Search by Drug Name or NDC
NDC 47335-0343-16 Cetirizine Hydrochloride 5 mg/1 Details
Cetirizine Hydrochloride 5 mg/1
Cetirizine Hydrochloride is a ORAL TABLET, CHEWABLE in the HUMAN OTC DRUG category. It is labeled and distributed by Sun Pharmaceutical Industries, Inc.. The primary component is CETIRIZINE HYDROCHLORIDE.
MedlinePlus Drug Summary
Cetirizine is used to temporarily relieve the symptoms of hay fever (allergy to pollen, dust, or other substances in the air) and allergy to other substances (such as dust mites, animal dander, cockroaches, and molds). These symptoms include sneezing; runny nose; itchy, red, watery eyes; and itchy nose or throat. Cetirizine is also used to treat itching and redness caused by hives. However, cetirizine does not prevent hives or other allergic skin reactions. Cetirizine is in a class of medications called antihistamines. It works by blocking the action of histamine, a substance in the body that causes allergic symptoms. Cetirizine is also available in combination with pseudoephedrine (Sudafed, others). This monograph only includes information about the use of cetirizine alone. If you are taking the cetirizine and pseudoephedrine combination product, read the information on the package label or ask your doctor or pharmacist for more information.
Related Packages: 47335-0343-16Last Updated: 11/30/2022
MedLinePlus Full Drug Details: Cetirizine
Product Information
| NDC | 47335-0343 |
|---|---|
| Product ID | 47335-343_82a6bc4d-2eed-434f-931a-ba338706ebbb |
| Associated GPIs | 41550020100510 |
| GCN Sequence Number | 053980 |
| GCN Sequence Number Description | cetirizine HCl TAB CHEW 5 MG ORAL |
| HIC3 | Z2Q |
| HIC3 Description | ANTIHISTAMINES - 2ND GENERATION |
| GCN | 21769 |
| HICL Sequence Number | 006544 |
| HICL Sequence Number Description | CETIRIZINE HCL |
| Brand/Generic | Generic |
| Proprietary Name | Cetirizine Hydrochloride |
| Proprietary Name Suffix | n/a |
| Non-Proprietary Name | Cetirizine Hydrochloride |
| Product Type | HUMAN OTC DRUG |
| Dosage Form | TABLET, CHEWABLE |
| Route | ORAL |
| Active Ingredient Strength | 5 |
| Active Ingredient Units | mg/1 |
| Substance Name | CETIRIZINE HYDROCHLORIDE |
| Labeler Name | Sun Pharmaceutical Industries, Inc. |
| Pharmaceutical Class | Histamine H1 Receptor Antagonists [MoA], Histamine-1 Receptor Antagonist [EPC] |
| DEA Schedule | n/a |
| Marketing Category | ANDA |
| Application Number | ANDA090142 |
| Listing Certified Through | 2024-12-31 |
Package
Package Images









NDC 47335-0343-16 (47335034316)
| NDC Package Code | 47335-343-16 |
|---|---|
| Billing NDC | 47335034316 |
| Package | 100 TABLET, CHEWABLE in 1 BOTTLE (47335-343-16) |
| Marketing Start Date | 2011-09-26 |
| NDC Exclude Flag | N |
| Pricing Information | N/A |
Standard Product Labeling (SPL)/Prescribing Information SPL 54812a5b-ce4a-47ff-a481-0ed36ca17f5d Details
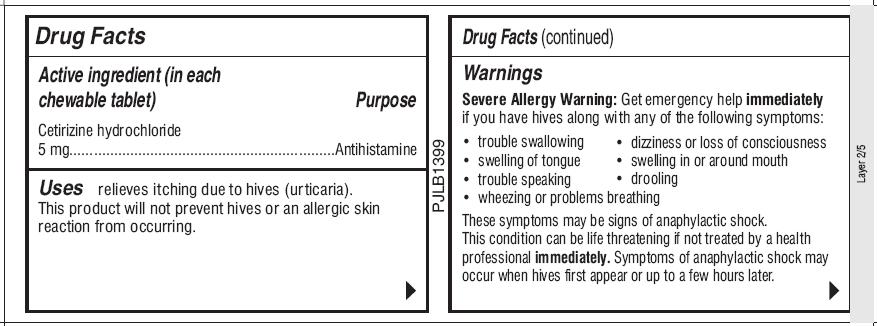
Active ingredient (in each chewable tablet)
Uses
Warnings
Severe Allergy Warning: Get emergency help immediately if you have hives along with any of the following symptoms:
- trouble swallowing
- dizziness or loss of consciousness
- swelling of tongue
- swelling in or around mouth
- trouble speaking
- drooling
- wheezing or problems breathing
These symptoms may be signs of anaphylactic shock. This condition can be life threatening if not treated by a health professional immediately. Symptoms of anaphylactic shock may occur when hives first appear or up to a few hours later.
Not a Substitute for Epinephrine. If your doctor has prescribed an epinephrine injector for “anaphylaxis” or severe allergy symptoms that could occur with your hives, never use this product as a substitute for the epinephrine injector. If you have been prescribed an epinephrine injector, you should carry it with you at all times.
Do not use
- to prevent hives from any known cause such as:
- foods
- insect stings
- medicines
- latex or rubber gloves because this product will not stop hives from occurring. Avoiding the cause of your hives is the only way to prevent them. Hives can sometimes be serious. If you do not know the cause of your hives, see your doctor for a medical exam. Your doctor may be able to help you find a cause.
- If you have ever had an allergic reaction to this product or any of its ingredients or to an antihistamine containing hydroxyzine.
Ask a doctor before use if you have
When using this product
Stop use and ask doctor if
If pregnant or breast-feeding:
Keep out of reach of children
Directions
- may be taken with or without water
For 5 mg:
| adults and children 6 years and over | 1 to 2 tablets once daily depending upon severity of symptoms; do not take more than 2 tablets in 24 hours. |
| adults 65 years and over | 1 tablet once a day; do not take more than 1 tablet in 24 hours |
| children under 6 years of age | ask a doctor |
| consumers with liver or kidney disease | ask a doctor |
For 10 mg:
| adults and children 6 years and over | one 10 mg tablet once daily; do not take more than one 10 mg tablet in 24 hours. A 5 mg product may be appropriate for less severe symptoms. |
| adults 65 years and over | ask a doctor |
| children under 6 years of age | ask a doctor |
| consumers with liver or kidney disease | ask a doctor |
Other information
Inactive ingredients
acesulfame potassium, colloidal silicon dioxide, compressible sugar, crospovidone, FD & C Blue No # 2 Aluminum Lake, FD & C Red No # 40 Aluminum Lake, guar gum, magnesium oxide light powder, magnesium stearate, mannitol, microcrystalline cellulose, pregelatinized starch, prosweet N & A flavor powder, talc, tutti frutti flavor
Principal Display Panel
For 5 mg Hives Relief:
Original Prescription Strength
NDC 47335-343-16
Children's
Cetirizine Hydrochloride Chewable Tablets
5 mg
HIVES Relief
Antihistamine
24 hour Relief of ITCHING Due to Hives
Tutti-frutti Flavor
6 yrs. & older
100 CHEWABLE TABLETS
SUN PHARMACEUTICAL INDUSTRIES LTD.





For 10 mg Hives Relief:
Original Prescription Strength
NDC 47335-344-16
Children's
Cetirizine Hydrochloride Chewable Tablets
10 mg
HIVES Relief
Antihistamine
Tutti-frutti Flavor
6 yrs. & older
100 CHEWABLE TABLETS
SUN PHARMACEUTICAL INDUSTRIES LTD.





INGREDIENTS AND APPEARANCE
| CETIRIZINE HYDROCHLORIDE
cetirizine hydrochloride tablet, chewable |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| CETIRIZINE HYDROCHLORIDE
cetirizine hydrochloride tablet, chewable |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Labeler - Sun Pharmaceutical Industries, Inc. (146974886) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Sun Pharmaceutical Industries Limited | 725959238 | ANALYSIS(47335-343, 47335-344) , MANUFACTURE(47335-343, 47335-344) | |