Search by Drug Name or NDC
NDC 45802-0151-53 Fluocinonide 1 mg/g Details
Fluocinonide 1 mg/g
Fluocinonide is a TOPICAL CREAM in the HUMAN PRESCRIPTION DRUG category. It is labeled and distributed by Padagis Israel Pharmaceuticals Ltd. The primary component is FLUOCINONIDE.
MedlinePlus Drug Summary
Fluocinolone topical is used to treat the itching, redness, dryness, crusting, scaling, inflammation, and discomfort of various skin conditions, including psoriasis (a skin disease in which red, scaly patches form on some areas of the body and eczema (a skin disease that causes the skin to be dry and itchy and to sometimes develop red, scaly rashes). Fluocinolone is in a class of medications called corticosteroids. It works by activating natural substances in the skin to reduce swelling, redness, and itching.
Related Packages: 45802-0151-53Last Updated: 11/30/2022
MedLinePlus Full Drug Details: Fluocinolone Topical
Fluocinonide topical is used to treat the itching, redness, dryness, crusting, scaling, inflammation, and discomfort of various skin conditions, including psoriasis (a skin disease in which red, scaly patches form on some areas of the body and eczema (a skin disease that causes the skin to be dry and itchy and to sometimes develop red, scaly rashes). Fluocinonide is in a class of medications called corticosteroids. It works by activating natural substances in the skin to reduce swelling, redness, and itching.
Related Packages: 45802-0151-53Last Updated: 11/30/2022
MedLinePlus Full Drug Details: Fluocinonide Topical
Product Information
| NDC | 45802-0151 |
|---|---|
| Product ID | 45802-151_72c72409-e1bc-4058-8957-4361d7f94d22 |
| Associated GPIs | 90550060003710 |
| GCN Sequence Number | 058794 |
| GCN Sequence Number Description | fluocinonide CREAM (G) 0.1 % TOPICAL |
| HIC3 | Q5P |
| HIC3 Description | TOPICAL ANTI-INFLAMMATORY STEROIDAL |
| GCN | 24306 |
| HICL Sequence Number | 003318 |
| HICL Sequence Number Description | FLUOCINONIDE |
| Brand/Generic | Generic |
| Proprietary Name | Fluocinonide |
| Proprietary Name Suffix | n/a |
| Non-Proprietary Name | Fluocinonide |
| Product Type | HUMAN PRESCRIPTION DRUG |
| Dosage Form | CREAM |
| Route | TOPICAL |
| Active Ingredient Strength | 1 |
| Active Ingredient Units | mg/g |
| Substance Name | FLUOCINONIDE |
| Labeler Name | Padagis Israel Pharmaceuticals Ltd |
| Pharmaceutical Class | Corticosteroid Hormone Receptor Agonists [MoA], Corticosteroid [EPC] |
| DEA Schedule | n/a |
| Marketing Category | ANDA |
| Application Number | ANDA090256 |
| Listing Certified Through | 2024-12-31 |
Package
Package Images


NDC 45802-0151-53 (45802015153)
| NDC Package Code | 45802-151-53 |
|---|---|
| Billing NDC | 45802015153 |
| Package | 1 TUBE in 1 CARTON (45802-151-53) / 120 g in 1 TUBE |
| Marketing Start Date | 2014-01-14 |
| NDC Exclude Flag | N |
| Pricing Information | N/A |
Standard Product Labeling (SPL)/Prescribing Information SPL b90275ff-6ab2-4e85-9095-d904abf59338 Details
HIGHLIGHTS OF PRESCRIBING INFORMATION
Fluocinonide Cream USP, 0.1%
For topical use
Initial U.S. Approval: 1971
INDICATIONS AND USAGE
Fluocinonide Cream USP, 0.1% is a corticosteroid indicated for the relief of the inflammatory and pruritic manifestations of corticosteroid responsive dermatoses in patients 12 years of age or older. (1)
Limitation of Use: (1)
- •
- Treatment beyond 2 consecutive weeks is not recommended and the total dosage should not exceed 60 g per week because of the potential for the drug to suppress the hypothalamic-pituitary-adrenal (HPA) axis. (1)
- •
- Avoid use on the face, groin, or axillae. (1.2)
- •
- Avoid use in perioral dermatitis or rosacea.
DOSAGE AND ADMINISTRATION
For topical use only. Fluocinonide Cream USP, 0.1% is not for ophthalmic, oral, or intravaginal use. (2)
Psoriasis: apply a thin layer once or twice daily to the affected skin areas. (2)
Atopic Dermatitis: apply a thin layer once daily to the affected skin areas. (2)
Corticosteroid Responsive Dermatoses, other than psoriasis or atopic dermatitis: (2)
apply a thin layer once or twice daily to the affected areas. (2)
DOSAGE FORMS AND STRENGTHS
Cream, 0.1% (3)
CONTRAINDICATIONS
None (4)
WARNINGS AND PRECAUTIONS
- •
- Fluocinonide Cream USP, 0.1% has been shown to suppress the HPA axis. Systemic absorption of Fluocinonide Cream USP, 0.1% may produce reversible hypothalamic-pituitary-adrenal (HPA) axis suppression, Cushing’s syndrome, hyperglycemia and unmask latent diabetes (5.1)
- •
- Systemic absorption may require evaluation for HPA axis suppression (5.1)
- •
- Modify use should HPA axis suppression develop (5.1)
- •
- Potent corticosteroids, use on large areas, prolonged use or occlusive use may increase systemic absorption (5.3)
- •
- Local adverse reactions with topical steroids may include atrophy, striae, irritation, acneiform eruptions, hypopigmentation and allergic contact dermatitis and may be more likely to occur with occlusive use or more potent corticosteroids (5.3)
- •
- Children may be more susceptible to systemic toxicity when treated with topical corticosteroids. (5.1, 8.4)
ADVERSE REACTIONS
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 9/2021
Table Of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
1.1 Indication
1.2 Limitation of Use
2 DOSAGE AND ADMINISTRATION
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Effect on Endocrine System
5.2 Local Adverse Reactions with Topical Corticosteroids
5.3 Concomitant Skin Infections
5.4 Allergic Contact Dermatitis
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Postmarketing Experience
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.3 Nursing Mothers
8.4 Pediatric Use
8.5 Geriatric Use
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- *
- Sections or subsections omitted from the full prescribing information are not listed.
1 INDICATIONS AND USAGE
1.1 Indication
Fluocinonide Cream USP, 0.1%, is indicated for the relief of the inflammatory and pruritic manifestations of corticosteroid responsive dermatoses in patients 12 years of age or older [see Use in Specific Populations (8.4)].
1.2 Limitation of Use
Treatment beyond 2 consecutive weeks is not recommended and the total dosage should not exceed 60 g per week because the safety of Fluocinonide Cream USP, 0.1% for longer than 2 weeks has not been established and because of the potential for the drug to suppress the hypothalamic-pituitary-adrenal (HPA) axis. Therapy should be discontinued when control of the disease is achieved. If no improvement is seen within 2 weeks, reassessment of the diagnosis may be necessary. Do not use more than half of the 120 g tube per week.
Fluocinonide Cream USP, 0.1% should not be used in the treatment of rosacea or perioral dermatitis, and should not be used on the face, groin, or axillae.
2 DOSAGE AND ADMINISTRATION
For topical use only. Fluocinonide Cream USP, 0.1% is not for ophthalmic, oral, or intravaginal use.
For psoriasis, apply a thin layer of Fluocinonide Cream USP, 0.1% once or twice daily to the affected skin areas as directed by a physician. Twice daily application for the treatment of psoriasis has been shown to be more effective in achieving treatment success during 2 weeks of treatment.
For atopic dermatitis, apply a thin layer of Fluocinonide Cream USP, 0.1% once daily to the affected skin areas as directed by a physician. Once daily application for the treatment of atopic dermatitis has been shown to be as effective as twice daily treatment in achieving treatment success during 2 weeks of treatment [see Clinical Studies (14)].
For corticosteroid responsive dermatoses, other than psoriasis or atopic dermatitis, apply a thin layer of Fluocinonide Cream USP, 0.1% once or twice daily to the affected areas as directed by a physician.
3 DOSAGE FORMS AND STRENGTHS
5 WARNINGS AND PRECAUTIONS
5.1 Effect on Endocrine System
Systemic absorption of topical corticosteroids, including Fluocinonide Cream USP, 0.1%, can produce reversible hypothalamic-pituitary-adrenal (HPA) axis suppression with the potential for clinical glucocorticosteroid insufficiency. This may occur during treatment or upon withdrawal of the topical corticosteroid. In addition, the use of Fluocinonide Cream USP, 0.1% for longer than 2 weeks may suppress the immune system [see Nonclinical Toxicology (13.1)].
HPA axis suppression has been observed with fluocinonide cream, 0.1%, applied once or twice daily in 2 out of 18 adult patients with plaque-type psoriasis, 1 out of 31 adult patients with atopic dermatitis and 4 out of 123 pediatric patients with atopic dermatitis [see Use in Specific Population (8.4) and Clinical Pharmacology (12.2)].
Because of the potential for systemic absorption, use of topical corticosteroids, including Fluocinonide Cream USP, 0.1%, may require that patients be periodically evaluated for HPA axis suppression. Factors that predispose a patient using a topical corticosteroid to HPA axis suppression include the use of more potent steroids, use over large surface areas, use over prolonged periods, use under occlusion, use on an altered skin barrier, and use in patients with liver failure.
An ACTH stimulation test may be helpful in evaluating patients for HPA axis suppression. If HPA axis suppression is documented, an attempt should be made to gradually withdraw the drug, to reduce the frequency of application, or to substitute a less potent steroid. Manifestations of adrenal insufficiency may require supplemental systemic corticosteroids. Recovery of HPA axis function is generally prompt and complete upon discontinuation of topical corticosteroids.
Cushing’s syndrome, hyperglycemia, and unmasking of latent diabetes mellitus can also result from systemic absorption of topical corticosteroids.
Use of more than one corticosteroid-containing product at the same time may increase the total systemic absorption of topical corticosteroids.
Studies conducted in pediatric patients demonstrated reversible HPA axis suppression after use of fluocinonide cream, 0.1%. Pediatric patients may be more susceptible than adults to systemic toxicity from equivalent doses of fluocinonide cream, 0.1% due to their larger skin surface-to-body-mass ratios [See Use in Specific Populations (8.4)].
5.2 Local Adverse Reactions with Topical Corticosteroids
Local adverse reactions may be more likely to occur with occlusive use, prolonged use or use of higher potency corticosteroids. Reactions may include atrophy, striae, telangiectasis, burning, itching, irritation, dryness, folliculitis, acneiform eruptions, hypopigmentation, perioral dermatitis, allergic contact dermatitis, secondary infection, and miliaria. Some local adverse reactions may be irreversible.
5.3 Concomitant Skin Infections
If concomitant skin infections are present or develop, an appropriate antifungal or antibacterial agent should be used. If a favorable response does not occur promptly, use of Fluocinonide Cream USP, 0.1% should be discontinued until the infection has been adequately controlled.
5.4 Allergic Contact Dermatitis
If irritation develops, Fluocinonide Cream USP, 0.1% should be discontinued and appropriate therapy instituted. Allergic contact dermatitis with corticosteroids is usually diagnosed by observing failure to heal rather than noting a clinical exacerbation as with most topical products not containing corticosteroids. Such an observation should be corroborated with appropriate diagnostic patch testing.
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
Because clinical studies are conducted under widely varying conditions, adverse reaction rates observed in the clinical studies of a drug cannot be directly compared to rates in the clinical studies of another drug and may not reflect the rates observed in practice.
In clinical trials, a total of 443 adult subjects with atopic dermatitis or plaque-type psoriasis were treated once daily or twice daily with fluocinonide cream, 0.1% for 2 weeks. The most commonly observed adverse reactions in these clinical trials were as follows:
Table 1: Most Commonly Observed Adverse Reactions (≥1%) in Adult Clinical Trials
|
Adverse Reaction |
Fluocinonide Cream once daily (n=216) |
Fluocinonide Cream twice daily (n=227) |
Vehicle Cream once or twice daily (n=211) |
|
Headache |
8 (3.7%) |
9 (4.0%) |
6 (2.8%) |
|
Application Site Burning |
5 (2.3%) |
4 (1.8%) |
14 (6.6%) |
|
Nasopharyngitis |
2 (0.9%) |
3 (1.3%) |
3 (1.4%) |
|
Nasal Congestion |
3 (1.4%) |
1 (0.4%) |
0 |
Safety in patients 12 to 17 years of age was similar to that observed in adults.
6.2 Postmarketing Experience
The following adverse reactions have been identified during post approval use of fluocinonide cream, 0.1%:
Administration Site Conditions: discoloration, erythema, irritation, pruritus, swelling, pain and condition aggravated.
Immune System Disorders: hypersensitivity.
Nervous System Disorders: headache and dizziness.
Skin and Subcutaneous Tissue Disorders: acne, dry skin, rash, skin exfoliation and skin tightness.
Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Teratogenic Effects: Pregnancy Category C
There are no adequate and well-controlled studies in pregnant women. Therefore, Fluocinonide Cream USP, 0.1% should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
Corticosteroids have been shown to be teratogenic in laboratory animals when administered systemically at relatively low dosage levels. Some corticosteroids have been shown to be teratogenic after dermal application in laboratory animals.
8.3 Nursing Mothers
Systemically administered corticosteroids appear in human milk and could suppress growth, interfere with endogenous corticosteroid production, or cause other untoward effects. It is not known whether topical administration of corticosteroids could result in sufficient systemic absorption to produce detectable quantities in breast milk. Nevertheless, a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother.
8.4 Pediatric Use
Safety and efficacy of Fluocinonide Cream USP, 0.1% in pediatric patients younger than 12 years of age have not been established; therefore use in pediatric patients younger than 12 years of age is not recommended.
HPA axis suppression was studied in 4 sequential cohorts of pediatric patients with atopic dermatitis covering at least 20% of the body surface area, treated once daily or twice daily with fluocinonide cream, 0.1%. The first cohort of 31 patients (mean 36.3% BSA) 12 to < 18 years old; the second cohort included 31 patients (mean 39.0% BSA) 6 to < 12 years old; the third cohort included 30 patients (mean 34.6% BSA) 2 to < 6 years old; the fourth cohort included 31 patients (mean 40.0% BSA) 3 months to < 2 years old. Fluocinonide cream, 0.1% caused HPA-axis suppression in 1 patient in the twice daily group in Cohort 1, 2 patients in the twice daily group in Cohort 2, and 1 patient in the twice daily group in Cohort 3. Follow-up testing 14 days after treatment discontinuation, available for all 4 suppressed patients, demonstrated a normally responsive HPA axis. Signs of skin atrophy were present at baseline and severity was not determined making it difficult to assess local skin safety. Therefore, the safety of fluocinonide cream, 0.1% in patients younger than 12 years of age has not been demonstrated [see Warnings and Precautions (5.1)].
HPA axis suppression has not been evaluated in patients with psoriasis who are less than 18 years of age.
Because of a higher ratio of skin surface area to body mass, pediatric patients are at a greater risk than adults of HPA-axis suppression and Cushing’s syndrome when they are treated with topical corticosteroids. They are therefore also at greater risk of adrenal insufficiency during or after withdrawal of treatment. Adverse effects including striae have been reported with inappropriate use of topical corticosteroids in infants and children.
HPA-axis suppression, Cushing’s syndrome, linear growth retardation, delayed weight gain, and intracranial hypertension have been reported in children receiving topical corticosteroids. Manifestations of adrenal suppression in children include low plasma cortisol levels and absence of response to cosyntropin (ACTH1-24) stimulation. Manifestations of intracranial hypertension include bulging fontanelles, headaches, and bilateral papilledema.
10 OVERDOSAGE
11 DESCRIPTION
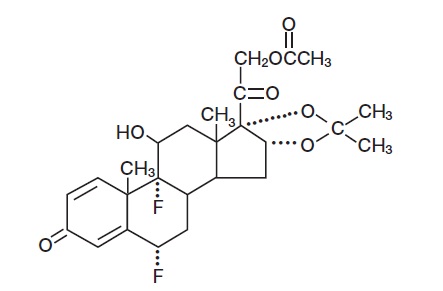
Fluocinonide Cream USP, 0.1% contains fluocinonide, a synthetic corticosteroid for topical dermatologic use. The corticosteroids constitute a class of primarily synthetic steroids used topically as anti-inflammatory and antipruritic agents. Fluocinonide has the chemical name 6α, 9-Difluoro-11β, 16α, 17, 21-tetrahydroxypregna-1, 4-diene-3, 20-dione, cyclic 16, 17-acetal with acetone, 21-acetate. Its chemical formula is C26H32F2O7 and it has a molecular weight of 494.52.
It has the following chemical structure:
Fluocinonide is an almost odorless white to creamy white crystalline powder. It is practically insoluble in water and slightly soluble in ethanol.
Each gram of Fluocinonide Cream USP, 0.1% contains 1 mg fluocinonide in a cream base of carbopol 980, citric acid, diisopropanolamine, glycerin, glyceryl monostearate, glyceryl stearate, isostearic acid, PEG-100 stearate, polyethylene glycol monomethyl ether, propylene glycol, and water.
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Corticosteroids play a role in cellular signaling, immune function, inflammation, and protein regulation; however, the precise mechanism of action of Fluocinonide Cream USP, 0.1% in corticosteroid responsive dermatoses is unknown.
12.2 Pharmacodynamics
Vasoconstrictor studies performed with fluocinonide cream, 0.1% in healthy subjects indicate that it is in the super-high range of potency as compared with other topical corticosteroids; however, similar blanching scores do not necessarily imply therapeutic equivalence.
Application of fluocinonide cream, 0.1% twice daily for 14 days in 18 adult subjects with plaque-type psoriasis (10–50% BSA, mean 19.6% BSA) and 31 adult subjects (17 treated once daily; 14 treated twice daily) with atopic dermatitis (2-10% BSA, mean 5% BSA) showed demonstrable HPA-axis suppression in 2 subjects with psoriasis (with 12% and 25% BSA) and 1 subject with atopic dermatitis (treated once daily, 4% BSA) where the criterion for HPA-axis suppression is a serum cortisol level of less than or equal to 18 micrograms per deciliter 30 minutes after stimulation with cosyntropin (ACTH1-24) [see Warnings and Precautions (5.1)].
HPA-axis suppression following application of fluocinonide cream, 0.1%, (once or twice daily) was also evaluated in 123 pediatric patients from 3 months to < 18 years of age with atopic dermatitis (mean BSA range 34.6 % - 40.0 %). HPA-axis suppression was observed in 4 patients in the twice daily groups. Follow-up testing 14 days after treatment discontinuation demonstrated a normally responsive HPA axis in all 4 suppressed patients [see Warnings and Precautions (5.1) and Use in Specific Populations (8.4)].
12.3 Pharmacokinetics
The extent of percutaneous absorption of topical corticosteroids is determined by many factors including the vehicle and the integrity of the epidermal barrier. Topical corticosteroids can be absorbed from normal intact skin. Inflammation and/or other disease processes in the skin may increase percutaneous absorption.
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Long-term animal studies have not been performed to evaluate the carcinogenic potential of fluocinonide cream, 0.1% because of severe immunosuppression induced in a 13-week dermal rat study. The effects of fluocinonide on fertility have not been evaluated.
Fluocinonide revealed no evidence of mutagenic or clastogenic potential based on the results of two in vitro genotoxicity tests (Ames test and chromosomal aberration assay using human lymphocytes). However, fluocinonide was positive for clastogenic potential when tested in the in vivo mouse micronucleus assay.
Topical (dermal) application of 0.0003%-0.03% fluocinonide cream to rats once daily for 13 weeks resulted in a toxicity profile generally associated with long term exposure to corticosteroids including decreased skin thickness, adrenal atrophy, and severe immunosuppression. A NOAEL could not be determined in this study. In addition, topical (dermal) application of 0.1% fluocinonide cream plus UVR exposure to hairless mice for 13 weeks and 150-900 mg/kg/day of 0.1% fluocinonide cream to minipigs (a model which more closely approximates human skin) for 13 weeks produced glucocorticoid-related suppression of the HPA axis, with some signs of immuno-suppression noted in the dermal minipig study. Although the clinical relevance of the findings in animals to humans is not clear, sustained glucocorticoid-related immune suppression may increase the risk of infection and possibly the risk for carcinogenesis.
Topical doses of 0% (fluocinonide cream vehicle), 0.0001%, 0.005% and 0.001% fluocinonide cream were evaluated in a 52 week dermal photo-carcinogenicity study (40 weeks of treatment followed by 12 weeks of observation) conducted in hairless albino mice with concurrent exposure to low level ultraviolet radiation. Topical treatment with increasing concentrations of fluocinonide cream did not have an adverse effect in this study. The results of this study suggest that topical treatment with fluocinonide cream would not enhance photo-carcinogenesis.
14 CLINICAL STUDIES
Two adequate and well-controlled efficacy and safety studies of fluocinonide cream, 0.1% have been completed, one in adult subjects with plaque-type psoriasis (Table 2), and one in adult subjects with atopic dermatitis (Table 3). In each of these studies, subjects with between 2% and 10% body surface area involvement at baseline treated all affected areas either once daily or twice daily with fluocinonide cream, 0.1% for 14 consecutive days. The primary measure of efficacy was the proportion of subjects whose condition was cleared or almost cleared at the end of treatment. The results of these studies are presented in the tables below as percent and number of patients achieving treatment success at Week 2.
Table 2: Plaque-type Psoriasis in Adults
|
Fluocinonide Cream, once daily (n=107) |
Vehicle, once daily (n=54) |
Fluocinonide Cream, twice daily (n=107) |
Vehicle, twice daily (n=55) |
|
|
Subjects cleared |
0 (0) |
0 (0) |
6 (6%) |
0 (0) |
|
Subjects achieving treatment success* |
19 (18%) |
4 (7%) |
33 (31%) |
3 (5%) |
*Cleared or almost cleared
Table 3: Atopic Dermatitis in Adults
|
Fluocinonide Cream, once daily (n=109) |
Vehicle, once daily (n=50) |
Fluocinonide Cream, twice daily (n=102) |
Vehicle, twice daily (n=52) |
|
|
Subjects cleared |
11 (10%) |
0 (0) |
17 (17%) |
0 (0) |
|
Subjects achieving treatment success* |
64 (59%) |
6 (12%) |
58 (57%) |
10 (19%) |
*Cleared or almost cleared
No efficacy studies have been conducted to compare fluocinonide cream, 0.1% with any other topical corticosteroid product, including fluocinonide cream 0.05%.
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
[See FDA-approved patient labeling (Patient Information)]
Patients using Fluocinonide Cream USP, 0.1% should receive the following information and instructions. This information is intended to aid in the safe and effective use of this medication. It is not a disclosure of all possible adverse or unintended effects:
- •
- Fluocinonide Cream USP, 0.1% is to be used as directed by the physician. It is for external use only. Avoid contact with the eyes. It should not be used on the face, groin, and underarms.
- •
- Fluocinonide Cream USP, 0.1% should not be used for any disorder other than that for which it was prescribed.
- •
- The treated skin area should not be bandaged or otherwise covered or wrapped, so as to be occlusive unless directed by the physician.
- •
- Patients should report to their physician any signs of local adverse reactions.
- •
- Other corticosteroid-containing products should not be used with Fluocinonide Cream USP, 0.1% without first talking to the physician.
- •
- As with other corticosteroids, therapy should be discontinued when control is achieved. If no improvement is seen in 2 weeks, the patient should be instructed to contact a physician. The safety of the use of Fluocinonide Cream USP, 0.1% for longer than 2 weeks has not been established.
- •
- Patients should be informed to not use more than 60 g per week of Fluocinonide Cream USP, 0.1%. Do not use more than half of the 120 g tube per week.
- •
- Patients should inform their physicians that they are using Fluocinonide Cream USP, 0.1% if surgery is contemplated.
- •
- Patients should wash their hands after applying medication.
Rx Only
Made in Israel
Manufactured By Perrigo
Yeruham, Israel
Distributed By
Perrigo®
Allegan, MI 49010
www.perrigo.com
Rev 01-19
1M100 RC J5
PATIENT INFORMATION
Fluocinonide Cream USP, 0.1%
Important: For skin use only. Do not get Fluocinonide Cream USP, 0.1% in your eyes, mouth, or vagina. Not for use on the face, groin, or underarms.
Read the Patient Information that comes with Fluocinonide Cream USP, 0.1% before you start using it and each time you get a refill. There may be new information. This leaflet does not take the place of talking to your doctor about your condition or treatment.
What is Fluocinonide Cream USP, 0.1%?
Fluocinonide Cream USP, 0.1% is a prescription corticosteroid medicine used on the skin (topical) to treat adults and children 12 years and older with certain skin conditions that cause red, flaky, and itchy skin.
- •
- You should not use Fluocinonide Cream USP, 0.1% for longer than 2 weeks in a row.
- •
- You should not use more than 60 grams of Fluocinonide Cream USP, 0.1% or more than half of the 120 gram tube in 1 week.
- •
- Fluocinonide Cream USP, 0.1% should not be used:
- •
- if you have skin swelling or redness on the nose of face (rosacea)
- •
- for a scaly or bumpy rash around your mouth (perioral dermatitis)
- •
- on your face, underarms, or groin area
It is not known if Fluocinonide Cream USP, 0.1% is safe and effective in children under 12 years of age.
What should I tell my doctor before using Fluocinonide Cream USP, 0.1%?
Before using Fluocinonide Cream USP, 0.1% , tell your doctor if you:
- •
- have had irritation or other skin reaction to a steroid medicine in the past
- •
- have adrenal gland problems
- •
- plan to have surgery
- •
- are pregnant or plan to become pregnant. It is not known if Fluocinonide Cream USP, 0.1% will harm your unborn baby. Talk to your doctor if you are pregnant or plan to become pregnant.
- •
- are breast-feeding or plan to breastfeed. It is not known if Fluocinonide Cream USP, 0.1% passes into your breast milk. Talk to your doctor about the best way to feed your baby if you use Fluocinonide Cream USP, 0.1% .
Tell your doctor about all the medicine you take including prescriptions and non-prescriptions medicines, vitamins, and herbal supplements. Especially tell your doctor if you take a corticosteroid medicine by mouth or use other products on your skin that contain corticosteroids. Ask your doctor or pharmacist if you are not sure.
Know the medicines you take. Keep a list of your medicines with you to show your doctor and pharmacist when you get a new medicine.
How should I use Fluocinonide Cream USP, 0.1%?
- •
- See “What is Fluocinonide Cream USP, 0.1%?”
- •
- Use Fluocinonide Cream USP, 0.1% exactly as your doctor tells you.
- •
- This medicine is for use on the skin only. Do not use Fluocinonide Cream USP, 0.1% in your eyes, mouth or vagina.
- •
- Wash your hands after you use Fluocinonide Cream USP, 0.1%.
- •
- Do not use Fluocinonide Cream USP, 0.1% for longer than 2 weeks in a row.
- •
- Talk to your doctor if your skin does not get better after 2 weeks of treatment with Fluocinonide Cream USP, 0.1% .
- •
- Do not bandage or cover the skin treated with Fluocinonide Cream USP, 0.1% unless your doctor tells you to.
What are the possible side effects with Fluocinonide Cream USP, 0.1%?
Fluocinonide Cream USP, 0.1% may cause side effects, including:
- •
- Symptoms of a disorder where the adrenal gland does not make enough of certain hormones (adrenal insufficiency) during treatment or after stopping treatment. Your doctor may do blood tests to check you for adrenal insufficiency while you are using Fluocinonide Cream USP, 0.1% . Tell your doctor if you have any of these symptoms of adrenal insufficiency:
- •
- tiredness that worsens and does not go away
- •
- nausea or vomiting
- •
- dizziness or fainting
- •
- muscle weakness
- •
- irritability and depression
- •
- loss of appetite
- •
- weight loss
- •
- Cushing’s syndrome, when the body is exposed to too much of the hormone cortisol.
Your doctor may do tests to check for this. Symptoms can include:
- •
- weight gain, especially around your upper back and midsection
- •
- slow healing of cuts, insect bites and infections
- •
- tiredness and muscle weakness
- •
- depression, anxiety and irritability
- •
- roundness of your face (moon face)
- •
- new or worsening high blood pressure
The most common side effect of Fluocinonide Cream USP, 0.1% is burning of your skin treated with Fluocinonide Cream USP, 0.1%.
Talk to your doctor about any side effect that bothers you or that does not go away.
These are not all the side effects with Fluocinonide Cream USP, 0.1%. Ask your doctor or pharmacist for more information.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
You may also report side effects to Perrigo at 1-866-634-9120.
How should I store Fluocinonide Cream USP, 0.1%?
- •
- Store Fluocinonide Cream USP, 0.1% at room temperature, between 20-25°C (68-77°F) [see USP Controlled Room Temperature].
- •
- Keep the tube tightly closed.
Keep Fluocinonide Cream USP, 0.1% and all medicines out of the reach of children.
General information about Fluocinonide Cream USP, 0.1%
Medicines are sometimes prescribed for purposes other than those listed in the Patient Information leaflet. Do not use Fluocinonide Cream USP, 0.1% for a condition for which it was not prescribed. Do not give Fluocinonide Cream USP, 0.1% to other people, even if they have the same symptoms you have. It may harm them.
This Patient Information leaflet summarizes the most important information about Fluocinonide Cream USP, 0.1% . If you would like more information, talk with your doctor. You can also ask your pharmacist or doctor for information about Fluocinonide Cream USP, 0.1% that is written for healthcare professionals.
What are the ingredients in Fluocinonide Cream USP, 0.1%?
Active ingredient: fluocinonide 0.1%
Inactive ingredients: carbopol 980, citric acid, diisopropanolamine, glycerin, glyceryl monostearate, glyceryl stearate, isostearic acid, PEG-100 stearate, polyethylene glycol monomethyl ether, propylene glycol, and water.
This Patient Information has been approved by the U.S. Food and Drug Administration.
Rx Only
Made in Israel
Manufactured By Perrigo
Yeruham, Israel
Distributed By
Perrigo®
Allegan, MI 49010
www.perrigo.com
Rev 01-19
1M100 RC J5
PACKAGE/LABEL PRINCIPAL DISPLAY PANEL – 30 g Carton
INGREDIENTS AND APPEARANCE
| FLUOCINONIDE
fluocinonide cream |
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
| Labeler - Padagis Israel Pharmaceuticals Ltd (600093611) |