Search by Drug Name or NDC
NDC 42571-0137-25 Ketorolac Tromethamine 5 mg/mL Details
Ketorolac Tromethamine 5 mg/mL
Ketorolac Tromethamine is a OPHTHALMIC SOLUTION in the HUMAN PRESCRIPTION DRUG category. It is labeled and distributed by Micro Labs Limited. The primary component is KETOROLAC TROMETHAMINE.
MedlinePlus Drug Summary
Ophthalmic ketorolac is used to treat itchy eyes caused by allergies. It also is used to treat swelling and redness (inflammation) that can occur after cataract surgery. Ketorolac is in a class of medications called nonsteroidal anti-inflammatory drugs (NSAIDs). It works by stopping the release of substances that cause allergy symptoms and inflammation.
Related Packages: 42571-0137-25Last Updated: 11/30/2022
MedLinePlus Full Drug Details: Ketorolac Ophthalmic
Product Information
| NDC | 42571-0137 |
|---|---|
| Product ID | 42571-137_d1aee3bb-346e-b6e9-e053-2995a90ad789 |
| Associated GPIs | 86805035102020 |
| GCN Sequence Number | 019067 |
| GCN Sequence Number Description | ketorolac tromethamine DROPS 0.5 % OPHTHALMIC |
| HIC3 | Q6P |
| HIC3 Description | EYE ANTI-INFLAMMATORY AGENTS |
| GCN | 52700 |
| HICL Sequence Number | 005175 |
| HICL Sequence Number Description | KETOROLAC TROMETHAMINE |
| Brand/Generic | Generic |
| Proprietary Name | Ketorolac Tromethamine |
| Proprietary Name Suffix | n/a |
| Non-Proprietary Name | Ketorolac Tromethamine |
| Product Type | HUMAN PRESCRIPTION DRUG |
| Dosage Form | SOLUTION |
| Route | OPHTHALMIC |
| Active Ingredient Strength | 5 |
| Active Ingredient Units | mg/mL |
| Substance Name | KETOROLAC TROMETHAMINE |
| Labeler Name | Micro Labs Limited |
| Pharmaceutical Class | Anti-Inflammatory Agents, Non-Steroidal [CS], Cyclooxygenase Inhibitor [EPC], Cyclooxygenase Inhibitors [MoA], Nonsteroidal Anti-inflammatory Drug [EPC] |
| DEA Schedule | n/a |
| Marketing Category | ANDA |
| Application Number | ANDA203410 |
| Listing Certified Through | 2024-12-31 |
Package
Package Images


NDC 42571-0137-25 (42571013725)
| NDC Package Code | 42571-137-25 |
|---|---|
| Billing NDC | 42571013725 |
| Package | 5 mL in 1 BOTTLE (42571-137-25) |
| Marketing Start Date | 2019-06-03 |
| NDC Exclude Flag | N |
| Pricing Information | |
| Price Per Unit | 1.41765 |
| Pricing Unit | ML |
| Effective Date | 2024-02-21 |
| NDC Description | KETOROLAC 0.5% OPHTH SOLUTION |
| Pharmacy Type Indicator | C/I |
| OTC | N |
| Explanation Code | 1, 5 |
| Classification for Rate Setting | G |
| As of Date | 2024-02-21 |
Standard Product Labeling (SPL)/Prescribing Information SPL 631c2c31-cd4f-4a17-85c6-be96746a753e Details
HIGHLIGHTS OF PRESCRIBING INFORMATION
KETOROLAC TROMETHAMINE ophthalmic solution 0.5%
Initial U.S. Approval: 1991
INDICATIONS AND USAGE
DOSAGE AND ADMINISTRATION
One drop of ketorolac tromethamine ophthalmic solution 0.5% should be applied to the affected eye(s) four times a day for relief of ocular itching due to seasonal allergic conjunctivitis.
For the treatment of postoperative inflammation in patients who have undergone cataract extraction, one drop of ketorolac tromethamine ophthalmic solution 0.5% should be applied to the affected eye four times daily beginning 24 hours after cataract surgery and continuing through the first 2 weeks of the postoperative period. ( 2.1)
DOSAGE FORMS AND STRENGTHS
Ophthalmic solution containing 5 mg/mL ketorolac tromethamine. ( 3)
- 5 mL size bottle filled with 3 mL, 5 mL size bottle filled with 5 mL and 10 mL size bottle filled with 10 mL of ketorolac tromethamine ophthalmic solution, 0.5% (5 mg/mL)
CONTRAINDICATIONS
Hypersensitivity to any component of this product. ( 4)
WARNINGS AND PRECAUTIONS
ADVERSE REACTIONS
The most frequent adverse reactions reported by up to 40% of
patients participating in clinical trials have been transient stinging and burning on instillation. ( 6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Micro Labs Limited at 1-855-839-8195 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 10/2021
Table Of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dosing
2.2 Use with Other Topical Ophthalmic Medications
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Delayed Healing
5.2 Cross-Sensitivity or Hypersensitivity
5.3 Increased Bleeding Time
5.4 Corneal Effects
5.5 Contact Lens Wear
6 ADVERSE REACTIONS
6.1 Clinical Studies Experience
6.2 Postmarketing Experience
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.3 Nursing Mothers
8.4 Pediatric Use
8.5 Geriatric Use
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
17.1 Slow or Delayed Healing
17.2 Avoiding Contamination of the Product
17.3 Contact Lens Wear
17.4 Intercurrent Ocular Conditions
17.5 Concomitant Topical Ocular Therapy
- *
- Sections or subsections omitted from the full prescribing information are not listed.
1 INDICATIONS AND USAGE
Ketorolac tromethamine ophthalmic solution 0.5% is indicated for the temporary relief of ocular itching due to seasonal allergic conjunctivitis. Ketorolac tromethamine ophthalmic solution 0.5% is also indicated for the treatment of postoperative inflammation in patients who have undergone cataract extraction.
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dosing
Patient Dosing
The recommended dose of ketorolac tromethamine ophthalmic solution 0.5% is one drop four times a day to the affected eye(s) for relief of ocular itching due to seasonal allergic conjunctivitis.
For the treatment of postoperative inflammation in patients who have undergone cataract extraction, one drop of ketorolac tromethamine ophthalmic solution 0.5% should be applied to the affected eye four times daily beginning 24 hours after cataract surgery and continuing through the first 2 weeks of the postoperative period.
2.2 Use with Other Topical Ophthalmic Medications
Ketorolac tromethamine ophthalmic solution 0.5% has been safely administered in conjunction with other ophthalmic medications such as antibiotics, alpha-agonists, beta blockers, carbonic anhydrase inhibitors, cycloplegics, and mydriatics. Drops should be administered at least 5 minutes apart.
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Delayed Healing
Topical nonsteroidal anti-inflammatory drugs (NSAIDs) may slow or delay healing. Topical corticosteroids are also known to slow or delay healing. Concomitant use of topical NSAIDs and topical steroids may increase the potential for healing problems.
5.2 Cross-Sensitivity or Hypersensitivity
There is the potential for cross-sensitivity to acetylsalicylic acid, phenylacetic acid derivatives, and other NSAIDs. There have been reports of bronchospasm or exacerbation of asthma associated with the use of ketorolac tromethamine ophthalmic solution in patients who have either a known hypersensitivity to aspirin/non-steroidal anti-inflammatory drugs or a past medical history of asthma. Therefore, caution should be used when treating individuals who have previously exhibited sensitivities to these drugs.
5.3 Increased Bleeding Time
With some NSAIDs, there exists the potential for increased bleeding time due to interference with thrombocyte aggregation. There have been reports that ocularly applied nonsteroidal anti- inflammatory drugs may cause increased bleeding of ocular tissues (including hyphemas) in conjunction with ocular surgery.
It is recommended that ketorolac tromethamine ophthalmic solution 0.5% be used with caution in patients with known bleeding tendencies or who are receiving other medications, which may prolong bleeding time.
5.4 Corneal Effects
Use of topical NSAIDs may result in keratitis. In some susceptible patients, continued use of topical NSAIDs may result in epithelial breakdown, corneal thinning, corneal erosion, corneal ulceration, or corneal perforation. These events may be sight threatening. Patients with evidence of corneal epithelial breakdown should immediately discontinue use of topical NSAIDs and should be closely monitored for corneal health.
Postmarketing experience with topical NSAIDs suggests that patients with complicated ocular surgeries, corneal denervation, corneal epithelial defects, diabetes mellitus, ocular surface diseases (e.g., dry eye syndrome), rheumatoid arthritis, or repeat ocular surgeries within a short period of time may be at increased risk for corneal adverse events which may become sight threatening. Topical NSAIDs should be used with caution in these patients.
Postmarketing experience with topical NSAIDs also suggests that use more than 1 day prior to surgery or use beyond 14 days post-surgery may increase patient risk for the occurrence and severity of corneal adverse events.
6 ADVERSE REACTIONS
6.1 Clinical Studies Experience
Because clinical studies are conducted under widely varying conditions, adverse reaction rates observed in the clinical studies of a drug cannot be directly compared to the rates in the clinical studies of another drug and may not reflect the rates observed in practice.
The most frequent adverse reactions reported with the use of ketorolac tromethamine ophthalmic solutions have been transient stinging and burning on instillation. These reactions were reported by up to 40% of patients participating in clinical trials.
Other adverse reactions occurring approximately 1 to 10% of the time during treatment with ketorolac tromethamine ophthalmic solutions included allergic reactions, corneal edema, iritis, ocular inflammation, ocular irritation, superficial keratitis, and superficial ocular infections.
Other adverse reactions reported rarely with the use of ketorolac tromethamine ophthalmic solutions included: corneal infiltrates, corneal ulcer, eye dryness, headaches , and visual disturbance (blurry vision).
6.2 Postmarketing Experience
The following adverse reactions have been identified during post-marketing use of ketorolac tromethamine ophthalmic solution 0.5% in clinical practice. Because they are reported voluntarily from a population of unknown size, estimates of frequency cannot be made. The reactions, which have been chosen for inclusion due to either their seriousness, frequency of reporting, possible causal connection to topical ketorolac tromethamine ophthalmic solution 0.5% or a combination of these factors, include bronchospasm or exacerbation of asthma, corneal erosion, corneal perforation, corneal thinning, and epithelial breakdown [see Warnings and Precautions ( 5.2, 5.4)] .
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Teratogenic Effects. Pregnancy Category C
Pregnancy Category C: Ketorolac tromethamine, administered during organogenesis, was not teratogenic in rabbits and rats at oral doses of 3.6 mg/kg/day and 10 mg/kg/day, respectively. These doses are approximately 100 times and 250 times higher respectively than the maximum recommended human topical ophthalmic daily dose of 2 mg (5 mg/mL x 0.05 mL/drop, x4 drops x 2 eyes) to affected eyes on a mg/kg basis. Additionally, when administered to rats after Day 17 of gestation at oral doses up to 1.5 mg/kg/day (approximately 40 times the typical human topical ophthalmic daily dose), ketorolac tromethamine resulted in dystocia and increased pup mortality. There are no adequate and well-controlled studies in pregnant women. Ketorolac tromethamine ophthalmic solution 0.5% should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
Nonteratogenic Effects: Because of the known effects of prostaglandin-inhibiting drugs on the fetal cardiovascular system (closure of the ductus arteriosus), the use of ketorolac tromethamine ophthalmic solution 0.5% during late pregnancy should be avoided.
8.3 Nursing Mothers
Because many drugs are excreted in human milk, caution should be exercised when ketorolac tromethamine ophthalmic solution 0.5% is administered to a nursing woman.
11 DESCRIPTION
Ketorolac tromethamine ophthalmic solution 0.5% is a member of the pyrrolo-pyrrole group of nonsteroidal anti-inflammatory drugs (NSAIDs) for ophthalmic use. Its chemical name is (±)-5-Benzoyl-2,3-dihydro-1H pyrrolizine-1-carboxylic acid compound with 2-amino-2-(hydroxymethyl)-1,3-propanediol (1:1) and it has the following structure:

Ketorolac tromethamine ophthalmic solution 0.5% is supplied as a sterile isotonic aqueous 0.5% solution, with a pH of 7.4. Ketorolac tromethamine ophthalmic solution 0.5% is a racemic mixture of R-(+) and S-(-)- ketorolac tromethamine. Ketorolac tromethamine may exist in three crystal forms. All forms are equally soluble in water. The pKa of ketorolac is 3.5. This white to off-white crystalline substance discolors on prolonged exposure to light. The molecular weight of ketorolac tromethamine is 376.41. The osmolality of ketorolac tromethamine ophthalmic solution 0.5% is 290 mOsmol/kg.
Each mL of ketorolac tromethamine ophthalmic solution contains: Active: ketorolac tromethamine 0.5%. Preservative: benzalkonium chloride 0.01%. Inactives: edetate disodium 0.1%; octoxynol 40; water for injection; sodium chloride; hydrochloric acid and/or sodium hydroxide to adjust the pH.
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Ketorolac tromethamine is a nonsteroidal anti-inflammatory drug which, when administered systemically, has demonstrated analgesic, anti-inflammatory, and anti-pyretic activity. The mechanism of its action is thought to be due to its ability to inhibit prostaglandin biosynthesis.
12.3 Pharmacokinetics
Two drops of 0.5% ketorolac tromethamine ophthalmic solution instilled into the eyes of patients 12 hours and 1 hour prior to cataract extraction achieved a mean ketorolac concentration of 95 ng/mL in the aqueous humor of 8 of 9 eyes tested (range 40 to 170 ng/mL).
One drop of 0.5% ketorolac tromethamine ophthalmic solution was instilled into 1 eye and 1 drop of vehicle into the other eye TID in 26 healthy subjects. Five (5) of 26 subjects had detectable concentrations of ketorolac in their plasma (range 11 to 23 ng/mL) at Day 10 during topical ocular treatment. The range of concentrations following TID dosing of 0.5% ketorolac tromethamine ophthalmic solution are approximately 4 to 8% of the steady state mean minimum plasma concentration observed following four times daily oral administration of 10 mg ketorolac in humans (290 ± 70 ng/mL).
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Ketorolac tromethamine was not carcinogenic in either rats given up to 5 mg/kg/day orally for 24 months or in mice given 2 mg/kg/day orally for 18 months. These doses are approximately 125 times and 50 times higher respectively than the maximum recommended human topical ophthalmic daily dose given as QID for itching to affected eyes on a mg/kg basis.
Ketorolac tromethamine was not mutagenic in vitro in the Ames assay or in forward mutation assays. Similarly, it did not result in an in vitro increase in unscheduled DNA synthesis or an in vivo increase in chromosome breakage in mice. However, ketorolac tromethamine did result in an increased incidence in chromosomal aberrations in Chinese hamster ovary cells.
Ketorolac tromethamine did not impair fertility when administered orally to male and female rats at doses up to 9 mg/kg/day and 16 mg/kg/day, respectively. These doses are respectively 225 and 400 times higher than the typical human topical ophthalmic daily dose.
14 CLINICAL STUDIES
Two controlled clinical studies showed that ketorolac tromethamine ophthalmic solution was significantly more effective than its vehicle in relieving ocular itching caused by seasonal allergic conjunctivitis.
Two controlled clinical studies showed that patients treated for two weeks with ketorolac tromethamine ophthalmic solution were less likely to have measurable signs of inflammation (cell and flare) than patients treated with its vehicle.
Results from clinical studies indicate that ketorolac tromethamine has no significant effect upon intraocular pressure; however, changes in intraocular pressure may occur following cataract surgery.
16 HOW SUPPLIED/STORAGE AND HANDLING
Ketorolac Tromethamine Ophthalmic solution 0.5% is supplied sterile, in white opaque LDPE bottles with white opaque LDPE Nozzles with HDPE grey caps as follows.
3 mL in 5 mL bottle NDC 42571-137-31
5 mL in 5 mL bottle NDC 42571-137-25
10 mL in 10 mL bottle NDC 42571-137-26
Storage: Store at 20° to 25°C (68° to 77°F) [see USP Controlled Room Temperature].
17 PATIENT COUNSELING INFORMATION
17.1 Slow or Delayed Healing
Patients should be informed of the possibility that slow or delayed healing may occur while using nonsteroidal anti-inflammatory drugs (NSAIDs).
17.2 Avoiding Contamination of the Product
Patients should be instructed to avoid allowing the tip of the bottle to contact the eye or surrounding structures because this could cause the tip to become contaminated by common bacteria known to cause ocular infections. Serious damage to the eye and subsequent loss of vision may result from using contaminated solutions.
Also, to avoid the potential for cross-contamination, the patient should be advised to use one bottle for each eye following bilateral ocular surgery. The use of the same bottle of topical eye drops for both eyes following bilateral ocular surgery is not recommended.
17.3 Contact Lens Wear
Patients should be advised that ketorolac tromethamine ophthalmic solution 0.5% should not be administered while wearing contact lenses.
17.4 Intercurrent Ocular Conditions
Patients should be advised that if they develop an intercurrent ocular condition (e.g., trauma or infection) or have ocular surgery, they should immediately seek their physician’s advice concerning the continued use of ketorolac tromethamine ophthalmic solution 0.5%.
17.5 Concomitant Topical Ocular Therapy
Patients should be advised that if more than one topical ophthalmic medication is being used, the medicines should be administered at least 5 minutes apart.
Manufactured by:
Micro Labs Limited
Bangalore-560099, INDIA.
Manufactured for:
Micro Labs USA Inc.
Somerset, NJ 08873
Rev.10/2021
INSTRUCTIONS FOR USE:
Before you use Ketorolac Tromethamine Ophthalmic solution 0.5% for the first time:
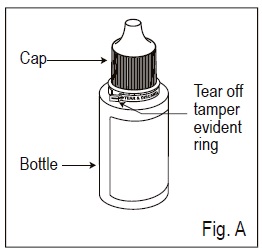
1.Check to make sure that the tamper evident ring between the bottle and the cap is not broken ( See Figure A). If the tamper evident ring is broken or missing, contact your pharmacist.
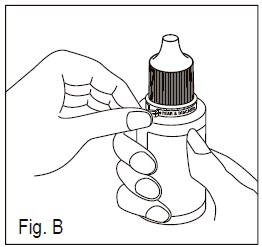
2.Tear off the tamper evident ring ( See Figure B).
3.To open the bottle, remove the cap by turning it in the counterclockwise direction ( See Figure C).

This Instructions for Use has been approved by the U.S. Food and Drug Administration.
Rev.10/2021
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
INGREDIENTS AND APPEARANCE
| KETOROLAC TROMETHAMINE
ketorolac tromethamine solution |
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
| Labeler - Micro Labs Limited (862174955) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Micro Labs Limited | 677600482 | analysis(42571-137) , label(42571-137) , manufacture(42571-137) , pack(42571-137) | |