Search by Drug Name or NDC
NDC 47335-0779-91 Azelastine Hydrochloride 137 ug/1 Details
Azelastine Hydrochloride 137 ug/1
Azelastine Hydrochloride is a NASAL SPRAY, METERED in the HUMAN PRESCRIPTION DRUG category. It is labeled and distributed by Sun Pharmaceutical Industries, Inc.. The primary component is AZELASTINE HYDROCHLORIDE.
MedlinePlus Drug Summary
Azelastine, an antihistamine, is used to treat hay fever and allergy symptoms including runny nose, sneezing, and itchy nose. This medication is sometimes prescribed for other uses; ask your doctor or pharmacist for more information.
Related Packages: 47335-0779-91Last Updated: 11/30/2022
MedLinePlus Full Drug Details: Azelastine Nasal Spray
Product Information
| NDC | 47335-0779 |
|---|---|
| Product ID | 47335-779_09652cb1-7315-4188-9f36-4e2b50fb764d |
| Associated GPIs | 42401015102020 |
| GCN Sequence Number | 029893 |
| GCN Sequence Number Description | azelastine HCl SPRAY/PUMP 137 MCG NASAL |
| HIC3 | Q7E |
| HIC3 Description | NASAL ANTIHISTAMINE |
| GCN | 60544 |
| HICL Sequence Number | 007607 |
| HICL Sequence Number Description | AZELASTINE HCL |
| Brand/Generic | Generic |
| Proprietary Name | Azelastine Hydrochloride |
| Proprietary Name Suffix | n/a |
| Non-Proprietary Name | Azelastine Hydrochloride |
| Product Type | HUMAN PRESCRIPTION DRUG |
| Dosage Form | SPRAY, METERED |
| Route | NASAL |
| Active Ingredient Strength | 137 |
| Active Ingredient Units | ug/1 |
| Substance Name | AZELASTINE HYDROCHLORIDE |
| Labeler Name | Sun Pharmaceutical Industries, Inc. |
| Pharmaceutical Class | Histamine H1 Receptor Antagonists [MoA], Histamine-1 Receptor Antagonist [EPC] |
| DEA Schedule | n/a |
| Marketing Category | ANDA |
| Application Number | ANDA090423 |
| Listing Certified Through | 2024-12-31 |
Package
Package Images

NDC 47335-0779-91 (47335077991)
| NDC Package Code | 47335-779-91 |
|---|---|
| Billing NDC | 47335077991 |
| Package | 1 BOTTLE, SPRAY in 1 BOX (47335-779-91) / 200 SPRAY, METERED in 1 BOTTLE, SPRAY |
| Marketing Start Date | 2012-05-24 |
| NDC Exclude Flag | N |
| Pricing Information | |
| Price Per Unit | 0.2952 |
| Pricing Unit | ML |
| Effective Date | 2024-02-21 |
| NDC Description | AZELASTINE 0.1% (137 MCG) SPRY |
| Pharmacy Type Indicator | C/I |
| OTC | N |
| Explanation Code | 1, 5 |
| Classification for Rate Setting | G |
| As of Date | 2024-02-21 |
Standard Product Labeling (SPL)/Prescribing Information SPL 305c6dc8-8247-473d-9975-b02903af6d53 Details
HIGHLIGHTS OF PRESCRIBING INFORMATION
See full prescribing information for AZELASTINE HYDROCHLORIDE NASAL SOLUTION.
AZELASTINE HYDROCHLORIDE nasal solution (nasal spray)
Initial U.S. Approval: 1996
INDICATIONS AND USAGE
Azelastine hydrochloride nasal solution (nasal spray) is an H1-receptor antagonist indicated for the treatment of the symptoms of seasonal allergic rhinitis in adults and pediatric patients 5 years and older and for the treatment of the symptoms of vasomotor rhinitis in adults and adolescent patients 12 years and older. (1)
DOSAGE AND ADMINISTRATION
DOSAGE FORMS AND STRENGTHS
Azelastine hydrochloride nasal solution (nasal spray): 137 mcg of azelastine hydrochloride in each 0.137 mL spray. (3)
CONTRAINDICATIONS
None. (4)
WARNINGS AND PRECAUTIONS
- Somnolence: Avoid engaging in hazardous occupations requiring complete mental alertness such as driving or operating machinery when taking azelastine hydrochloride nasal solution. (5.1)
- Alcohol and other central nervous system (CNS) depressants: Avoid concurrent use with azelastine hydrochloride nasal solution because further decreased alertness and impairment of CNS performance may occur. (5.1)
ADVERSE REACTIONS
The most common adverse reactions (≥2% incidence) are: bitter taste, headache, somnolence, dysesthesia, rhinitis, nasal burning, pharyngitis, epistaxis, sinusitis, paroxysmal sneezing, nausea, dry mouth, fatigue, dizziness, and weight increase. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Sun Pharmaceutical Industries, Inc. at 1-800-818-4555 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 12/2018
Table Of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Seasonal Allergic Rhinitis
2.2 Vasomotor Rhinitis
2.3 Important Administration Instructions
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Somnolence in Activities Requiring Mental Alertness
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Postmarketing Experience
7 DRUG INTERACTIONS
7.1 Central Nervous System Depressants
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
14.1 Seasonal Allergic Rhinitis
14.2 Vasomotor Rhinitis
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- *
- Sections or subsections omitted from the full prescribing information are not listed.
1 INDICATIONS AND USAGE
Azelastine hydrochloride nasal solution (nasal spray), 0.1% (137 mcg per spray) is indicated for the treatment of the symptoms of seasonal allergic rhinitis in adults and pediatric patients 5 years and older, and for the treatment of the symptoms of vasomotor rhinitis in adults and adolescent patients 12 years and older.
2 DOSAGE AND ADMINISTRATION
2.1 Seasonal Allergic Rhinitis
The recommended dosage of azelastine hydrochloride nasal solution in adults and adolescent patients 12 years and older with seasonal allergic rhinitis is one or two sprays per nostril twice daily. The recommended dosage of azelastine hydrochloride nasal solution in pediatric patients 5 years to 11 years of age is one spray per nostril twice daily.
2.2 Vasomotor Rhinitis
The recommended dosage of azelastine hydrochloride nasal solution in adults and adolescent patients 12 years and older with vasomotor rhinitis is two sprays per nostril twice daily.
2.3 Important Administration Instructions
Administer azelastine hydrochloride nasal solution by the intranasal route only.
Priming: Prime azelastine hydrochloride nasal solution before initial use by releasing 4 sprays or until a fine mist appears. When azelastine hydrochloride nasal solution has not been used for 3 or more days, reprime with 2 sprays or until a fine mist appears. Avoid spraying azelastine hydrochloride nasal solution into the eyes.
3 DOSAGE FORMS AND STRENGTHS
5 WARNINGS AND PRECAUTIONS
5.1 Somnolence in Activities Requiring Mental Alertness
In clinical trials, the occurrence of somnolence has been reported in some patients taking azelastine hydrochloride nasal solution [see Adverse Reactions (6.1)]. Patients should be cautioned against engaging in hazardous occupations requiring complete mental alertness and motor coordination such as operating machinery or driving a motor vehicle after administration of azelastine hydrochloride nasal solution. Concurrent use of azelastine hydrochloride nasal solution with alcohol or other central nervous system depressants should be avoided because additional reductions in alertness and additional impairment of central nervous system performance may occur [see Drug Interactions (7.1)].
6 ADVERSE REACTIONS
Use of azelastine hydrochloride nasal solution has been associated with somnolence [see Warnings and Precautions (5.1)].
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect rates observed in practice.
Seasonal Allergic Rhinitis
Azelastine Hydrochloride Nasal Solution Two Sprays Per Nostril Twice Daily
Adverse experience information for azelastine hydrochloride nasal solution is derived from six placebo- and active- controlled, 2-day to 8-week clinical trials which included 391 patients, 12 years of age and older, with seasonal allergic rhinitis who received azelastine hydrochloride nasal solution at a dose of 2 sprays per nostril twice daily. In placebo-controlled efficacy trials, the incidence of discontinuation due to adverse reactions in patients receiving azelastine hydrochloride nasal solution and vehicle placebo was 2.2% and 2.8%, respectively.
Table 1 contains adverse reactions that were reported with frequencies ≥2% in the azelastine hydrochloride nasal solution 2 sprays per nostril twice daily treatment group and more frequently than placebo.
| Table 1: Adverse Reactions Reported in ≥2% Incidence in Placebo-Controlled Trials in Patients with Seasonal Allergic Rhinitis [n (%)]
|
||
| | Azelastine Hydrochloride Nasal Solution N = 391 | Vehicle Placebo N = 353 |
| Bitter Taste | 77 (19.7%) | 2 (0.6%) |
| Headache | 58 (14.8%) | 45 (12.7%) |
| Somnolence | 45 (11.5%) | 19 (5.4%) |
| Nasal Burning | 16 (4.1%) | 6 (1.7%) |
| Pharyngitis | 15 (3.8%) | 10 (2.8%) |
| Paroxysmal Sneezing | 12 (3.1%) | 4 (1.1%) |
| Dry Mouth | 11 (2.8%) | 6 (1.7%) |
| Nausea | 11 (2.8%) | 4 (1.1%) |
| Rhinitis | 9 (2.3%) | 5 (1.4%) |
| Fatigue | 9 (2.3%) | 5 (1.4%) |
| Dizziness | 8 (2%) | 5 (1.4%) |
| Epistaxis | 8 (2%) | 5 (1.4%) |
| Weight Increase | 8 (2%) | 0 (0%) |
Azelastine Hydrochloride Nasal Solution One Spray Per Nostril Twice Daily
Adverse experience information for azelastine hydrochloride nasal solution at a dose of one spray per nostril twice daily is derived from two placebo-controlled 2-week clinical studies which included 276 patients 12 years of age and older with seasonal allergic rhinitis. The incidence of discontinuation due to adverse reactions in patients receiving azelastine hydrochloride nasal solution and vehicle placebo was 0% and 0.8%, respectively. Bitter taste was reported in 8.3% of patients compared to none in the placebo group. Somnolence was reported in 0.4% of patients compared to none in the placebo group.
A total of 176 patients 5 to 11 years of age were exposed to azelastine hydrochloride nasal solution at a dose of 1 spray each nostril twice daily in 3 placebo-controlled studies. In these studies, adverse reactions that occurred more frequently in patients treated with azelastine hydrochloride nasal solution than with placebo, and that were not represented in the adult adverse reactions table above include rhinitis/cold symptoms (17% vs. 9.5%), cough (11.4% vs. 8.3%), conjunctivitis (5.1% vs. 1.8%), and asthma (4.5% vs. 4.1%).
Adverse Reactions <2% in Azelastine Hydrochloride Nasal Solution One or Two Sprays Per Nostril Twice Daily
The following reactions were observed infrequently (<2% and exceeding placebo incidence) in patients who received azelastine hydrochloride nasal solution dosed at 1 or 2 sprays per nostril twice daily in U.S. clinical trials.
Cardiovascular: flushing, hypertension, tachycardia.
Dermatological: contact dermatitis, eczema, hair and follicle infection, furunculosis, skin laceration.
Digestive: constipation, gastroenteritis, glossitis, ulcerative stomatitis, vomiting, increased SGPT, aphthous stomatitis, diarrhea, toothache.
Metabolic and Nutritional: increased appetite.
Musculoskeletal: myalgia, temporomandibular dislocation, rheumatoid arthritis.
Neurological: hyperkinesia, hypoesthesia, vertigo.
Psychological: anxiety, depersonalization, depression, nervousness, sleep disorder, thinking abnormal.
Respiratory: bronchospasm, coughing, throat burning, laryngitis, bronchitis, dry throat, nocturnal dyspnea, nasopharyngitis, nasal congestion, pharyngolaryngeal pain, sinusitis, nasal dryness, paranasal sinus hypersecretion, post nasal drip.
Special Senses: conjunctivitis, eye abnormality, eye pain, watery eyes, taste loss.
Urogenital: albuminuria, amenorrhea, breast pain, hematuria, increased urinary frequency.
Whole Body: allergic reaction, back pain, herpes simplex, viral infection, malaise, pain in extremities, abdominal pain, pyrexia.
Vasomotor Rhinitis
Adverse experience information for azelastine hydrochloride nasal solution is derived from two placebo-controlled clinical studies which included 216 patients 12 years and older with vasomotor rhinitis who received azelastine hydrochloride nasal solution at a dose of 2 sprays per nostril twice daily for up to 28 days. The incidence of discontinuation due to adverse reactions in patients receiving azelastine hydrochloride nasal solution and vehicle placebo was 2.8% and 2.9%, respectively.
The following adverse reactions were reported with frequencies ≥ 2% in the azelastine hydrochloride nasal solution treatment group and more frequently than placebo.
| Table 2: Adverse Reactions Reported in ≥2% Incidence in Placebo-Controlled Trials in Patients with Vasomotor Rhinitis [n (%)]
|
||
| | Azelastine Hydrochloride Nasal Solution N = 216 | Vehicle Placebo N = 210 |
| Bitter Taste | 42 (19.4%) | 5 (2.4%) |
| Headache | 17 (7.9%) | 16 (7.6%) |
| Dysesthesia | 17 (7.9%) | 7 (3.3%) |
| Rhinitis | 12 (5.6%) | 5 (2.4%) |
| Epistaxis | 7 (3.2%) | 5 (2.4%) |
| Sinusitis | 7 (3.2%) | 4 (1.9%) |
| Somnolence | 7 (3.2%) | 2 (1%) |
Reactions observed infrequently (<2% and exceeding placebo incidence) in patients who received azelastine hydrochloride nasal solution (2 sprays/nostril twice daily) in U.S. clinical trials in vasomotor rhinitis were similar to those observed in U.S. clinical trials in seasonal allergic rhinitis.
In controlled trials involving nasal and oral azelastine hydrochloride formulations, there were infrequent occurrences of hepatic transaminase elevations.
6.2 Postmarketing Experience
During the post approval use of azelastine hydrochloride nasal solution, the following adverse reactions have been identified. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure. Adverse reactions reported include: anaphylaxis, application site irritation, atrial fibrillation, chest pain, confusion, dyspnea, facial edema, involuntary muscle contractions, nasal sores, palpitations, paresthesia, parosmia, pruritus, rash, disturbance or loss of sense of smell and/or taste, tolerance, urinary retention, vision abnormal and xerophthalmia.
7 DRUG INTERACTIONS
7.1 Central Nervous System Depressants
Concurrent use of azelastine hydrochloride nasal solution with alcohol or other central nervous system depressants should be avoided because reductions in alertness and impairment of central nervous system performance may occur [see Warnings and Precautions (5.1)].
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
Limited data from postmarketing experience over decades of use with azelastine hydrochloride nasal solution in pregnant women have not identified any drug associated risks of miscarriage, birth defects, or other adverse maternal or fetal outcomes. In animal reproduction studies, there was no evidence of fetal harm at oral doses approximately 5 times the clinical daily dose. Oral administration of azelastine hydrochloride to pregnant mice, rats, and rabbits, during the period of organogenesis, produced developmental toxicity that included structural abnormalities, decreased embryo-fetal survival, and decreased fetal body weights at doses 270 times and higher than the maximum recommended human daily intranasal dose (MRHDID) of 1.096 mg. However, the relevance of these findings in animals to pregnant women was considered questionable based upon the high animal to human dose multiple.
The estimated background risk of major birth defects and miscarriage for the indicated populations is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2-4% and 15-20%, respectively.
Data
Animal Data
In an embryo-fetal development study in mice dosed during the period of organogenesis, azelastine hydrochloride caused embryo-fetal death, structural abnormalities (cleft palate; short or absent tail; fused, absent or branched ribs), delayed ossification, and decreased fetal weight at approximately 300 times the maximum recommended human daily intranasal dose (MRHDID) in adults (on a mg/m2 basis at a maternal oral dose of 68.6 mg/kg/day), which also caused maternal toxicity as evidenced by decreased maternal body weight. Neither fetal nor maternal effects occurred in mice at approximately 15 times the MRHDID in adults (on a mg/m2 basis at a maternal oral dose of 3 mg/kg/day).
In an embryo-fetal development study in pregnant rats dosed during the period of organogenesis from gestation days 7 to 17, azelastine hydrochloride caused structural abnormalities (oligo-and brachydactylia), delayed ossification, and skeletal variations, in the absence of maternal toxicity, at approximately 270 times the MRHDID in adults (on a mg/m2 basis at a maternal oral dose of 30 mg/kg/day). Azelastine hydrochloride caused embryo-fetal death and decreased fetal weight and severe maternal toxicity at approximately 610 times the MRHDID (on a mg/m2 basis at a maternal oral dose of 68.6 mg/kg/day). Neither fetal nor maternal effects occurred at approximately 20 times the MRHDID (on a mg/m2 basis at a maternal oral dose of 2 mg/kg/day).
In an embryo-fetal development study in pregnant rabbits dosed during the period of organogenesis from gestation days 6 to 18, azelastine hydrochloride caused abortion, delayed ossification and decreased fetal weight and severe maternal toxicity at approximately 530 times the MRHDID in adults (on a mg/m2 basis at a maternal oral dose of 30 mg/kg/day). Neither fetal nor maternal effects occurred at approximately 5 times the MRHDID (on a mg/m2 basis at a maternal oral dose of 0.3 mg/kg/day).
In a prenatal and postnatal development study in pregnant rats dosed from late in the gestation period and through the lactation period from gestation day 17 through lactation day 21, azelastine hydrochloride produced no adverse developmental effects on pups at maternal doses up to approximately 270 times the MRHDID (on mg/m2 basis at a maternal dose of 30 mg/kg/day).
8.2 Lactation
Risk Summary
There are no data on the presence of azelastine hydrochloride in human milk, the effects on the breastfed infant, or the effects on milk production. Breastfed infants should be monitored for signs of milk rejection during azelastine hydrochloride nasal solution use by lactating women (see Clinical Considerations). The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for azelastine hydrochloride nasal solution and any potential adverse effects on the breastfed infant from azelastine hydrochloride nasal solution or from the underlying maternal condition.
Clinical Considerations
Monitoring for Adverse Reactions
Breastfed infants of lactating women treated with azelastine hydrochloride nasal solution should be monitored for possible signs of milk rejection related to the bitter taste of azelastine hydrochloride.
8.4 Pediatric Use
The safety and effectiveness of azelastine hydrochloride nasal solution for the treatment of symptoms of seasonal allergic rhinitis have been established for patients 5 years and older [see Adverse Reactions (6.1) and Clinical Studies (14.1)]. The safety and effectiveness of azelastine hydrochloride nasal solution for the treatment of vasomotor rhinitis have been established for patients 12 years and older [see Adverse Reactions (6.1) and Clinical Studies (14.2)]. The safety and effectiveness of azelastine hydrochloride nasal solution in pediatric patients below the age of 5 years with seasonal allergic rhinitis and in pediatric patients below the age of 12 years with vasomotor rhinitis have not been established.
8.5 Geriatric Use
Clinical trials of azelastine hydrochloride nasal solution did not include sufficient numbers of patients aged 65 and over to determine whether they respond differently from younger patients. Other reported clinical experience has not identified differences in responses between the elderly and younger patients. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy.
10 OVERDOSAGE
There have been no reported overdosages with azelastine hydrochloride nasal solution. Acute overdosage by adults with this dosage form is unlikely to result in clinically significant adverse reactions, other than increased somnolence, since one bottle of azelastine hydrochloride nasal solution contains 30 mg of azelastine hydrochloride. Clinical trials in adults with single doses of the oral formulation of azelastine hydrochloride (up to 16 mg) have not resulted in increased incidence of serious adverse reactions. General supportive measures should be employed if overdosage occurs. There is no known antidote to azelastine hydrochloride nasal solution. Oral ingestion of antihistamines has the potential to cause serious adverse effects in young children. Accordingly, azelastine hydrochloride nasal solution should be kept out of the reach of children.
11 DESCRIPTION
Azelastine Hydrochloride Nasal Solution (Nasal Spray), 0.1% (137 mcg per spray), is an antihistamine formulated as a metered-spray solution for intranasal administration. Azelastine hydrochloride occurs as a white, almost odorless, crystalline powder with a bitter taste. It has a molecular weight of 418.37. It is sparingly soluble in water, methanol, and propylene glycol and slightly soluble in ethanol, octanol, and glycerine. It has a melting point of about 225°C and the pH of a saturated solution is between 5 and 5.4. Its chemical name is (±)-1-(2H)-phthalazinone,4-[(4-chlorophenyl) methyl]-2-(hexahydro-1-methyl-1H-azepin-4-yl)-, monohydrochloride. Its molecular formula is C22H24ClN3O•HCl with the following chemical structure:

Azelastine hydrochloride nasal solution contains 0.1% azelastine hydrochloride in an aqueous solution at pH 6.8 ± 0.3. It also contains benzalkonium chloride 50% solution (250 mcg/mL), edetate disodium, hypromellose, citric acid anhydrous, dibasic sodium phosphate, sodium chloride, and water for injection.
After priming [see Dosage and Administration (2.3)], each metered spray delivers a 0.137 mL mean volume containing 137 mcg of azelastine hydrochloride (equivalent to 125 mcg of azelastine base). The bottle can deliver 200 metered sprays.
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Azelastine hydrochloride, a phthalazinone derivative, exhibits histamine H1-receptor antagonist activity in isolated tissues, animal models, and humans. Azelastine hydrochloride nasal solution is administered as a racemic mixture with no difference in pharmacologic activity noted between the enantiomers in in vitro studies. The major metabolite, desmethylazelastine, also possesses H1-receptor antagonist activity.
12.2 Pharmacodynamics
Cardiac Electrophysiology:
In a placebo-controlled study (95 subjects with allergic rhinitis), there was no evidence of an effect of azelastine hydrochloride nasal solution (2 sprays per nostril twice daily for 56 days) on cardiac repolarization as represented by the corrected QT interval (QTc) of the electrocardiogram. Following multiple dose oral administration of azelastine 4 mg or 8 mg twice daily, the mean change in QTc was 7.2 msec and 3.6 msec, respectively.
Interaction studies investigating the cardiac repolarization effects of concomitantly administered oral azelastine hydrochloride and erythromycin or ketoconazole were conducted. These drugs had no effect on QTc based on analysis of serial electrocardiograms. At a dose approximately 8 times the maximum recommended dose, azelastine hydrochloride does not prolong the QTc interval to any clinically relevant extent.
12.3 Pharmacokinetics
Absorption: After intranasal administration, the systemic bioavailability of azelastine hydrochloride is approximately 40%. Maximum plasma concentrations (Cmax) are achieved in 2 to 3 hours.
Azelastine hydrochloride administered intranasally at doses above two sprays per nostril twice daily for 29 days resulted in greater than proportional increases in Cmax and area under the curve (AUC) for azelastine.
Distribution: Based on intravenous and oral administration, the steady-state volume of distribution is 14.5 L/kg. In vitro studies with human plasma indicate that the plasma protein binding of azelastine and its metabolite, desmethylazelastine, are approximately 88% and 97%, respectively.
Metabolism: Azelastine is oxidatively metabolized to the principal active metabolite, desmethylazelastine, by the cytochrome P450 enzyme system. The specific P450 isoforms responsible for the biotransformation of azelastine have not been identified. After intranasal dosing of azelastine hydrochloride to steady-state, plasma concentrations of desmethylazelastine range from 20 to 50% of azelastine concentrations. Limited data indicate that the metabolite profile is similar when azelastine hydrochloride is administered via the intranasal or oral route.
Elimination: Based on intravenous and oral administration, the elimination half-life and plasma clearance are 22 hours and 0.5 L/h/kg, respectively. Approximately 75% of an oral dose of radiolabeled azelastine hydrochloride was excreted in the feces with less than 10% as unchanged azelastine.
Special Populations:
Hepatic Impairment: Following oral administration, pharmacokinetic parameters were not influenced by hepatic impairment.
Renal Impairment: Based on oral, single-dose studies, renal insufficiency (creatinine clearance <50 mL/min) resulted in a 70 to 75% higher Cmax and AUC compared to normal subjects. Time to maximum concentration was unchanged.
Age: Following oral administration, pharmacokinetic parameters were not influenced by age.
Gender: Following oral administration, pharmacokinetic parameters were not influenced by gender.
Race: The effect of race has not been evaluated.
Drug-Drug Interactions:
Erythromycin: No significant pharmacokinetic interaction was observed with the coadministration of orally administered azelastine (4 mg twice daily) with erythromycin (500 mg three times daily for 7 days). In this study, coadministration of orally administered azelastine with erythromycin resulted in Cmax of 5.36 ± 2.6 ng/mL and AUC of 49.7 ± 24 ng•h/mL for azelastine, whereas, administration of azelastine alone resulted in Cmax of 5.57 ± 2.7 ng/mL and AUC of 48.4 ± 24 ng•h/mL for azelastine.
Cimetidine and Ranitidine: In a multiple-dose, steady-state drug interaction trial in healthy subjects, cimetidine (400 mg twice daily) increased orally administered mean azelastine (4 mg twice daily) concentrations by approximately 65%. No pharmacokinetic interaction was observed with coadministration of orally administered azelastine (4 mg twice daily) with ranitidine hydrochloride (150 mg twice daily). Oral coadministration of azelastine with ranitidine resulted in Cmax of 8.89 ±3.28 ng/mL and AUC of 88.22 ± 40.43 ng•h/mL for azelastine, whereas, azelastine when administered alone resulted in Cmax of 7.83 ± 4.06 ng/mL and AUC of 80.09 ± 43.55 ng•h/mL for azelastine.
Theophylline: No significant pharmacokinetic interaction was observed with the coadministration of an oral 4 mg dose of azelastine hydrochloride twice daily and theophylline 300 mg or 400 mg twice daily.
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Two-year carcinogenicity studies in Crl:CD(SD)BR rats and NMRI mice were conducted to assess the carcinogenic potential of azelastine hydrochloride. No evidence of tumorigenicity was observed in rats at doses up to 30 mg/kg day (approximately 270 and 240 times the MRHDID for adults and children, respectively, on a mg/m2 basis). No evidence for tumorigenicity was observed in mice at doses up to 25 mg/kg (approximately 110 and 100 times the MRHDID for adults and children, respectively, on a mg/m2 basis).
Azelastine hydrochloride showed no genotoxic effects in the Ames test, DNA repair test, mouse lymphoma forward mutation assay, mouse micronucleus test, or chromosomal aberration test in rat bone marrow.
There were no effects on male or female fertility and reproductive performance in male and female rats at oral doses up to 30 mg/kg (approximately 270 times the MRHDID in adults on a mg/m2 basis). At 68.6 mg/kg (approximately 610 times the MRHDID on a mg/m2 basis), the duration of estrous cycles was prolonged and copulatory activity and the number of pregnancies were decreased. The numbers of corpora lutea and implantations were decreased; however, preimplantation loss was not increased.
14 CLINICAL STUDIES
14.1 Seasonal Allergic Rhinitis
Two Sprays Per Nostril Twice Daily
The efficacy and safety of azelastine hydrochloride nasal solution were evaluated in three placebo-controlled clinical trials of azelastine hydrochloride nasal solution including 322 patients with seasonal allergic rhinitis who received two sprays per nostril twice a day for up to 4 weeks. These trials included 55 pediatric patients ages 12 to 16 years. Assessment of efficacy was based on the 12-hour reflective Total Symptom Complex (TSC) and Major Symptom Complex (MSC). The MSC was calculated as the average of individual symptoms of nose blows, sneezes, runny nose/sniffles, itchy nose, and watery eyes as assessed by patients on a 0 to 5 categorical scale. Azelastine hydrochloride nasal solution two sprays per nostril twice daily demonstrated a greater decrease in the MSC than placebo (Table 3).
|
||||||
| | Treatment
| N
| Baseline LS Mean (SD)
| Change from Baseline (SD)
| TreatmentDifference
| P-value
|
| Trial 1: 12 Hour AM and PM Reflective MSC
|
||||||
| | Azelastine Hydrochloride Nasal Solution (Nasal Spray) | 63 | 11.48 (4.13) | -3.05 (3.51) | 1.98 | <0.01 |
| | Placebo Nasal Spray | 60 | 10.84 (4.53) | -1.07 (3.52) |
||
| Trial 2: 12 Hour AM and PM Reflective MSC
|
||||||
| | Azelastine Hydrochloride Nasal Solution (Nasal Spray) | 63 | 12.5 (4.5) | -4.10 (3.46) | 2.03 | <0.01 |
| | Placebo Nasal Spray | 63 | 12.18 (4.64) | -2.07 (4.01) |
||
| Trial 3: 12 Hour AM and PM Reflective MSC
|
||||||
| | Azelastine Hydrochloride Nasal Solution (Nasal Spray) | 66 | 12.04 (4.03) | -3.31 (3.74) | 1.35 | 0.04 |
| | Placebo Nasal Spray | 66 | 11.66 (3.96) | -1.96 (3.57) |
||
In dose-ranging trials, administration of azelastine hydrochloride nasal solution two sprays per nostril twice daily resulted in a statistically significant decrease in symptoms compared to saline placebo within 3 hours after initial dosing and persisted over the 12-hour dosing interval.
One Spray Per Nostril Twice Daily
The efficacy and safety of azelastine hydrochloride nasal solution were evaluated in two placebo-controlled clinical trials of azelastine hydrochloride nasal solution including 275 patients with seasonal allergic rhinitis who received one spray per nostril twice a day for up to 2 weeks. Assessment of efficacy was based on the 12-hour reflective Total Nasal Symptom Score [rTNSS]. rTNSS is calculated as the sum of the patients scoring of four individual nasal symptoms (runny nose, sneezing, itchy nose, and nasal congestion) as assessed by patients on a 0 to 3 categorical scale. The primary efficacy endpoint was the change from Baseline to Day 14 in rTNSS. The mean change from baseline in rTNSS was greater in patients receiving azelastine hydrochloride nasal solution one spray per nostril twice daily than those receiving placebo (Table 4).
|
||||||
| | Treatment
| N
| Baseline LS Mean (SD)
| Change from Baseline (SD)
| TreatmentDifference
| P-value
|
| Trial 4: 12 Hour AM and PM Reflective TNSS
|
||||||
| | Azelastine Hydrochloride Nasal Solution (Nasal Spray) | 138 | 16.34 (4.22) | -2.69 (4.79) | 1.38 | 0.01 |
| | Placebo Nasal Spray | 141 | 17.21 (4.32) | -1.31 (4.29) |
||
| Trial 5: 12 Hour AM and PM Reflective TNSS
|
||||||
| | Azelastine Hydrochloride Nasal Solution (Nasal Spray) | 137 | 16.62 (4.2) | -3.68 (4.16) | 1.18 | 0.02 |
| | Placebo Nasal Spray | 136 | 16.84 (4.77) | -2.50 (4.01) |
||
Two-week studies comparing the efficacy (and safety) of azelastine hydrochloride nasal solution two sprays per nostril twice daily versus one spray per nostril twice daily were not conducted.
14.2 Vasomotor Rhinitis
The efficacy and safety of azelastine hydrochloride nasal solution were evaluated in two placebo-controlled clinical trials of azelastine hydrochloride nasal solution including 216 patients with vasomotor rhinitis who received two sprays per nostril twice a day for up to 4 weeks. These patients had vasomotor rhinitis for at least one year, negative skin tests to indoor and outdoor aeroallergens, negative nasal smears for eosinophils, and negative sinus X-rays. Azelastine hydrochloride nasal solution demonstrated a significantly greater decrease in a symptom complex comprised of rhinorrhea, post nasal drip, nasal congestion, and sneezing compared to placebo.
16 HOW SUPPLIED/STORAGE AND HANDLING
Azelastine hydrochloride nasal solution (Nasal Spray), 0.1% (137 mcg per spray) is supplied as a 30 mL package (NDC 47335-779-91) delivering 200 metered sprays in a high-density polyethylene (HDPE) bottle fitted with a metered-dose spray pump unit. The spray pump unit consists of a nasal spray pump fitted with a blue safety clip and a blue plastic dust cover. The net content of the bottle is 30 mL (net weight 30 gm of solution). Each bottle contains 30 mg (1 mg/mL) of azelastine hydrochloride. After priming [see Dosage and Administration (2.3)], each spray delivers a fine mist containing a mean volume of 0.137 mL solution containing 137 mcg of azelastine hydrochloride. The correct amount of medication in each spray cannot be assured before the initial priming and after 200 sprays have been used, even though the bottle is not completely empty. The bottle should be discarded after 200 sprays have been used. Azelastine hydrochloride nasal solution (nasal spray) should not be used after the expiration date "Exp" printed on the medicine label and carton.
Storage: Store at 20° to 25°C (68° to 77°F); excursions permitted between 15° and 30°C (59° and 86°F) [see USP Controlled Room Temperature]. Protect from freezing.
17 PATIENT COUNSELING INFORMATION
See FDA-approved patient labeling (Patient Information and Instructions for Use).
Activities Requiring Mental Alertness
Somnolence has been reported in some patients taking azelastine hydrochloride nasal solution. Caution patients against engaging in hazardous occupations requiring complete mental alertness and motor coordination such as driving or operating machinery after administration of azelastine hydrochloride nasal solution [see Warnings and Precautions (5.1)].
Concurrent Use of Alcohol and other Central Nervous System Depressants
Instruct patients to avoid concurrent use of azelastine hydrochloride nasal solution with alcohol or other central nervous system depressants because additional reductions in alertness and additional impairment of central nervous system performance may occur [see Warnings and Precautions (5.1)].
Common Adverse Reactions
Inform patients that the treatment with azelastine hydrochloride nasal solution may lead to adverse reactions, which include bitter taste, headache, somnolence, dysesthesia, rhinitis, nasal burning, pharyngitis, epistaxis, sinusitis, paroxysmal sneezing, nausea, dry mouth, fatigue, dizziness, and weight increase [see Adverse Reactions (6.1)].
Priming
Instruct patients to prime the pump before initial use and when azelastine hydrochloride nasal solution has not been used for 3 or more days [see Dosage and Administration (2.3)].
Keep Spray Out of Eyes
Instruct patients to avoid spraying azelastine hydrochloride nasal solution into their eyes.
Keep Out of Children's Reach
Instruct patients to keep azelastine hydrochloride nasal solution out of the reach of children. If a child accidentally ingests azelastine hydrochloride nasal solution, seek medical help or call a poison control center immediately.
PATIENT INFORMATION
Azelastine Hydrochloride Nasal Solution
(Nasal Spray), 0.1% (137 mcg per spray)
(a-ZEL-as-teen HYE-droe-KLOR-ide)
Important: For use in your nose only.
What is azelastine hydrochloride nasal solution?
- Azelastine hydrochloride nasal solution is a prescription medicine used to treat symptoms of seasonal allergic rhinitis in people age 5 and older and vasomotor rhinitis in people age 12 and older.
- Azelastine hydrochloride nasal solution may help to reduce your nasal symptoms including stuffy nose, runny nose, itching and sneezing.
It is not known if azelastine hydrochloride nasal solution is safe and effective in children with seasonal allergic rhinitis under 5 years of age or in children with vasomotor rhinitis under 12 years of age.
What should I tell my healthcare provider before using azelastine hydrochloride nasal solution?
Before using azelastine hydrochloride nasal solution, tell your healthcare provider if you are:
- allergic to any of the ingredients in azelastine hydrochloride nasal solution. See the end of this leaflet for a complete list of ingredients in azelastine hydrochloride nasal solution.
- pregnant, or plan to become pregnant
- breastfeeding, or plan to breastfeed. It is not known if azelastine hydrochloride passes into your breast milk. You and your healthcare provider should decide if you will use azelastine hydrochloride nasal solution if you plan to breastfeed.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. Azelastine hydrochloride nasal solution and other medicines may affect each other, causing side effects.
How should I use azelastine hydrochloride nasal solution?
- Read the Instructions for Use at the end of this leaflet for information about the right way to use azelastine hydrochloride nasal solution.
- Spray azelastine hydrochloride nasal solution in your nose only. Do not spray it into your eyes or mouth.
- Use azelastine hydrochloride nasal solution exactly as your healthcare provider tells you to use it.
- Do not use more than your healthcare provider tells you.
- Throw away your azelastine hydrochloride nasal solution bottle after using 200 sprays. Even though the bottle may not be completely empty, you may not get the correct dose of medicine.
- If you use too much or a child accidentally swallows azelastine hydrochloride nasal solution, call your healthcare provider or go to the nearest hospital emergency room right away.
What should I avoid while using azelastine hydrochloride nasal solution?
Azelastine hydrochloride nasal solution can cause sleepiness:
- Do not drive, operate machinery, or do other dangerous activities until you know how azelastine hydrochloride nasal solution affects you.
- Do not drink alcohol or take other medicines that may cause you to feel sleepy while using azelastine hydrochloride nasal solution. It may make your sleepiness worse.
What are the possible side effects of azelastine hydrochloride nasal solution?
The most common side effects of azelastine hydrochloride nasal solution include:
- unusual bitter taste
- headache
- sleepiness
- nose burning, pain or discomfort
- runny nose
- scratchy or sore throat
- nosebleeds
- inflammation or swelling of the sinuses
- sneezing
- nausea
- dry mouth
- fatigue
- dizziness
- weight increase
Tell your healthcare provider if you have any side effect that bothers you or that does not go away.
These are not all of the possible side effects of azelastine hydrochloride nasal solution. For more information, ask your healthcare provider or pharmacist.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
How should I store azelastine hydrochloride nasal solution?
- Keep azelastine hydrochloride nasal solution upright at 20° to 25°C (68° to 77°F).
- Do not freeze azelastine hydrochloride nasal solution.
- Do not use azelastine hydrochloride nasal solution after the expiration date “Exp” on the medicine label and box.
Keep azelastine hydrochloride nasal solution and all medicines out of reach of children.
General information about the safe and effective use of azelastine hydrochloride nasal solution.
Medicines are sometimes prescribed for conditions other than those listed in a Patient Information leaflet. Do not use azelastine hydrochloride nasal solution for a condition for which it was not prescribed. Do not give azelastine hydrochloride nasal solution to other people, even if they have the same symptoms that you have. It may harm them.
This Patient Information leaflet summarizes the most important information about azelastine hydrochloride nasal solution. If you would like more information, talk with your healthcare provider. You can ask your pharmacist or healthcare provider for information about azelastine hydrochloride nasal solution that is written for health professionals.
For more information call 1-800-818-4555.
What are the ingredients in azelastine hydrochloride nasal solution?
Active ingredient: azelastine hydrochloride
Inactive ingredients: benzalkonium chloride 50% solution (250 mcg/mL), edetate disodium, hypromellose, citric acid anhydrous, dibasic sodium phosphate, sodium chloride, and water for injection.
Instructions for Use
Azelastine Hydrochloride Nasal Solution
(Nasal Spray), 0.1% (137 mcg per spray)
(a-ZEL-as-teen HYE-droe-KLOR-ide)
Important: For use in your nose only.
For the correct dose of medicine:
- Keep your head tilted downward when spraying into your nostril.
- Change nostrils each time you use the spray.
- Breathe gently and do not tip your head back after using the spray. This will keep the medicine from running down into your throat. You may get a bitter taste in your mouth.
Figure A identifies the parts of your azelastine hydrochloride nasal solution pump

Before you use azelastine hydrochloride nasal solution for the first time, you will need to prime the bottle.
Priming your azelastine hydrochloride nasal solution
Remove the blue dust cover over the tip of the pump and the blue safety clip just under the “shoulders” of the pump (See Figure B).

Hold the bottle upright with 2 fingers on the shoulders of the spray pump unit and
- put your thumb on the bottom of the bottle. Press upward with your thumb and release for the pumping action. Repeat this until you see a fine mist (See Figure C).
- To get a fine mist you must pump the spray fast and use firm pressure against the bottom of the bottle. If you see a stream of liquid, the pump is not working correctly and you may have nasal discomfort.
- This should happen in 4 sprays or less.
Now your pump is primed and ready to use.

- Do not use azelastine hydrochloride nasal solution unless you see a fine mist after you do the priming sprays. If you do not see a fine mist, clean the tip of the spray nozzle. See the “Cleaning the SprayTip of your azelastine hydrochloride nasal solution” section below.
- If you do not use azelastine hydrochloride nasal solution for 3 or more days, you will need to prime the pump with 2 sprays or until you see a fine mist.
Using your azelastine hydrochloride nasal solution
Step 1. Blow your nose to clear your nostrils.
Step 2. Keep your head tilted downward toward your toes.
Step 3. Place the spray tip about ¼ inch to ½ inch into 1 nostril. Hold bottle upright and aim the spray tip toward the back of your nose (See Figure D).

Step 4. Close your other nostril with a finger. Press the pump 1 time and sniff gently at the same time, keeping your head tilted forward and down (See Figure E).
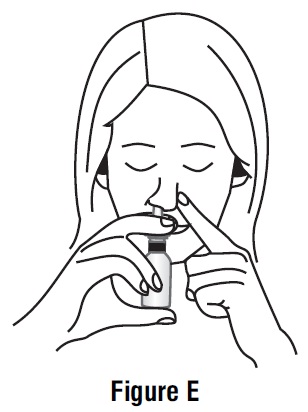
Step 5. Repeat Step 3 and Step 4 in your other nostril.
Step 6. If your healthcare provider tells you to use 2 sprays in each nostril, repeat Steps 2 through 4 above for the second spray in each nostril.
Step 7. Breathe in gently, and do not tilt your head back after using azelastine hydrochloride nasal solution. This will help to keep the medicine from going into your throat.
Step 8. When you finish using your azelastine hydrochloride nasal solution, wipe the spray tip with a clean tissue or cloth. Put the safety clip and dust cover back on the bottle.
Cleaning the Spray Tip of your azelastine hydrochloride nasal solution
- If the spray tip opening is clogged, do not use a pin or pointed object to unclog the tip. Unscrew the spray pump unit from the bottle by turning it to the left (counter-clockwise) (See Figure F).
- Soak only the spray pump unit in warm water. Squirt the spray unit several times while holding it under water. Use the pumping action to clear the opening in the tip (See Figure G).

- Let the spray pump unit air dry. Make sure it is dry before you put it back onto the bottle.
- Put the spray pump unit back into the open bottle and tighten it by turning clockwise (to the right).
- To keep the medicine from leaking out, use firm pressure when you put the pump back onto the bottle.
- After cleaning, follow the instructions for priming.
This Patient Information and Instructions for Use has been approved by the U.S. Food and Drug Administration.
Distributed by:
Sun Pharmaceutical Industries, Inc.
Cranbury, NJ 08512
Manufactured by:
Sun Pharmaceutical Industries Ltd.
Halol-Baroda Highway,
Halol-389 350, Gujarat, India.
PJPI0385E
ISS. 12/2018
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
INGREDIENTS AND APPEARANCE
| AZELASTINE HYDROCHLORIDE
azelastine hydrochloride spray, metered |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Sun Pharmaceutical Industries, Inc. (146974886) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Sun Pharmaceutical Industries Limited | 725959238 | ANALYSIS(47335-779) , MANUFACTURE(47335-779) | |