Search by Drug Name or NDC
NDC 42571-0112-30 LOSARTAN POTASSIUM 100 mg/1 Details
LOSARTAN POTASSIUM 100 mg/1
LOSARTAN POTASSIUM is a ORAL TABLET, FILM COATED in the HUMAN PRESCRIPTION DRUG category. It is labeled and distributed by Micro Labs Limited. The primary component is LOSARTAN POTASSIUM.
MedlinePlus Drug Summary
Losartan is used alone or in combination with other medications to treat high blood pressure. Losartan is also used to decrease the risk of stroke in people who have high blood pressure and a heart condition called left ventricular hypertrophy (enlargement of the walls of the left side of the heart). Losartan may not decrease the risk of stroke in African Americans who have these conditions. This medication is also used to treat kidney disease in people who have type 2 diabetes (condition in which the body does not use insulin normally and therefore cannot control the amount of sugar in the blood) and high blood pressure. Losartan is in a class of medications called angiotensin II receptor antagonists. It works by blocking the action of certain natural substances that tighten the blood vessels, allowing the blood to flow more smoothly and the heart to pump more efficiently. High blood pressure is a common condition, and when not treated it can cause damage to the brain, heart, blood vessels, kidneys, and other parts of the body. Damage to these organs may cause heart disease, a heart attack, heart failure, stroke, kidney failure, loss of vision, and other problems. In addition to taking medication, making lifestyle changes will also help to control your blood pressure. These changes include eating a diet that is low in fat and salt, maintaining a healthy weight, exercising at least 30 minutes most days, not smoking, and using alcohol in moderation.
Related Packages: 42571-0112-30Last Updated: 11/30/2022
MedLinePlus Full Drug Details: Losartan
Product Information
| NDC | 42571-0112 |
|---|---|
| Product ID | 42571-112_d279edfe-37b8-fbf9-e053-2995a90a0c24 |
| Associated GPIs | 36150040200340 |
| GCN Sequence Number | 038686 |
| GCN Sequence Number Description | losartan potassium TABLET 100 MG ORAL |
| HIC3 | A4F |
| HIC3 Description | ANTIHYPERTENSIVES, ANGIOTENSIN RECEPTOR ANTAGONIST |
| GCN | 14853 |
| HICL Sequence Number | 009829 |
| HICL Sequence Number Description | LOSARTAN POTASSIUM |
| Brand/Generic | Generic |
| Proprietary Name | LOSARTAN POTASSIUM |
| Proprietary Name Suffix | n/a |
| Non-Proprietary Name | losartan potassium |
| Product Type | HUMAN PRESCRIPTION DRUG |
| Dosage Form | TABLET, FILM COATED |
| Route | ORAL |
| Active Ingredient Strength | 100 |
| Active Ingredient Units | mg/1 |
| Substance Name | LOSARTAN POTASSIUM |
| Labeler Name | Micro Labs Limited |
| Pharmaceutical Class | Angiotensin 2 Receptor Antagonists [MoA], Angiotensin 2 Receptor Blocker [EPC] |
| DEA Schedule | n/a |
| Marketing Category | ANDA |
| Application Number | ANDA091541 |
| Listing Certified Through | 2024-12-31 |
Package
Package Images


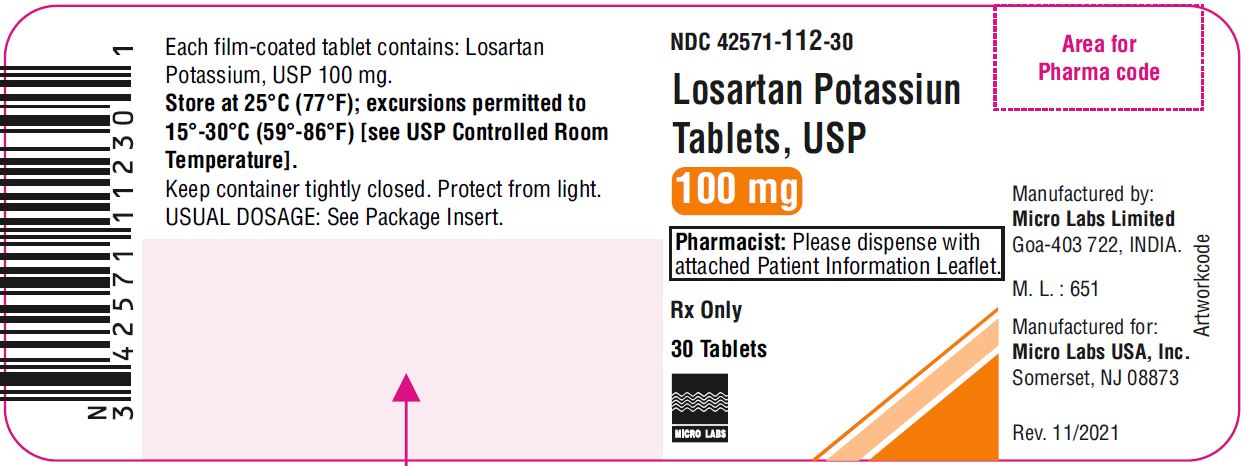
NDC 42571-0112-30 (42571011230)
| NDC Package Code | 42571-112-30 |
|---|---|
| Billing NDC | 42571011230 |
| Package | 30 TABLET, FILM COATED in 1 BOTTLE (42571-112-30) |
| Marketing Start Date | 2012-12-06 |
| NDC Exclude Flag | N |
| Pricing Information | N/A |
Standard Product Labeling (SPL)/Prescribing Information SPL f2f2b6c6-0ab1-483f-b4a2-78dfe3200153 Details
HIGHLIGHTS OF PRESCRIBING INFORMATION
LOSARTAN POTASSIUM tablets, for oral use
Initial U.S. Approval: 1995
WARNING: FETAL TOXICITY
See full prescribing information for complete boxed warning.
When pregnancy is detected, discontinue losartan potassium as soon as possible. Drugs that act directly on the renin-angiotensin system can cause injury and death to the developing fetus. ( 5.1)
INDICATIONS AND USAGE
Losartan potassium tablet is an angiotensin II receptor blocker (ARB) indicated for:
- Treatment of hypertension, to lower blood pressure in adults and children greater than 6 years old. Lowering blood pressure reduces the risk of fatal and nonfatal cardiovascular events, primarily strokes and myocardial infarctions. ( 1.1)
- Reduction of the risk of stroke in patients with hypertension and left ventricular hypertrophy. There is evidence that this benefit does not apply to Black patients. ( 1.2)
- Treatment of diabetic nephropathy with an elevated serum creatinine and proteinuria in patients with type 2 diabetes and a history of hypertension. ( 1.3)
DOSAGE AND ADMINISTRATION
Hypertension
- Usual adult dose: 50 mg once daily. ( 2.1)
- Usual pediatric starting dose: 0.7 mg per kg once daily (up to 50 mg). ( 2.1)
Hypertensive Patients with Left Ventricular Hypertrophy
- Usual starting dose: 50 mg once daily. ( 2.2)
- Add hydrochlorothiazide 12.5 mg and/or increase losartan potassium to 100 mg followed by an increase to hydrochlorothiazide 25 mg if further blood pressure response is needed. ( 2.2, 14.2)
Nephropathy in Type 2 Diabetic Patients
DOSAGE FORMS AND STRENGTHS
Tablets: 25 mg; 50 mg; and 100 mg. ( 3)
CONTRAINDICATIONS
WARNINGS AND PRECAUTIONS
ADVERSE REACTIONS
Most common adverse reactions (incidence ≥2% and greater than placebo) are: dizziness, upper respiratory infection, nasal congestion, and back pain. ( 6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Micro Labs USA, Inc. at 1-855-839-8195 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS
- Agents Increasing Serum Potassium: Risk of hyperkalemia. ( 7.1)
- Lithium: Risk of lithium toxicity. ( 7.2)
- NSAIDs: Increased risk of renal impairment and reduced diuretic, natriuretic, and antihypertensive effects. ( 7.3)
- Dual Inhibition of the Renin-Angiotensin System: Increased risk of renal impairment, hypotension, syncope, and hyperkalemia. ( 7.4)
USE IN SPECIFIC POPULATIONS
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 11/2021
Table Of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
WARNING: FETAL TOXICITY
1 INDICATIONS AND USAGE
1.1 Hypertension
1.2 Hypertensive Patients with Left Ventricular Hypertrophy
1.3 Nephropathy in Type 2 Diabetic Patients
2 DOSAGE AND ADMINISTRATION
2.1 Hypertension
2.2 Hypertensive Patients with Left Ventricular Hypertrophy
2.3 Nephropathy in Type 2 Diabetic Patients
2.4 Dosage Modifications in Patients with Hepatic Impairment
2.5 Preparation of Suspension (for 200 mL of a 2.5 mg/mL suspension)
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Fetal Toxicity
5.2 Hypotension in Volume- or Salt-Depleted Patients
5.3 Renal Function Deterioration
5.4 Hyperkalemia
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Postmarketing Experience
7 DRUG INTERACTIONS
7.1 Agents Increasing Serum Potassium
7.2 Lithium
7.3 Non-Steroidal Anti-Inflammatory Drugs (NSAIDs) Including Selective Cyclooxygenase-2 Inhibitors (COX-2 Inhibitors)
7.4 Dual Blockade of the Renin-Angiotensin System (RAS)
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Race
8.7 Renal Impairment
8.8 Hepatic Impairment
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
14.1 Hypertension
14.2 Hypertensive Patients with Left Ventricular Hypertrophy
14.3 Nephropathy in Type 2 Diabetic Patients
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- *
- Sections or subsections omitted from the full prescribing information are not listed.
WARNING
When pregnancy is detected, discontinue losartan potassium as soon as possible. Drugs that act directly on the renin-angiotensin system can cause injury and death to the developing fetus [see Warnings and Precautions ( 5.1)].
1 INDICATIONS AND USAGE
1.1 Hypertension
Losartan potassium tablets are indicated for the treatment of hypertension in adults and pediatric patients 6 years of age and older, to lower blood pressure. Lowering blood pressure lowers the risk of fatal and nonfatal cardiovascular (CV) events, primarily strokes and myocardial infarction. These benefits have been seen in controlled trials of antihypertensive drugs from a wide variety of pharmacologic classes including losartan.
Control of high blood pressure should be part of comprehensive cardiovascular risk management, including, as appropriate, lipid control, diabetes management, antithrombotic therapy, smoking cessation, exercise, and limited sodium intake. Many patients will require more than 1 drug to achieve blood pressure goals. For specific advice on goals and management, see published guidelines, such as those of the National High Blood Pressure Education Program’s Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC).
Numerous antihypertensive drugs, from a variety of pharmacologic classes and with different mechanisms of action, have been shown in randomized controlled trials to reduce cardiovascular morbidity and mortality, and it can be concluded that it is blood pressure reduction, and not some other pharmacologic property of the drugs, that is largely responsible for those benefits. The largest and most consistent cardiovascular outcome benefit has been a reduction in the risk of stroke, but reductions in myocardial infarction and cardiovascular mortality also have been seen regularly.
Elevated systolic or diastolic pressure causes increased cardiovascular risk, and the absolute risk increase per mmHg is greater at higher blood pressures, so that even modest reductions of severe hypertension can provide substantial benefit. Relative risk reduction from blood pressure reduction is similar across populations with varying absolute risk, so the absolute benefit is greater in patients who are at higher risk independent of their hypertension (for example, patients with diabetes or hyperlipidemia), and such patients would be expected to benefit from more aggressive treatment to a lower blood pressure goal.
Some antihypertensive drugs have smaller blood pressure effects (as monotherapy) in Black patients, and many antihypertensive drugs have additional approved indications and effects (e.g., on angina, heart failure, or diabetic kidney disease). These considerations may guide selection of therapy.
Losartan potassium tablets may be administered with other antihypertensive agents.
1.2 Hypertensive Patients with Left Ventricular Hypertrophy
Losartan potassium tablets are indicated to reduce the risk of stroke in patients with hypertension and left ventricular hypertrophy, but there is evidence that this benefit does not apply to Black patients [see Use in Specific Populations ( 8.6) and Clinical Pharmacology ( 12.3)].
1.3 Nephropathy in Type 2 Diabetic Patients
Losartan potassium tablets are indicated for the treatment of diabetic nephropathy with an elevated serum creatinine and proteinuria (urinary albumin to creatinine ratio ≥300 mg/g) in patients with type 2 diabetes and a history of hypertension. In this population, losartan potassium tablets reduces the rate of progression of nephropathy as measured by the occurrence of doubling of serum creatinine or end stage renal disease (need for dialysis or renal transplantation) [see Clinical Studies ( 14.3)].
2 DOSAGE AND ADMINISTRATION
2.1 Hypertension
Adult Hypertension
The usual starting dose of losartan potassium tablet is 50 mg once daily. The dosage can be increased to a maximum dose of 100 mg once daily as needed to control blood pressure [see Clinical Studies ( 14.1)]. A starting dose of 25 mg is recommended for patients with possible intravascular depletion (e.g., on diuretic therapy).
Pediatric Hypertension
The usual recommended starting dose is 0.7 mg per kg once daily (up to 50 mg total) administered as a tablet or a suspension [see Dosage and Administration ( 2.5)]. Dosage should be adjusted according to blood pressure response. Doses above 1.4 mg per kg (or in excess of 100 mg) daily have not been studied in pediatric patients [see Clinical Pharmacology ( 12.3), Clinical Studies ( 14.1), and Warnings and Precautions ( 5.2)].
Losartan potassium tablets is not recommended in pediatric patients less than 6 years of age or in pediatric patients with estimated glomerular filtration rate less than 30 mL/min/1.73 m 2 [see Use in Specific Populations ( 8.4), Clinical Pharmacology ( 12.3), and Clinical Studies ( 14)].
2.2 Hypertensive Patients with Left Ventricular Hypertrophy
The usual starting dose is 50 mg of losartan potassium tablets once daily. Hydrochlorothiazide 12.5 mg daily should be added and/or the dose of Losartan potassium tablets should be increased to 100 mg once daily followed by an increase in hydrochlorothiazide to 25 mg once daily based on blood pressure response [see Clinical Studies ( 14.2)].
2.3 Nephropathy in Type 2 Diabetic Patients
The usual starting dose is 50 mg once daily. The dose should be increased to 100 mg once daily based on blood pressure response [see Clinical Studies ( 14.3)].
2.4 Dosage Modifications in Patients with Hepatic Impairment
In patients with mild-to-moderate hepatic impairment the recommended starting dose of losartan potassium tablet is 25 mg once daily. Losartan potassium tablets has not been studied in patients with severe hepatic impairment [see Use in Specific Populations (8.8) and Clinical Pharmacology (12.3)].
2.5 Preparation of Suspension (for 200 mL of a 2.5 mg/mL suspension)
Add 10 mL of Purified Water USP to an 8 ounce (240 mL) amber polyethylene terephthalate (PET) bottle containing ten 50 mg losartan potassium tablets. Immediately shake for at least 2 minutes. Let the concentrate stand for 1 hour and then shake for 1 minute to disperse the tablet contents. Separately prepare a 50/50 volumetric mixture of Ora-Plus TM and Ora-Sweet SF TM. Add 190 mL of the 50/50 Ora-Plus TM /Ora- Sweet SF TM mixture to the tablet and water slurry in the PET bottle and shake for 1 minute to disperse the ingredients. The suspension should be refrigerated at 2 to 8°C (36 to 46°F) and can be stored for up to 4 weeks. Shake the suspension prior to each use and return promptly to the refrigerator.
3 DOSAGE FORMS AND STRENGTHS
- Losartan potassium tablets USP 25 mg are white to off-white, oval shaped, film-coated tablets with “A” engraved on one side and “25” on the other side.
- Losartan potassium tablets USP 50 mg are white to off-white, oval shaped, film-coated tablets with “A50” engraved on one side and breakline on the other side.
- Losartan potassium tablets USP 100 mg are white to off-white, oval shaped, film-coated tablets with “A100” engraved on one side and plain on the other side.
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Fetal Toxicity
Losartan potassium can cause fetal harm when administered to a pregnant woman. Use of drugs that act on the renin-angiotensin system during the second and third trimesters of pregnancy reduces fetal renal function and increases fetal and neonatal morbidity and death. Resulting oligohydramnios can be associated with fetal lung hypoplasia and skeletal deformations. Potential neonatal adverse effects include skull hypoplasia, anuria, hypotension, renal failure, and death. When pregnancy is detected, discontinue losartan potassium as soon as possible [see Use in Specific Populations (8.1)].
5.2 Hypotension in Volume- or Salt-Depleted Patients
In patients with an activated renin-angiotensin system, such as volume- or salt-depleted patients (e.g., those being treated with high doses of diuretics), symptomatic hypotension may occur after initiation of treatment with losartan potassium. Correct volume or salt depletion prior to administration of losartan potassium [see Dosage and Administration ( 2.1)].
5.3 Renal Function Deterioration
Changes in renal function including acute renal failure can be caused by drugs that inhibit the renin-angiotensin system and by diuretics. Patients whose renal function may depend in part on the activity of the renin-angiotensin system (e.g., patients with renal artery stenosis, chronic kidney disease, severe congestive heart failure, or volume depletion) may be at particular risk of developing acute renal failure on losartan potassium. Monitor renal function periodically in these patients. Consider withholding or discontinuing therapy in patients who develop a clinically significant decrease in renal function on losartan potassium [see Drug Interactions ( 7.3) and Use in Specific Populations ( 8.7)].
5.4 Hyperkalemia
Monitor serum potassium periodically and treat appropriately. Dosage reduction or discontinuation of losartan potassium may be required [see Adverse Reactions ( 6.1)].
Concomitant use of other drugs that may increase serum potassium may lead to hyperkalemia [see Drug Interactions (7.1)].
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Hypertension
Losartan potassium has been evaluated for safety in more than 3300 adult patients treated for essential hypertension and 4058 patients/subjects overall. Over 1200 patients were treated for over 6 months and more than 800 for over one year.
Treatment with losartan potassium was well-tolerated with an overall incidence of adverse events similar to that of placebo. In controlled clinical trials, discontinuation of therapy for adverse events occurred in 2.3% of patients treated with losartan potassium and 3.7% of patients given placebo. In 4 clinical trials involving over 1000 patients on various doses (10 to 150 mg) of losartan potassium and over 300 patients given placebo, the adverse events that occurred in ≥2% of patients treated with losartan potassium and more commonly than placebo were: dizziness (3% vs. 2%), upper respiratory infection (8% vs. 7%), nasal congestion (2% vs. 1%), and back pain (2% vs. 1%).
The following less common adverse reactions have been reported:
Blood and lymphatic system disorders: Anemia.
Psychiatric disorders: Depression.
Nervous system disorders: Somnolence, headache, sleep disorders, paresthesia, migraine.
Ear and labyrinth disorders: Vertigo, tinnitus.
Cardiac disorders: Palpitations, syncope, atrial fibrillation, CVA.
Respiratory, thoracic and mediastinal disorders: Dyspnea.
Gastrointestinal disorders: Abdominal pain, constipation, nausea, vomiting.
Skin and subcutaneous tissue disorders: Urticaria, pruritus, rash, photosensitivity.
Musculoskeletal and connective tissue disorders: Myalgia, arthralgia.
Reproductive system and breast disorders: Impotence.
General disorders and administration site conditions: Edema.
Cough
Persistent dry cough (with an incidence of a few percent) has been associated with ACE-inhibitor use and in practice can be a cause of discontinuation of ACE-inhibitor therapy. Two prospective, parallel-group, double-blind, randomized, controlled trials were conducted to assess the effects of losartan on the incidence of cough in hypertensive patients who had experienced cough while receiving ACE-inhibitor therapy. Patients who had typical ACE-inhibitor cough when challenged with lisinopril, whose cough disappeared on placebo, were randomized to losartan 50 mg, lisinopril 20 mg, or either placebo (one study, n=97) or 25 mg hydrochlorothiazide (n=135). The double-blind treatment period lasted up to 8 weeks. The incidence of cough is shown in Table 1 below.
Table 1:
| Study 1
*
| HCTZ
| Losartan
| Lisinopril
|
| Cough
| 25%
| 17%
| 69%
|
| Study 2
†
| Placebo
| Losartan
| Lisinopril
|
| Cough
| 35%
| 29%
| 62%
|
* Demographics = (89% Caucasian, 64% female)
† Demographics = (90% Caucasian, 51% female)
These studies demonstrate that the incidence of cough associated with losartan therapy, in a population that all had cough associated with ACE-inhibitor therapy, is similar to that associated with hydrochlorothiazide or placebo therapy.
Cases of cough, including positive re-challenges, have been reported with the use of losartan in postmarketing experience.
Hypertensive Patients with Left Ventricular Hypertrophy
In the Losartan Intervention for Endpoint (LIFE) study, adverse reactions with losartan potassium were similar to those reported previously for patients with hypertension.
Nephropathy in Type 2 Diabetic Patients
In the Reduction of Endpoints in NIDDM with the Angiotensin II Receptor Antagonist Losartan (RENAAL) study involving 1513 patients treated with losartan potassium or placebo, the overall incidences of reported adverse events were similar for the two groups. Discontinuations of losartan potassium because of side effects were similar to placebo (19% for losartan potassium, 24% for placebo). The adverse events, regardless of drug relationship, reported with an incidence of ≥4% of patients treated with losartan potassium and occurring with ≥2% difference in the losartan group vs. placebo on a background of conventional antihypertensive therapy, were asthenia/fatigue, chest pain, hypotension, orthostatic hypotension, diarrhea, anemia, hyperkalemia, hypoglycemia, back pain, muscular weakness, and urinary tract infection.
6.2 Postmarketing Experience
The following additional adverse reactions have been reported in postmarketing experience with losartan potassium. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to estimate their frequency reliably or to establish a causal relationship to drug exposure:
Digestive: Hepatitis.
General Disorders and Administration Site Conditions: Malaise.
Hematologic: Thrombocytopenia.
Hypersensitivity: Angioedema, including swelling of the larynx and glottis, causing airway obstruction and/or swelling of the face, lips, pharynx, and/or tongue has been reported rarely in patients treated with losartan; some of these patients previously experienced angioedema with other drugs including ACE inhibitors. Vasculitis, including Henoch-Schönlein purpura, has been reported. Anaphylactic reactions have been reported.
Metabolic and Nutrition: Hyponatremia.
Musculoskeletal: Rhabdomyolysis.
Nervous System Disorders: Dysgeusia.
Skin: Erythroderma.
7 DRUG INTERACTIONS
7.1 Agents Increasing Serum Potassium
Coadministration of losartan with other drugs that raise serum potassium levels may result in hyperkalemia. Monitor serum potassium in such patients.
7.2 Lithium
Increases in serum lithium concentrations and lithium toxicity have been reported during concomitant administration of lithium with angiotensin II receptor antagonists. Monitor serum lithium levels during concomitant use.
7.3 Non-Steroidal Anti-Inflammatory Drugs (NSAIDs) Including Selective Cyclooxygenase-2 Inhibitors (COX-2 Inhibitors)
In patients who are elderly, volume-depleted (including those on diuretic therapy), or with compromised renal function, coadministration of NSAIDs, including selective COX-2 inhibitors, with angiotensin II receptor antagonists (including losartan) may result in deterioration of renal function, including possible acute renal failure. These effects are usually reversible. Monitor renal function periodically in patients receiving losartan and NSAID therapy.
The antihypertensive effect of angiotensin II receptor antagonists, including losartan, may be attenuated by NSAIDs, including selective COX-2 inhibitors.
7.4 Dual Blockade of the Renin-Angiotensin System (RAS)
Dual blockade of the RAS with angiotensin receptor blockers, ACE inhibitors, or aliskiren is associated with increased risks of hypotension, syncope, hyperkalemia, and changes in renal function (including acute renal failure) compared to monotherapy.
The Veterans Affairs Nephropathy in Diabetes (VA NEPHRON-D) trial enrolled 1448 patients with type 2 diabetes, elevated urinary-albumin-to-creatinine ratio, and decreased estimated glomerular filtration rate (GFR 30 to 89.9 mL/min), randomized them to lisinopril or placebo on a background of losartan therapy and followed them for a median of 2.2 years. Patients receiving the combination of losartan and lisinopril did not obtain any additional benefit compared to monotherapy for the combined endpoint of decline in GFR, end stage renal disease, or death, but experienced an increased incidence of hyperkalemia and acute kidney injury compared with the monotherapy group.
In most patients no benefit has been associated with using two RAS inhibitors concomitantly. In general, avoid combined use of RAS inhibitors. Closely monitor blood pressure, renal function, and electrolytes in patients on losartan potassium and other agents that affect the RAS.
Do not coadminister aliskiren with losartan potassium in patients with diabetes. Avoid use of aliskiren with losartan potassium in patients with renal impairment (GFR <60 mL/min).
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
Losartan potassium can cause fetal harm when administered to a pregnant woman. Use of drugs that act on the renin-angiotensin system during the second and third trimesters of pregnancy reduces fetal renal function and increases fetal and neonatal morbidity and death. Most epidemiologic studies examining fetal abnormalities after exposure to antihypertensive use in the first trimester have not distinguished drugs affecting the renin-angiotensin system from other antihypertensive agents. When pregnancy is detected, discontinue losartan potassium as soon as possible ( see Clinical Considerations).
The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively.
Clinical Considerations
Disease-associated Maternal and/or Embryo/Fetal Risk Hypertension in pregnancy increases the maternal risk for pre-eclampsia, gestational diabetes, premature delivery, and delivery complications (e.g., need for cesarean section, post-partum hemorrhage). Hypertension increases the fetal risk for intrauterine growth restriction and intrauterine death. Pregnant women with hypertension should be carefully monitored and managed accordingly.
Fetal/Neonatal Adverse Reactions
Oligohydramnios in pregnant women who use drugs affecting the renin-angiotensin system in the second and third trimesters of pregnancy can result in the following: reduced fetal renal function leading to anuria and renal failure, fetal lung hypoplasia, skeletal deformations, including skull hypoplasia, hypotension, and death. In the unusual case that there is no appropriate alternative to therapy with drugs affecting the renin-angiotensin system for a particular patient, apprise the mother of the potential risk to the fetus.
In patients taking losartan potassium during pregnancy, perform serial ultrasound examinations to assess the intra-amniotic environment. Fetal testing may be appropriate, based on the week of gestation. If oligohydramnios is observed, discontinue losartan potassium, unless it is considered lifesaving for the mother. Patients and physicians should be aware, however, that oligohydramnios may not appear until after the fetus has sustained irreversible injury.
Closely observe neonates with histories of in utero exposure to losartan potassium for hypotension, oliguria, and hyperkalemia. In neonates with a history of in utero exposure to losartan potassium, if oliguria or hypotension occurs, support blood pressure and renal perfusion. Exchange transfusions or dialysis may be required as a means of reversing hypotension and replacing renal function.
Data
Animal Data
Losartan potassium was administered orally to rats during the period of late gestation through lactation (Gestation Day 15 through Lactation Day 20) at doses of 10, 25, and 100 mg/kg/day. Losartan potassium has been shown to produce adverse effects in rat fetuses and neonates, including decreased body weight, delayed physical and behavioral development, mortality and renal toxicity. With the exception of neonatal weight gain (which was affected at doses as low as 10 mg/kg/day), doses associated with these effects exceeded 25 mg/kg/day (approximately three times the maximum recommended human dose of 100 mg on a mg/m 2 basis). These findings are attributed to drug exposure in late gestation and during lactation. Significant levels of losartan and its active metabolite were shown to be present in rat fetal plasma during late gestation and in rat milk.
8.2 Lactation
Risk Summary
It is not known whether losartan is excreted in human milk, but significant levels of losartan and its active metabolite were shown to be present in rat milk. Because of the potential for adverse effects on the nursing infant, a decision should be made whether to discontinue nursing or discontinue the drug, taking into account the importance of the drug to the mother.
8.4 Pediatric Use
Antihypertensive effects of losartan potassium have been established in hypertensive pediatric patients aged 6 to 16 years. Safety and effectiveness have not been established in pediatric patients under the age of 6 or in pediatric patients with glomerular filtration rate <30 mL/min/1.73 m 2 [see Dosage and Administration ( 2.1), Clinical Pharmacology ( 12.3), and Clinical Studies ( 14.1)].
8.5 Geriatric Use
Of the total number of patients receiving losartan potassium in controlled clinical studies for hypertension, 391 patients (19%) were 65 years and over, while 37 patients (2%) were 75 years and over. In a controlled clinical study for renal protection in type 2 diabetic patients with proteinuria, 248 patients (33%) were 65 years and over. In a controlled clinical study for the reduction in the combined risk of cardiovascular death, stroke and myocardial infarction in hypertensive patients with left ventricular hypertrophy, 2857 patients (62%) were 65 years and over, while 808 patients (18%) were 75 years and over. No overall differences in effectiveness or safety were observed between these patients and younger patients, but greater sensitivity of some older individuals cannot be ruled out.
8.6 Race
In the LIFE study, Black patients with hypertension and left ventricular hypertrophy treated with atenolol were at lower risk of experiencing the primary composite endpoint compared with Black patients treated with losartan potassium (both cotreated with hydrochlorothiazide in the majority of patients). The primary endpoint was the first occurrence of stroke, myocardial infarction or cardiovascular death, analyzed using an intention-to-treat (ITT) approach. In the subgroup of Black patients (n=533, 6% of the LIFE study patients), there were 29 primary endpoints among 263 patients on atenolol (11%, 26 per 1000 patient-years) and 46 primary endpoints among 270 patients (17%, 42 per 1000 patient-years) on losartan potassium. This finding could not be explained on the basis of differences in the populations other than race or on any imbalances between treatment groups. In addition, blood pressure reductions in both treatment groups were consistent between Black and non-Black patients. Given the difficulty in interpreting subset differences in large trials, it cannot be known whether the observed difference is the result of chance. However, the LIFE study provides no evidence that the benefits of losartan potassium on reducing the risk of cardiovascular events in hypertensive patients with left ventricular hypertrophy apply to Black patients [see Clinical Studies ( 14.2)].
8.7 Renal Impairment
Patients with renal insufficiency have elevated plasma concentrations of losartan and its active metabolite compared to subjects with normal renal function. No dose adjustment is necessary in patients with renal impairment unless a patient with renal impairment is also volume depleted [see Dosage and Administration ( 2.3), Warnings and Precautions ( 5.3) and Clinical Pharmacology ( 12.3)].
8.8 Hepatic Impairment
The recommended starting dose of losartan potassium is 25 mg in patients with mild-to-moderate hepatic impairment. Following oral administration in patients with mild-to-moderate hepatic impairment, plasma concentrations of losartan and its active metabolite were, respectively, 5 times and 1.7 times those seen in healthy volunteers. Losartan potassium has not been studied in patients with severe hepatic impairment [see Dosage and Administration ( 2.4) and Clinical Pharmacology ( 12.3)].
10 OVERDOSAGE
Significant lethality was observed in mice and rats after oral administration of 1000 mg/kg and 2000 mg/kg, respectively, about 44 and 170 times the maximum recommended human dose on a mg/m 2 basis.
Limited data are available in regard to overdosage in humans. The most likely manifestation of overdosage would be hypotension and tachycardia; bradycardia could occur from parasympathetic (vagal) stimulation. If symptomatic hypotension should occur, supportive treatment should be instituted.
Neither losartan nor its active metabolite can be removed by hemodialysis.
11 DESCRIPTION
Losartan potassium tablets USP is an angiotensin II receptor blocker acting on the AT 1 receptor subtype. Losartan potassium, a non-peptide molecule, is chemically described as 2-butyl-4-chloro-1-[ p-( o-1 Htetrazol-5-ylphenyl)benzyl]imidazole-5-methanol monopotassium salt.
Its molecular formula is C 22H 22ClKN 6O, and its structural formula is:

Losartan potassium USP is a white to off-white free-flowing crystalline powder with a molecular weight of 461.01. It is freely soluble in water, soluble in alcohols, and slightly soluble in common organic solvents, such as acetonitrile and methyl ethyl ketone. Oxidation of the 5-hydroxymethyl group on the imidazole ring results in the active metabolite of losartan.
Losartan potassium is available as tablets for oral administration containing either 25 mg, 50 mg or 100 mg of losartan potassium and the following inactive ingredients: Microcrystalline cellulose, lactose monohydrate, pregelatinized starch, magnesium stearate, Opadry white (hydroxypropyl cellulose, hypromellose and titanium dioxide).
Losartan potassium 25 mg, 50 mg and 100 mg tablets contain potassium in the following amounts: 2.12 mg (0.054 mEq), 4.24 mg (0.108 mEq) and 8.48 mg (0.216 mEq), respectively.
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Angiotensin II [formed from angiotensin I in a reaction catalyzed by angiotensin converting enzyme (ACE, kininase II)] is a potent vasoconstrictor, the primary vasoactive hormone of the renin-angiotensin system, and an important component in the pathophysiology of hypertension. It also stimulates aldosterone secretion by the adrenal cortex. Losartan and its principal active metabolite block the vasoconstrictor and aldosterone-secreting effects of angiotensin II by selectively blocking the binding of angiotensin II to the AT 1 receptor found in many tissues, (e.g., vascular smooth muscle, adrenal gland). There is also an AT 2 receptor found in many tissues but it is not known to be associated with cardiovascular homeostasis. Neither losartan nor its principal active metabolite exhibits any partial agonist activity at the AT 1 receptor, and both have much greater affinity (about 1000-fold) for the AT 1 receptor than for the AT 2 receptor. In vitro binding studies indicate that losartan is a reversible, competitive inhibitor of the AT 1 receptor. The active metabolite is 10 to 40 times more potent by weight than losartan and appears to be a reversible, non-competitive inhibitor of the AT 1 receptor.
Neither losartan nor its active metabolite inhibits ACE (kininase II, the enzyme that converts angiotensin I to angiotensin II and degrades bradykinin), nor do they bind to or block other hormone receptors or ion channels known to be important in cardiovascular regulation.
12.2 Pharmacodynamics
Losartan inhibits the pressor effect of angiotensin II (as well as angiotensin I) infusions. A dose of 100 mg inhibits the pressor effect by about 85% at peak with 25 to 40% inhibition persisting for 24 hours. Removal of the negative feedback of angiotensin II causes a doubling to tripling in plasma renin activity and consequent rise in angiotensin II plasma concentration in hypertensive patients. Losartan does not affect the response to bradykinin, whereas ACE inhibitors increase the response to bradykinin. Aldosterone plasma concentrations fall following losartan administration. In spite of the effect of losartan on aldosterone secretion, very little effect on serum potassium was observed.
The effect of losartan is substantially present within one week but in some studies the maximal effect occurred in 3 to 6 weeks. In long-term follow-up studies (without placebo control) the effect of losartan appeared to be maintained for up to a year. There is no apparent rebound effect after abrupt withdrawal of losartan. There was essentially no change in average heart rate in losartan-treated patients in controlled trials.
12.3 Pharmacokinetics
Absorption: Following oral administration, losartan is well absorbed and undergoes substantial first-pass metabolism. The systemic bioavailability of losartan is approximately 33%. Mean peak concentrations of losartan and its active metabolite are reached in 1 hour and in 3 to 4 hours, respectively. While maximum plasma concentrations of losartan and its active metabolite are approximately equal, the AUC (area under the curve) of the metabolite is about 4 times as great as that of losartan. A meal slows absorption of losartan and decreases its C max but has only minor effects on losartan AUC or on the AUC of the metabolite (~10% decrease). The pharmacokinetics of losartan and its active metabolite are linear with oral losartan doses up to 200 mg and do not change over time.
Distribution: The volume of distribution of losartan and the active metabolite is about 34 liters and 12 liters, respectively. Both losartan and its active metabolite are highly bound to plasma proteins, primarily albumin, with plasma free fractions of 1.3% and 0.2%, respectively. Plasma protein binding is constant over the concentration range achieved with recommended doses. Studies in rats indicate that losartan crosses the blood-brain barrier poorly, if at all.
Metabolism: Losartan is an orally active agent that undergoes substantial first-pass metabolism by cytochrome P450 enzymes. It is converted, in part, to an active carboxylic acid metabolite that is responsible for most of the angiotensin II receptor antagonism that follows losartan treatment. About 14% of an orally-administered dose of losartan is converted to the active metabolite. In addition to the active carboxylic acid metabolite, several inactive metabolites are formed. In vitro studies indicate that cytochrome P450 2C9 and 3A4 are involved in the biotransformation of losartan to its metabolites.
Elimination: Total plasma clearance of losartan and the active metabolite is about 600 mL/min and 50 mL/min, respectively, with renal clearance of about 75 mL/min and 25 mL/min, respectively. The terminal half-life of losartan is about 2 hours and of the metabolite is about 6 to 9 hours. After single doses of losartan administered orally, about 4% of the dose is excreted unchanged in the urine and about 6% is excreted in urine as active metabolite. Biliary excretion contributes to the elimination of losartan and its metabolites. Following oral 14C-labeled losartan, about 35% of radioactivity is recovered in the urine and about 60% in the feces. Following an intravenous dose of 14C-labeled losartan, about 45% of radioactivity is recovered in the urine and 50% in the feces. Neither losartan nor its metabolite accumulates in plasma upon repeated once-daily dosing.
Specific Populations
Pediatric: Pharmacokinetic parameters after multiple doses of losartan (average dose 0.7 mg/kg, range 0.36 to 0.97 mg/kg) as a tablet to 25 hypertensive patients aged 6 to 16 years are shown in Table 4 below. Pharmacokinetics of losartan and its active metabolite were generally similar across the studied age groups and similar to historical pharmacokinetic data in adults. The principal pharmacokinetic parameters in adults and children are shown in the table below.
|
|
Adults given 50 mg once daily for 7 days
N=12 |
Age 6 to 16 given 0.7 mg/kg once daily for 7 days
N=25 |
||
|
|
Parent
|
Active Metabolite
|
Parent
|
Active Metabolite
|
| AUC
0 to 24 (ng•hr/mL)
*
| 442 ± 173
| 1685 ± 452
| 368 ± 169
| 1866 ± 1076
|
| C
MAX (ng/mL)
*
| 224 ± 82
| 212 ± 73
| 141 ± 88
| 222 ± 127
|
| T
1/2 (h)
†
| 2.1 ± 0.70
| 7.4 ± 2.4
| 2.3 ± 0.8
| 5.6 ± 1.2
|
| T
PEAK (h)
‡
| 0.9
| 3.5
| 2
| 4.1
|
| CL
REN(mL/min)
*
| 56 ± 23
| 20 ± 3
| 53 ± 33
| 17 ± 8
|
* Mean ± standard deviation
† Harmonic mean and standard deviation
‡ Median
The bioavailability of the suspension formulation was compared with losartan tablets in healthy adults. The suspension and tablet are similar in their bioavailability with respect to both losartan and the active metabolite [see Dosage and Administration ( 2.5)] .
Geriatric and Gender: Losartan pharmacokinetics have been investigated in the elderly (65 to 75 years) and in both genders. Plasma concentrations of losartan and its active metabolite are similar in elderly and young hypertensives. Plasma concentrations of losartan were about twice as high in female hypertensives as male hypertensives, but concentrations of the active metabolite were similar in males and females. No dosage adjustment is necessary [see Dosage and Administration ( 2.1)] .
Race: Pharmacokinetic differences due to race have not been studied [see Use in Specific Populations ( 8.6)].
Renal Insufficiency: Following oral administration, plasma concentrations and AUCs of losartan and its active metabolite are increased by 50 to 90% in patients with mild (creatinine clearance of 50 to 74 mL/min) or moderate (creatinine clearance 30 to 49 mL/min) renal insufficiency. In this study, renal clearance was reduced by 55 to 85% for both losartan and its active metabolite in patients with mild or moderate renal insufficiency. Neither losartan nor its active metabolite can be removed by hemodialysis [see Warnings and Precautions ( 5.3) and Use in Specific Populations ( 8.7)] .
Hepatic Insufficiency: Following oral administration in patients with mild to moderate alcoholic cirrhosis of the liver, plasma concentrations of losartan and its active metabolite were, respectively, 5-times and about 1.7-times those in young male volunteers. Compared to normal subjects the total plasma clearance of losartan in patients with hepatic insufficiency was about 50% lower and the oral bioavailability was about doubled. Use a starting dose of 25 mg for patients with mild to moderate hepatic impairment. Losartan potassium has not been studied in patients with severe hepatic impairment [see Dosage and Administration ( 2.4) and Use in Specific Populations ( 8.8)].
Drug Interactions
No clinically significant drug interactions have been found in studies of losartan potassium with hydrochlorothiazide, digoxin, warfarin, cimetidine and phenobarbital. However, rifampin has been shown to decrease the AUC of losartan and its active metabolite by 30% and 40%, respectively. Fluconazole, an inhibitor of cytochrome P450 2C9, decreased the AUC of the active metabolite by approximately 40%, but increased the AUC of losartan by approximately 70% following multiple doses. Conversion of losartan to its active metabolite after intravenous administration is not affected by ketoconazole, an inhibitor of P450 3A4. The AUC of active metabolite following oral losartan was not affected by erythromycin, an inhibitor of P450 3A4, but the AUC of losartan was increased by 30%.
The pharmacodynamic consequences of concomitant use of losartan and inhibitors of P450 2C9 have not been examined. Subjects who do not metabolize losartan to active metabolite have been shown to have a specific, rare defect in cytochrome P450 2C9. These data suggest that the conversion of losartan to its active metabolite is mediated primarily by P450 2C9 and not P450 3A4.
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Losartan potassium was not carcinogenic when administered at maximally tolerated dosages to rats and mice for 105 and 92 weeks, respectively. Female rats given the highest dose (270 mg/kg/day) had a slightly higher incidence of pancreatic acinar adenoma. The maximally tolerated dosages (270 mg/kg/day in rats, 200 mg/kg/day in mice) provided systemic exposures for losartan and its pharmacologically active metabolite that were approximately 160 and 90 times (rats) and 30 and 15 times (mice) the exposure of a 50 kg human given 100 mg per day.
Losartan potassium was negative in the microbial mutagenesis and V-79 mammalian cell mutagenesis assays and in the in vitro alkaline elution and in vitro and in vivo chromosomal aberration assays. In addition, the active metabolite showed no evidence of genotoxicity in the microbial mutagenesis, in vitro alkaline elution, and in vitro chromosomal aberration assays.
Fertility and reproductive performance were not affected in studies with male rats given oral doses of losartan potassium up to approximately 150 mg/kg/day. The administration of toxic dosage levels in females (300/200 mg/kg/day) was associated with a significant (p<0.05) decrease in the number of corpora lutea/female, implants/female, and live fetuses/female at C-section. At 100 mg/kg/day only a decrease in the number of corpora lutea/female was observed. The relationship of these findings to drug-treatment is uncertain since there was no effect at these dosage levels on implants/pregnant female, percent post-implantation loss, or live animals/litter at parturition. In nonpregnant rats dosed at 135 mg/kg/day for 7 days, systemic exposure (AUCs) for losartan and its active metabolite were approximately 66 and 26 times the exposure achieved in man at the maximum recommended human daily dosage (100 mg).
14 CLINICAL STUDIES
14.1 Hypertension
Adult Hypertension
The antihypertensive effects of losartan potassium were demonstrated principally in 4 placebo-controlled, 6- to- 12 week trials of dosages from 10 to 150 mg per day in patients with baseline diastolic blood pressures of 95 to 115. The studies allowed comparisons of two doses (50 to 100 mg/day) as once-daily or twice-daily regimens, comparisons of peak and trough effects, and comparisons of response by gender, age, and race. Three additional studies examined the antihypertensive effects of losartan and hydrochlorothiazide in combination.
The 4 studies of losartan monotherapy included a total of 1075 patients randomized to several doses of losartan and 334 to placebo. The 10 mg and 25 mg doses produced some effect at peak (6 hours after dosing) but small and inconsistent trough (24 hour) responses. Doses of 50, 100 and 150 mg once daily gave statistically significant systolic/diastolic mean decreases in blood pressure, compared to placebo in the range of 5.5 to 10.5/3.5 to 7.5 mmHg, with the 150 mg dose giving no greater effect than 50 to 100 mg. Twice-daily dosing at 50 to 100 mg/day gave consistently larger trough responses than once-daily dosing at the same total dose. Peak (6 hour) effects were uniformly, but moderately, larger than trough effects, with the trough-to-peak ratio for systolic and diastolic responses 50 to 95% and 60 to 90%, respectively.
Addition of a low dose of hydrochlorothiazide (12.5 mg) to losartan 50 mg once daily resulted in placebo-adjusted blood pressure reductions of 15.5/9.2 mmHg.
Analysis of age, gender, and race subgroups of patients showed that men and women, and patients over and under 65, had generally similar responses. Losartan potassium was effective in reducing blood pressure regardless of race, although the effect was somewhat less in Black patients (usually a low-renin population).
Pediatric Hypertension
The antihypertensive effect of losartan was studied in one trial enrolling 177 hypertensive pediatric patients aged 6 to 16 years old. Children who weighed <50 kg received 2.5, 25 or 50 mg of losartan daily and patients who weighed ≥50 kg received 5, 50 or 100 mg of losartan daily. Children in the lowest dose group were given losartan in a suspension formulation [see Dosage and Administration ( 2.1)]. The majority of the children had hypertension associated with renal and urogenital disease. The sitting diastolic blood pressure (SiDBP) on entry into the study was higher than the 95th percentile level for the patient’s age, gender, and height. At the end of three weeks, losartan reduced systolic and diastolic blood pressure, measured at trough, in a dose-dependent manner. Overall, the two higher doses (25 to 50 mg in patients <50 kg; 50 to 100 mg in patients ≥50 kg) reduced diastolic blood pressure by 5 to 6 mmHg more than the lowest dose used (2.5 mg in patients <50 kg; 5 mg in patients ≥50 kg). The lowest dose, corresponding to an average daily dose of 0.07 mg/kg, did not appear to offer consistent antihypertensive efficacy. When patients were randomized to continue losartan at the two higher doses or to placebo after 3 weeks of therapy, trough diastolic blood pressure rose in patients on placebo between 5 and 7 mmHg more than patients randomized to continuing losartan. When the low dose of losartan was randomly withdrawn, the rise in trough diastolic blood pressure was the same in patients receiving placebo and in those continuing losartan, again suggesting that the lowest dose did not have significant antihypertensive efficacy. Overall, no significant differences in the overall antihypertensive effect of losartan were detected when the patients were analyzed according to age (<, ≥12 years old) or gender. While blood pressure was reduced in all racial subgroups examined, too few non-White patients were enrolled to compare the dose-response of losartan in the non-White subgroup.
14.2 Hypertensive Patients with Left Ventricular Hypertrophy
The LIFE study was a multinational, double-blind study comparing losartan potassium and atenolol in 9193 hypertensive patients with ECG-documented left ventricular hypertrophy. Patients with myocardial infarction or stroke within six months prior to randomization were excluded. Patients were randomized to receive once daily losartan potassium 50 mg or atenolol 50 mg. If goal blood pressure (<140/90 mmHg) was not reached, hydrochlorothiazide (12.5 mg) was added first and, if needed, the dose of losartan potassium or atenolol was then increased to 100 mg once daily. If necessary, other antihypertensive treatments (e.g., increase in dose of hydrochlorothiazide therapy to 25 mg or addition of other diuretic therapy, calcium-channel blockers, alpha-blockers, or centrally acting agents, but not ACE inhibitors, angiotensin II antagonists, or beta-blockers) were added to the treatment regimen to reach the goal blood pressure.
Of the randomized patients, 4963 (54%) were female and 533 (6%) were Black. The mean age was 67 with 5704 (62%) age ≥65. At baseline, 1195 (13%) had diabetes, 1326 (14%) had isolated systolic hypertension, 1469 (16%) had coronary heart disease, and 728 (8%) had cerebrovascular disease. Baseline mean blood pressure was 174/98 mmHg in both treatment groups. The mean length of follow-up was 4.8 years. At the end of study or at the last visit before a primary endpoint, 77% of the group treated with losartan potassium and 73% of the group treated with atenolol were still taking study medication. Of the patients still taking study medication, the mean doses of losartan potassium and atenolol were both about 80 mg/day, and 15% were taking atenolol or losartan as monotherapy, while 77% were also receiving hydrochlorothiazide (at a mean dose of 20 mg/day in each group). Blood pressure reduction measured at trough was similar for both treatment groups but blood pressure was not measured at any other time of the day. At the end of study or at the last visit before a primary endpoint, the mean blood pressures were 144.1/81.3 mmHg for the group treated with losartan potassium and 145.4/80.9 mmHg for the group treated with atenolol; the difference in systolic blood pressure (SBP) of 1.3 mmHg was significant (p<0.001), while the difference of 0.4 mmHg in diastolic blood pressure (DBP) was not significant (p=0.098).
The primary endpoint was the first occurrence of cardiovascular death, nonfatal stroke, or nonfatal myocardial infarction. Patients with nonfatal events remained in the trial, so that there was also an examination of the first event of each type even if it was not the first event (e.g., a stroke following an initial myocardial infarction would be counted in the analysis of stroke). Treatment with losartan potassium resulted in a 13% reduction (p=0.021) in risk of the primary endpoint compared to the atenolol group (see Figure 1 and Table 3); this difference was primarily the result of an effect on fatal and nonfatal stroke. Treatment with losartan potassium reduced the risk of stroke by 25% relative to atenolol (p=0.001) (see Figure 2 and Table 3).

Figure 1: Kaplan-Meier estimates of the primary endpoint of time to cardiovascular death, nonfatal stroke, or nonfatal myocardial infarction in the groups treated with losartan potassium and atenolol. The Risk Reduction is adjusted for baseline Framingham risk score and level of electrocardiographic left ventricular hypertrophy.

Figure 2: Kaplan-Meier estimates of the time to fatal/nonfatal stroke in the groups treated with losartan potassium and atenolol. The Risk Reduction is adjusted for baseline Framingham risk score and level of electrocardiographic left ventricular hypertrophy.
Table 3 shows the results for the primary composite endpoint and the individual endpoints. The primary endpoint was the first occurrence of stroke, myocardial infarction or cardiovascular death, analyzed using an ITT approach. The table shows the number of events for each component in two different ways. The Components of Primary Endpoint (as a first event) counts only the events that define the primary endpoint, while the Secondary Endpoints count all first events of a particular type, whether or not they were preceded by a different type of event.
Table 3: Incidence of Primary Endpoint Events
|
| Losartan potassium
| Atenolol
| Risk Reduction
†
| 95% CI
| p-Value
|
||
|
| N (%)
| Rate*
| N (%)
| Rate*
|
|
|
|
| Primary Composite Endpoint
| 508 (11)
| 23.8
| 588 (13)
| 27.9
| 13%
| 2% to 23%
| 0.021
|
| Components of Primary Composite Endpoint (as a first event)
|
|
||||||
| Stroke (nonfatal)
| 209 (5)
|
| 286 (6)
|
|
|
|
|
| Myocardial infarction (nonfatal)
| 174 (4)
|
| 168 (4)
|
|
|
|
|
| Cardiovascular mortality
| 125 (3)
|
| 134 (3)
|
|
|
|
|
| Secondary Endpoints (any time in study)
|
|
||||||
| Stroke (fatal/nonfatal)
| 232 (5)
| 10.8
| 309 (7)
| 14.5
| 25%
| 11% to 37%
| 0.001
|
| Myocardial infarction (fatal/nonfatal)
| 198 (4)
| 9.2
| 188 (4)
| 8.7
| -7%
| -13% to 12%
| 0.491
|
| Cardiovascular mortality
| 204 (4)
| 9.2
| 234 (5)
| 10.6
| 11%
| -7% to 27%
| 0.206
|
| Due to CHD
| 125 (3)
| 5.6
| 124 (3)
| 5.6
| -3%
| -32% to 20%
| 0.839
|
| Due to Stroke
| 40 (1)
| 1.8
| 62 (1)
| 2.8
| 35%
| 4% to 67%
| 0.032
|
| Other
‡
| 39 (1)
| 1.8
| 48 (1)
| 2.2
| 16%
| -28% to 45%
| 0.411
|
*Rate per 1000 patient-years of follow-up
†Adjusted for baseline Framingham risk score and level of electrocardiographic left ventricular hypertrophy
‡Death due to heart failure, non-coronary vascular disease, pulmonary embolism, or a cardiovascular cause other than stroke or coronary heart disease
Although the LIFE study favored losartan potassium over atenolol with respect to the primary endpoint (p=0.021), this result is from a single study and, therefore, is less compelling than the difference between losartan potassium and placebo. Although not measured directly, the difference between losartan potassium and placebo is compelling because there is evidence that atenolol is itself effective (vs. placebo) in reducing cardiovascular events, including stroke, in hypertensive patients.
Other clinical endpoints of the LIFE study were: total mortality, hospitalization for heart failure or angina pectoris, coronary or peripheral revascularization procedures, and resuscitated cardiac arrest. There were no significant differences in the rates of these endpoints between the losartan potassium and atenolol groups.
For the primary endpoint and stroke, the effects of losartan potassium in patient subgroups defined by age, gender, race and presence or absence of isolated systolic hypertension (ISH), diabetes, and history of cardiovascular disease (CVD) are shown in Figure 3 below. Subgroup analyses can be difficult to interpret and it is not known whether these represent true differences or chance effects.
Figure 3: Primary Endpoint Events† within Demographic Subgroups

14.3 Nephropathy in Type 2 Diabetic Patients
The RENAAL study was a randomized, placebo-controlled, double-blind, multicenter study conducted worldwide in 1513 patients with type 2 diabetes with nephropathy (defined as serum creatinine 1.3 to 3 mg/dL in females or males ≤60 kg and 1.5 to 3 mg/dL in males >60 kg and proteinuria [urinary albumin to creatinine ratio ≥300 mg/g]).
Patients were randomized to receive losartan potassium 50 mg once daily or placebo on a background of conventional antihypertensive therapy excluding ACE inhibitors and angiotensin II antagonists. After one month, investigators were instructed to titrate study drug to 100 mg once daily if the trough blood pressure goal (140/90 mmHg) was not achieved. Overall, 72% of patients received the 100 mg daily dose more than 50% of the time they were on study drug. Because the study was designed to achieve equal blood pressure control in both groups, other antihypertensive agents (diuretics, calcium-channel blockers, alpha- or beta-blockers, and centrally acting agents) could be added as needed in both groups. Patients were followed for a mean duration of 3.4 years.
The study population was diverse with regard to race (Asian 16.7%, Black 15.2%, Hispanic 18.3%, White 48.6%). Overall, 63.2% of the patients were men, and 66.4% were under the age of 65 years. Almost all of the patients (96.6%) had a history of hypertension, and the patients entered the trial with a mean serum creatinine of 1.9 mg/dL and mean proteinuria (urinary albumin/creatinine) of 1808 mg/g at baseline.
The primary endpoint of the study was the time to first occurrence of any one of the following events: doubling of serum creatinine, end-stage renal disease (ESRD) (need for dialysis or transplantation), or death. Treatment with losartan potassium resulted in a 16% risk reduction in this endpoint (see Figure 4 and Table 4). Treatment with losartan potassium also reduced the occurrence of sustained doubling of serum creatinine by 25% and ESRD by 29% as separate endpoints, but had no effect on overall mortality (see Table 4).
The mean baseline blood pressures were 152/82 mmHg for losartan potassium plus conventional antihypertensive therapy and 153/82 mmHg for placebo plus conventional antihypertensive therapy. At the end of the study, the mean blood pressures were 143/76 mmHg for the group treated with losartan potassium and 146/77 mmHg for the group treated with placebo.

Figure 4: Kaplan-Meier curve for the primary composite endpoint of doubling of serum creatinine, end stage renal disease (need for dialysis or transplantation) or death.
Table 4: Incidence of Primary Endpoint Events
|
| Incidence
| Risk Reduction
| 95% C.I
| p-Value
|
|||
| Losartan
| Placebo
|
| |||||
| Primary Composite Endpoint
| 43.5%
| 47.1%
| 16.1%
| 2.3% to 27.9%
| 0.022
|
||
| Doubling of Serum Creatinine, ESRD and Death Occurring as a First Event
|
|||||||
| Doubling of Serum Creatinine
| 21.6%
| 26%
|
|
|
|
||
| ESRD
| 8.5%
| 8.5%
|
|
|
|
||
| Death
| 13.4%
| 12.6%
|
|
|
|
||
| Overall Incidence of Doubling of Serum Creatinine, ESRD and Death
|
|||||||
| Doubling of Serum Creatinine
| 21.6%
| 26%
| 25.3%
| 7.8% to 39.4%
| 0.006
|
||
| ESRD
| 19.6%
| 25.5%
| 28.6%
| 11.5% to 42.4%
| 0.002
|
||
| Death
| 21%
| 20.3%
| -1.7%
| -26.9% to 18.6%
| 0.884
|
||
The secondary endpoints of the study were change in proteinuria, change in the rate of progression of renal disease, and the composite of morbidity and mortality from cardiovascular causes (hospitalization for heart failure, myocardial infarction, revascularization, stroke, hospitalization for unstable angina, or cardiovascular death). Compared with placebo, losartan potassium significantly reduced proteinuria by an average of 34%, an effect that was evident within 3 months of starting therapy, and significantly reduced the rate of decline in glomerular filtration rate during the study by 13%, as measured by the reciprocal of the serum creatinine concentration. There was no significant difference in the incidence of the composite endpoint of cardiovascular morbidity and mortality.
The favorable effects of losartan potassium were seen in patients also taking other anti-hypertensive medications (angiotensin II receptor antagonists and angiotensin converting enzyme inhibitors were not allowed), oral hypoglycemic agents and lipid-lowering agents.
For the primary endpoint and ESRD, the effects of losartan potassium in patient subgroups defined by age, gender and race are shown in Table 5 below. Subgroup analyses can be difficult to interpret and it is not known whether these represent true differences or chance effects.
Table 5: Efficacy Outcomes within Demographic Subgroups
| No. of Patients
| Primary Composite Endpoint
| ESRD
|
|||||
| Losartan potassium
Event Rate % | Placebo
Event Rate % | Hazard Ratio (95% CI)
| Losartan potassium Event Rate %
| Placebo Event Rate
% | Hazard Ratio (95% CI)
|
||
| Overall Results
| 1513
| 43.5
| 47.1
| 0.84 (0.72, 0.98)
| 19.6
| 25.5
| 0.71 (0.58, 0.89)
|
| Age
|
|
|
|
|
|
|
|
| <65 years
| 1005
| 44.1
| 49
| 0.78 (0.65, 0.94)
| 21.1
| 28.5
| 0.67 (0.52, 0.86)
|
| ≥65 years
| 508
| 42.3
| 43.5
| 0.98 (0.75, 1.28)
| 16.5
| 19.6
| 0.85 (0.56, 1.28)
|
| Gender
|
|
|
|
|
|
|
|
| Female
| 557
| 47.8
| 54.1
| 0.76 (0.60, 0.96)
| 22.8
| 32.8
| 0.60 (0.44, 0.83)
|
| Male
| 956
| 40.9
| 43.3
| 0.89 (0.73, 1.09)
| 17.5
| 21.5
| 0.81 (0.60, 1.08)
|
| Race
|
|
|
|
|
|
|
|
| Asian
| 252
| 41.9
| 54.8
| 0.66 (0.45, 0.95)
| 18.8
| 27.4
| 0.63 (0.37, 1.07)
|
| Black
| 230
| 40
| 39
| 0.98 (0.65, 1.50)
| 17.6
| 21
| 0.83 (0.46, 1.52)
|
| Hispanic
| 277
| 55
| 54
| 1 (0.73, 1.38)
| 30
| 28.5
| 1.02 (0.66, 1.59)
|
| White
| 735
| 40.5
| 43.2
| 0.81 (0.65, 1.01)
| 16.2
| 23.9
| 0.60 (0.43, 0.83)
|
16 HOW SUPPLIED/STORAGE AND HANDLING
Losartan Potassium USP 25 mg tablets are white to off-white, oval shaped, film-coated tablets with “A” engraved on one side and “25” on the other side. They are supplied as follows:
NDC 42571-110-30 unit of use bottles of 30
NDC 42571-110-90 unit of use bottles of 90
NDC 42571-110-10 unit of use bottles of 1000
Losartan Potassium USP 50 mg tablets are white to off-white, oval shaped, film-coated tablets with “A50” engraved on one side and break line on the other side. They are supplied as follows:
NDC 42571-111-30 unit of use bottles of 30
NDC 42571-111-90 unit of use bottles of 90
NDC 42571-111-10 unit dose packages of 1000
Losartan Potassium USP 100 mg tablets are white to off-white, oval shaped, film-coated tablets with “A100” engraved on one side and plain on the other side. They are supplied as follows:
NDC 42571-112-30 unit of use bottles of 30
NDC 42571-112-90 unit of use bottles of 90
NDC 42571-112-10 unit dose packages of 1000
Store at 25ºC (77ºF); excursions permitted to 15 to 30ºC (59 to 86ºF) [see USP Controlled Room Temperature]. Keep container tightly closed. Protect from light.
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Patient Information).
Pregnancy
Advise female patients of childbearing age about the consequences of exposure to losartan potassium during pregnancy. Discuss treatment options with women planning to become pregnant. Tell patients to report pregnancies to their physicians as soon as possible [see Warnings and Precautions (5.1) and Use in Specific Populations (8.1)].
Potassium Supplements
Advise patients receiving losartan potassium not to use potassium supplements or salt substitutes containing potassium without consulting their healthcare provider [see Drug Interactions (7.1)].
Manufactured by:
Micro Labs Limited
Goa-403 722, INDIA.
Manufactured for:
Micro Labs USA, Inc.
Somerset, NJ 08873
Rev.11/2021
Patient Information
Losartan potassium (loe-SAR-tan poe-TAS-ee-um) tablets, USP
25 mg, 50 mg, 100 mg
Rx only
Read the Patient Information that comes with losartan potassium tablets before you start taking it and each time you get a refill. There may be new information. This leaflet does not take the place of talking with your doctor about your condition and treatment.
What is the most important information I should know about losartan potassium tablets?
- Losartan potassium tablets can cause harm or death to an unborn baby.
- Talk to your doctor about other ways to lower your blood pressure if you plan to become pregnant.
- If you get pregnant while taking losartan potassium tablets, tell your doctor right away.
What are losartan potassium tablets?
Losartan potassium tablet is a prescription medicine called an angiotensin receptor blocker (ARB). It is used:
- alone or with other blood pressure medicines to lower high blood pressure (hypertension).
- to lower the chance of stroke in patients with high blood pressure and a heart problem called left ventricular hypertrophy. Losartan potassium tablets may not help Black patients with this problem.
- to slow the worsening of diabetic kidney disease (nephropathy) in patients with type 2 diabetes who have or had high blood pressure.
Losartan potassium tablets has not been studied in children less than 6 years old or in children with certain kidney problems.
High Blood Pressure (hypertension). Blood pressure is the force in your blood vessels when your heart beats and when your heart rests. You have high blood pressure when the force is too much. Losartan potassium tablets can help your blood vessels relax so your blood pressure is lower.
Left Ventricular Hypertrophy (LVH) is an enlargement of the walls of the left chamber of the heart (the heart’s main pumping chamber). LVH can happen from several things. High blood pressure is the most common cause of LVH.
Type 2 Diabetes with Nephropathy. Type 2 diabetes is a type of diabetes that happens mainly in adults. If you have diabetic nephropathy it means that your kidneys do not work properly because of damage from the diabetes.
Who should not take losartan potassium tablets?
- Do not take losartan potassium tablets if you are allergic to any of the ingredients in losartan potassium tablets. See the end of this leaflet for a complete list of ingredients in losartan potassium tablets.
- Do not take losartan potassium tablets if you have diabetes and are taking a medicine called aliskiren to reduce blood pressure.
What should I tell my doctor before taking losartan potassium tablets?
Tell your doctor about all of your medical conditions including if you:
- are pregnant or planning to become pregnant. See “What is the most important information I should know about losartan potassium tablets?”
- are breastfeeding. It is not known if losartan potassium passes into your breast milk. You should choose either to take losartan potassium tablets or breastfeed, but not both.
- are vomiting a lot or having a lot of diarrhea
- have liver problems
- have kidney problems
Tell your doctor about all the medicines you take, including prescription and non-prescription medicines, vitamins, and herbal supplements. Losartan potassium tablets and certain other medicines may interact with each other. Especially tell your doctor if you are taking:
- potassium supplements
- salt substitutes containing potassium
- other medicines that may increase serum potassium
- water pills (diuretics)
- lithium (a medicine used to treat a certain kind of depression)
- medicines used to treat pain and arthritis, called non-steroidal anti-inflammatory drugs (NSAIDs), including COX-2 inhibitors
- other medicines to reduce blood pressure
How should I take losartan potassium tablets?
- Take losartan potassium tablets exactly as prescribed by your doctor. Your doctor may change your dose if needed.
- Losartan potassium tablets can be taken with or without food.
- If you miss a dose, take it as soon as you remember. If it is close to your next dose, do not take the missed dose. Just take the next dose at your regular time.
- If you take too much losartan potassium tablets, call your doctor or Poison Control Center, or go to the nearest hospital emergency room right away.
What are the possible side effects of losartan potassium tablets?
Losartan potassium tablets may cause the following side effects that may be serious:
· Injury or death of unborn babies. See “What is the most important information I should know about losartan potassium tablets?”· Allergic reaction. Symptoms of an allergic reaction are swelling of the face, lips, throat or tongue. Get emergency medical help right away and stop taking losartan potassium tablets.· Low blood pressure (hypotension). Low blood pressure may cause you to feel faint or dizzy. Lie down if you feel faint or dizzy. Call your doctor right away.· For people who already have kidney problems, you may see a worsening in how well your kidneys work. Call your doctor if you get swelling in your feet, ankles, or hands, or unexplained weight gain.· High blood levels of potassium
The most common side effects of losartan potassium tablets in people with high blood pressure are:
- “colds” (upper respiratory infection)
- dizziness
- stuffy nose
- back pain
The most common side effects of losartan potassium tablets in people with type 2 diabetes with diabetic kidney disease are:
- diarrhea
- tiredness
- low blood sugar
- chest pain
- high blood potassium
- low blood pressure
Tell your doctor if you get any side effect that bothers you or that won’t go away.
This is not a complete list of side effects. For a complete list, ask your doctor or pharmacist.
How do I store losartan potassium tablets?
- Store losartan potassium tablets at 59°F to 86°F (15°C to 30°C).
- Keep losartan potassium tablets in a tightly closed container that protects the medicine from light.
- Keep losartan potassium tablets and all medicines out of the reach of children.
General information about losartan potassium tablets
Medicines are sometimes prescribed for conditions that are not mentioned in patient information leaflets. Do not use losartan potassium tablets for a condition for which it was not prescribed. Do not give losartan potassium tablets to other people, even if they have the same symptoms that you have. It may harm them.
This leaflet summarizes the most important information about losartan potassium tablets. If you would like more information, talk with your doctor. You can ask your pharmacist or doctor for information about losartan potassium tablets that is written for health professionals.
What are the ingredients in losartan potassium tablets? Active ingredients: losartan potassium Inactive ingredients: Microcrystalline cellulose, lactose monohydrate, pregelatinized starch, magnesium stearate, Opadry white (hydroxypropyl cellulose, hypromellose and titanium dioxide). Losartan potassium 25 mg, 50 mg and 100 mg tablets contain potassium in the following amounts: 2.12 mg (0.054 mEq), 4.24 mg (0.108 mEq) and 8.48 mg (0.216 mEq), respectively.
Manufactured by:
Micro Labs Limited
Goa-403 722, INDIA.
Manufactured for:
Micro Labs USA, Inc.
Somerset, NJ 08873
Rev.11/2021
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
NDC 42571-110-30
LOSARTAN POTASSIUM TABLETS USP
25 mg
Pharmacist: Please dispense with
attached Patient Information Leaflet.
Rx Only
30 Tablets
MICRO LABS

NDC 42571-111-30
LOSARTAN POTASSIUM TABLETS USP
50 mg
Pharmacist: Please dispense with
attached Patient Information Leaflet.
Rx Only
30 Tablets
MICRO LABS

NDC 42571-112-30
LOSARTAN POTASSIUM TABLETS USP
100 mg
Pharmacist: Please dispense with
attached Patient Information Leaflet.
Rx Only
30 Tablets
MICRO LABS

INGREDIENTS AND APPEARANCE
| LOSARTAN POTASSIUM
losartan potassium tablet, film coated |
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
| LOSARTAN POTASSIUM
losartan potassium tablet, film coated |
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
| LOSARTAN POTASSIUM
losartan potassium tablet, film coated |
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
| Labeler - Micro Labs Limited (862174955) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Micro Labs Limited | 915793658 | manufacture(42571-110, 42571-111, 42571-112) | |