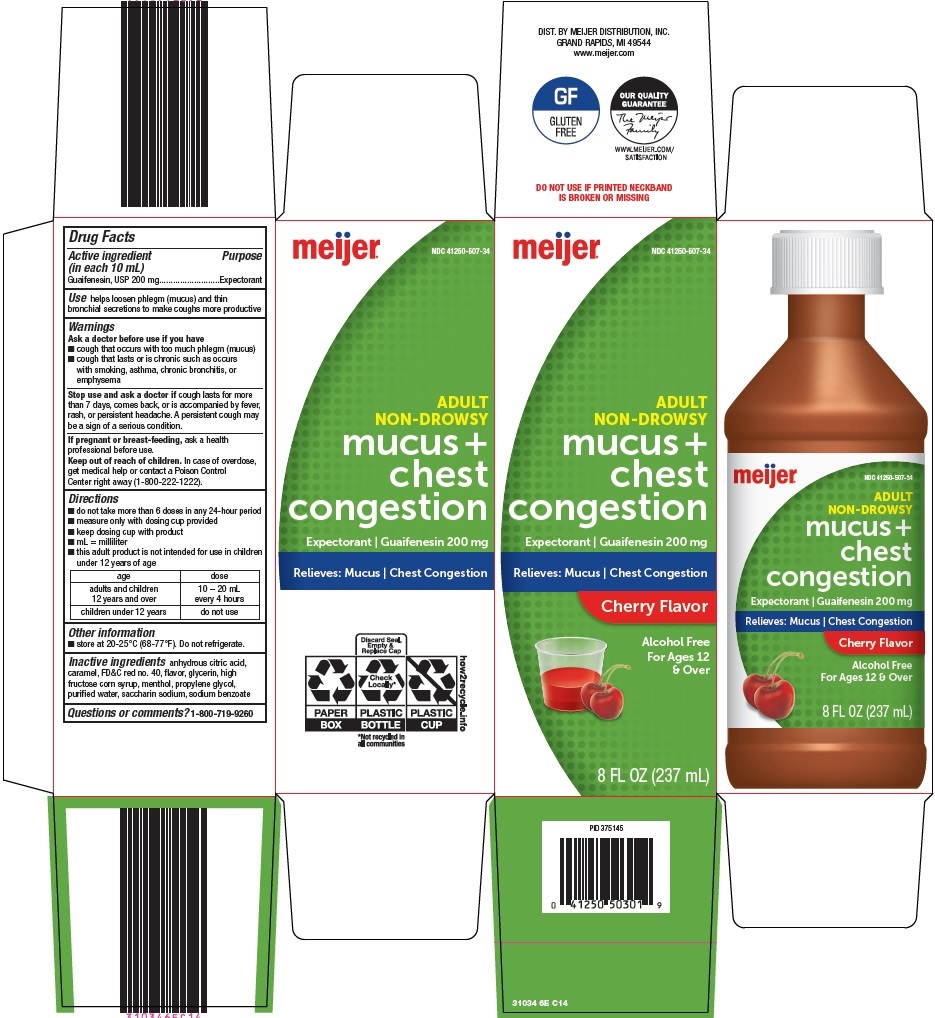
NDC 41250-0507-34 (41250050734)
| NDC Package Code | 41250-507-34 |
|---|---|
| Billing NDC | 41250050734 |
| Package | 1 BOTTLE in 1 CARTON (41250-507-34) / 237 mL in 1 BOTTLE |
| Marketing Start Date | 2014-07-15 |
| NDC Exclude Flag | N |
| Pricing Information | N/A |
Standard Product Labeling (SPL)/Prescribing Information SPL a1213e26-bbd3-4aef-9fe5-55325e768afc Details
Warnings
Ask a doctor before use if you have
- •
- cough that occurs with too much phlegm (mucus)
- •
- cough that lasts or is chronic such as occurs with smoking, asthma, chronic bronchitis, or emphysema
Directions
- •
- do not take more than 6 doses in any 24-hour period
- •
- measure only with dosing cup provided
- •
- keep dosing cup with product
- •
- mL = milliliter
- •
- this adult product is not intended for use in children under 12 years of age
|
age |
dose |
|
adults and children 12 years and over |
10 – 20 mL every 4 hours |
|
children under 12 years |
do not use |
Inactive ingredients
Principal Display Panel
INGREDIENTS AND APPEARANCE
| MUCUS AND CHEST CONGESTION
guaifenesin solution |
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
| Labeler - Meijer Distribution Inc (006959555) |
Revised: 4/2021
Document Id: 7c92afa0-dd2a-43c9-bf5a-9ea9db475420
Set id: a1213e26-bbd3-4aef-9fe5-55325e768afc
Version: 5
Effective Time: 20210429