Search by Drug Name or NDC
NDC 49884-0158-02 Everolimus 0.25 mg/1 Details
Everolimus 0.25 mg/1
Everolimus is a ORAL TABLET in the HUMAN PRESCRIPTION DRUG category. It is labeled and distributed by Par Pharmaceutical, Inc.. The primary component is EVEROLIMUS.
MedlinePlus Drug Summary
Everolimus (Afinitor) is used to treat advanced renal cell carcinoma (RCC; cancer that begins in the kidneys) that has already been treated unsuccessfully with other medications. Everolimus (Afinitor) is also used to treat a certain type of advanced breast cancer that has already been treated with at least one other medication. Everolimus (Afinitor) is also used to treat a certain type of cancer of the pancreas, stomach, intestines, or lungs that has spread or progressed and that cannot be treated with surgery. Everolimus (Afinitor) is also used to treat kidney tumors in people with tuberous sclerosis complex (TSC; a genetic condition that causes tumors to grow in many organs). Everolimus (Afinitor and Afinitor Disperz) is also used to treat subependymal giant cell astrocytoma (SEGA; a type of brain tumor) in adults and children 1 year of age and older who have TSC. Everolimus (Afinitor Disperz) is also used along with other medications to treat certain types of seizures in adults and children 2 years of age and older who have TSC. Everolimus (Zortress) is used with other medications to prevent transplant rejection (attack of the transplanted organ by the immune system of the person who received the organ) in certain adults who have received kidney transplants. Everolimus is in a class of medications called kinase inhibitors. Everolimus treats cancer by stopping cancer cells from reproducing and by decreasing blood supply to the cancer cells. Everolimus prevents transplant rejection by decreasing the activity of the immune system.
Related Packages: 49884-0158-02Last Updated: 11/30/2022
MedLinePlus Full Drug Details: Everolimus
Product Information
| NDC | 49884-0158 |
|---|---|
| Product ID | 49884-158_9a6bc6cc-b9da-4fe1-9ee1-c9a393bb5dec |
| Associated GPIs | |
| GCN Sequence Number | 059247 |
| GCN Sequence Number Description | everolimus TABLET 0.25 MG ORAL |
| HIC3 | Z2E |
| HIC3 Description | IMMUNOSUPPRESSIVES |
| GCN | 24825 |
| HICL Sequence Number | 032975 |
| HICL Sequence Number Description | EVEROLIMUS |
| Brand/Generic | Generic |
| Proprietary Name | Everolimus |
| Proprietary Name Suffix | n/a |
| Non-Proprietary Name | Everolimus |
| Product Type | HUMAN PRESCRIPTION DRUG |
| Dosage Form | TABLET |
| Route | ORAL |
| Active Ingredient Strength | 0.25 |
| Active Ingredient Units | mg/1 |
| Substance Name | EVEROLIMUS |
| Labeler Name | Par Pharmaceutical, Inc. |
| Pharmaceutical Class | Cytochrome P450 2D6 Inhibitors [MoA], Cytochrome P450 3A4 Inhibitors [MoA], Decreased Immunologic Activity [PE], Kinase Inhibitor [EPC], Protein Kinase Inhibitors [MoA], mTOR Inhibitor Immunosuppressant [EPC], mTOR Inhibitors [MoA] |
| DEA Schedule | n/a |
| Marketing Category | ANDA |
| Application Number | ANDA205775 |
| Listing Certified Through | 2024-12-31 |
Package
Package Images




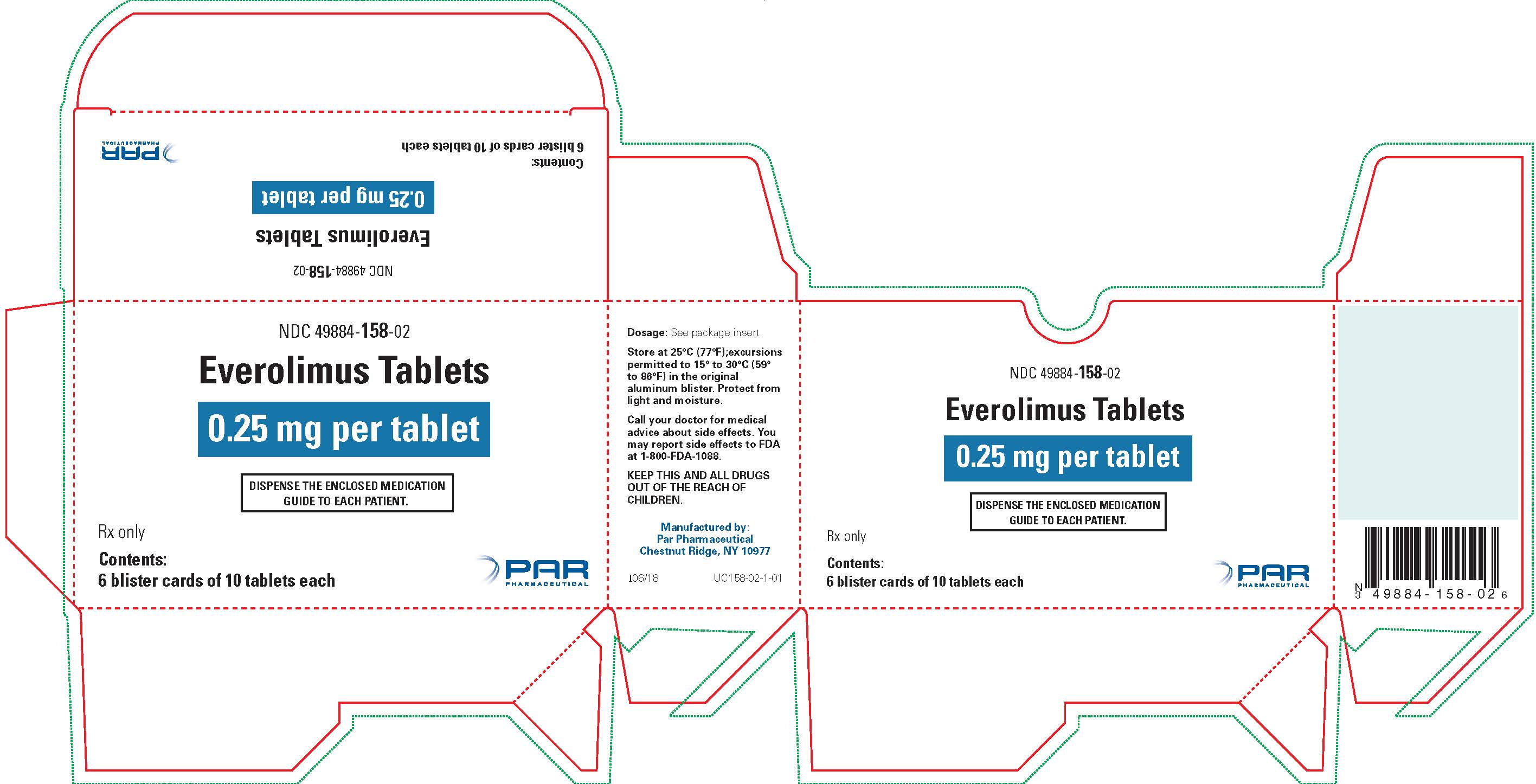



NDC 49884-0158-02 (49884015802)
| NDC Package Code | 49884-158-02 |
|---|---|
| Billing NDC | 49884015802 |
| Package | 6 BLISTER PACK in 1 CARTON (49884-158-02) / 10 TABLET in 1 BLISTER PACK (49884-158-52) |
| Marketing Start Date | 2022-03-31 |
| NDC Exclude Flag | N |
| Pricing Information | N/A |
Standard Product Labeling (SPL)/Prescribing Information SPL 59cb86d5-8706-4619-b074-af253d3e0b68 Details
HIGHLIGHTS OF PRESCRIBING INFORMATION
EVEROLIMUS tablets, for oral use
Initial U.S. Approval: 2009
WARNING: MALIGNANCIES AND SERIOUS INFECTIONS, KIDNEY GRAFT THROMBOSIS; NEPHROTOXICITY; AND MORTALITY IN HEART TRANSPLANTATION
See Full Prescribing Information for Complete Boxed Warning
- Only physicians experienced in immunosuppressive therapy and management of transplant patients should use Everolimus. (5.1)
- Increased susceptibility to infection and the possible development of malignancies may result from immunosuppression. (5.2, 5.3)
- Increased incidence of kidney graft thrombosis. (5.4)
- Reduced doses of cyclosporine are required for use in combination with everolimus in order to reduce nephrotoxicity. (2.4, 2.5, 5.6, 12.7, 12.8)
- Increased mortality in a heart transplant clinical trial. Use in heart transplantation is not recommended. (5.7)
INDICATIONS AND USAGE
- Everolimus is a mTOR inhibitor immunosuppressant indicated for the prophylaxis of organ rejection in adult patients:
- Kidney transplant: at low-moderate immunologic risk. Use in combination with basiliximab, cyclosporine (reduced doses) and corticosteroids. (1.1)
- Liver transplant: Administer no earlier than 30 days post-transplant. Use in combination with tacrolimus (reduced doses) and corticosteroids. (1.2, 5.5)
Limitations of Use (1.3)
Safety and efficacy has not been established in the following:
DOSAGE AND ADMINISTRATION
- Kidney transplantation: starting oral dose of 0.75 mg twice daily as soon as possible after transplantation. (2.1)
- Liver transplantation: starting oral dose of 1 mg twice daily starting 30 days after transplantation. (2.2)
- Monitor everolimus concentrations: Adjust maintenance dose to achieve trough concentrations within the 3-8 ng/mL target range using LC/MS/MS assay method (2.1, 2.2, 2.3)
- Administer consistently with or without food at the same time as cyclosporine or tacrolimus. (2.6, 12.3)
- Mild hepatic impairment: Reduce initial daily dose by one-third (2.7)
- Moderate or Severe hepatic impairment: Reduce initial daily dose by one- half. (2.7, 12.6)
DOSAGE FORMS AND STRENGTHS
Everolimus Tablets is available as 0.25 mg, 0.5 mg, 0.75 mg and 1 mg tablets. (3)
CONTRAINDICATIONS
- Hypersensitivity to everolimus, sirolimus, or to components of the drug product. (4)
WARNINGS AND PRECAUTIONS
- Angioedema [increased risk with concomitant angiotensin converting enzyme (ACE inhibitors)]: Monitor for symptoms and treat promptly. (5.8)
- Delayed Wound Healing/Fluid Accumulation: Monitor symptoms; treat promptly to minimize complications. (5.9)
- Interstitial Lung Disease/Non-Infectious Pneumonitis: Monitor for symptoms or radiologic changes; manage by dose reduction or discontinuation until symptoms resolve; consider use of corticosteroids. (5.10)
- Hyperlipidemia (elevations of serum cholesterol and triglycerides): Monitor and consider anti-lipid therapy. (5.11)
- Proteinuria (increased risk with higher trough concentrations): Monitor urine protein. (5.12)
- Polyoma Virus Infections (activation of latent viral infections; BK-virus associated nephropathy): Consider reducing immunosuppression. (5.13).
- TMA/TTP/HUS (concomitant use with cyclosporine may increase risk): Monitor for hematological changes or symptoms. (5.15)
- New Onset Diabetes After Transplantation: Monitor serum glucose. (5.16)
- Male Infertility: Azospermia or oligospermia may occur. (5.18, 13.1)
- Immunizations: Avoid live vaccines. (5.19)
- Embryo-Fetal Toxicity: Advise females of reproductive potential of the potential risk to a fetus and to use effective contraception during treatment with everolimus and for 8 weeks after final dose (5.17, 8.1, 8.3)
ADVERSE REACTIONS
Most common adverse reactions were as follows:
Kidney transplantation (incidence greater than or equal to 20%): peripheral edema, constipation, hypertension, nausea, anemia, UTI, and hyperlipidemia. (6.1);
Liver transplantation (incidence greater than 10%): diarrhea, headache, peripheral edema, hypertension, nausea, pyrexia, abdominal pain, leukopenia and hypercholesterolemia (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Par Pharmaceutical at 1-800-828-9393 or www.parpharm.com or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS
USE IN SPECIFIC POPULATIONS
See 17 for PATIENT COUNSELING INFORMATION and Medication Guide.
Revised: 9/2021
Table Of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
WARNING: MALIGNANCIES AND SERIOUS INFECTIONS, KIDNEY GRAFT THROMBOSIS; NEPHROTOXICITY; AND MORTALITY IN HEART TRANSPLANTATION
1 INDICATIONS AND USAGE
1.1 Prophylaxis of Organ Rejection in Kidney Transplantation
1.2 Prophylaxis of Organ Rejection in Liver Transplantation
1.3 Limitations of Use
2 DOSAGE AND ADMINISTRATION
2.1 Dosage in Adult Kidney Transplant Patients
2.2 Dosage in Adult Liver Transplant Patients
2.3 Therapeutic Drug Monitoring (TDM) - Everolimus
2.4 Therapeutic Drug Monitoring (TDM) - Cyclosporine in Kidney Transplant Patients
2.5 Therapeutic Drug Monitoring (TDM) - Tacrolimus in Liver Transplant Patients
2.6 Administration
2.7 Hepatic Impairment
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
4.1 Hypersensitivity Reactions
5 WARNINGS AND PRECAUTIONS
5.1 Management of Immunosuppression
5.2 Lymphomas and Other Malignancies
5.3 Serious Infections
5.4 Kidney Graft Thrombosis
5.5 Hepatic Artery Thrombosis
5.6 Everolimus and Calcineurin Inhibitor-Induced Nephrotoxicity
5.7 Heart Transplantation
5.8 Angioedema
5.9 Wound Healing and Fluid Accumulation
5.10 Interstitial Lung Disease (ILD)/Non-Infectious Pneumonitis
5.11 Hyperlipidemia
5.12 Proteinuria
5.13 Polyoma Virus Infections
5.14 Interaction with Strong Inhibitors and Inducers of CYP3A4
5.15 Thrombotic Microangiopathy/Thrombotic Thrombocytopenic Purpura/Hemolytic Uremic Syndrome (TMA/TTP/HUS)
5.16 New Onset Diabetes after Transplant
5.17 Embryo-Fetal Toxicity
5.18 Male Infertility
5.19 Immunizations
5.20 Interaction with Grapefruit Juice
5.21 Patients with Hereditary Disorders/Other
6 ADVERSE REACTIONS
6.1 Serious and Otherwise Important Adverse Reactions
6.2 Clinical Trials Experience
6.3 Post Marketing Experience
7 DRUG INTERACTIONS
7.1 Interactions with Strong Inhibitors or Inducers of CYP3A4 and P-glycoprotein
7.2 Cyclosporine (CYP3A4/P-gp Inhibitor and CYP3A4 Substrate)
7.3 Ketoconazole and Other Strong CYP3A4 Inhibitors
7.4 Erythromycin (Moderate CYP3A4 Inhibitor)
7.5 Verapamil (CYP3A4 and P-gp Substrate)
7.6 Atorvastatin (CYP3A4 substrate) and Pravastatin (P-gp substrate)
7.7 Simvastatin and Lovastatin
7.8 Rifampin (Strong CYP3A4/P-gp Inducers)
7.9 Midazolam (CYP3A4/5 substrate)
7.10 Other Possible Interactions
7.11 Octreotide
7.12 Tacrolimus
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.3 Females and Males of Reproductive Potential
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Hepatic Impairment
8.7 Renal Impairment
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.3 Pharmacokinetics
12.5 Drug-Drug Interactions
12.6 Specific Populations
12.7 Everolimus Whole Blood Concentrations Observed in Kidney and in Liver Transplant Patients
12.8 Cyclosporine Concentrations Observed in Kidney Transplant Patients
12.9 Tacrolimus Concentrations in Liver Transplant
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis and Mutagenesis and Impairment of Fertility
13.2 Animal Toxicology and/or Pharmacology
14 CLINICAL STUDIES
14.1 Prevention of Organ Rejection after Kidney Transplantation
14.2 Prevention of Organ Rejection after Liver Transplantation
16 HOW SUPPLIED
17 PATIENT COUNSELING INFORMATION
- *
- Sections or subsections omitted from the full prescribing information are not listed.
WARNING
Malignancies and Serious Infections
- Only physicians experienced in immunosuppressive therapy and management of transplant patients should prescribe everolimus tablets. Patients receiving the drug should be managed in facilities equipped and staffed with adequate laboratory and supportive medical resources. The physician responsible for maintenance therapy should have complete information requisite for the follow-up of the patient. [See Warnings and Precautions (5.1)]
- Increased susceptibility to infection and the possible development of malignancies such as lymphoma and skin cancer may result from immunosuppression. [See Warnings and Precautions (5.2 and 5.3)]
Kidney Graft Thrombosis
- An increased risk of kidney arterial and venous thrombosis, resulting in graft loss, was reported, mostly within the first 30 days post-transplantation [see Warnings and Precautions (5.4)]
Nephrotoxicity
- Increased nephrotoxicity can occur with use of standard doses of cyclosporine in combination with evrolimus. Therefore reduced doses of cyclosporine should be used in combination with everolimus in order to reduce renal dysfunction. It is important to monitor the cyclosporine and everolimus whole blood trough concentrations[see Dosage and Administration (2.4, 2.5),Warnings and Precautions (5.6), Clinical Pharmacology (12.7, 12.8)]
Mortality in Heart Transplantation
- Increased mortality, often associated with serious infections, within the first three months post-transplantation was observed in a clinical trial of de novo heart transplant patients receiving immunosuppressive regimens with or without induction therapy. Use in heart transplantation is not recommended. [See Warnings and Precautions (5.7)]
1 INDICATIONS AND USAGE
1.1 Prophylaxis of Organ Rejection in Kidney Transplantation
Everolimus tablets are indicated for the prophylaxis of organ rejection in adult patients at low-moderate immunologic risk receiving a kidney transplant [see Clinical Studies (14.1)] Everolimus is to be administered in combination with basiliximab induction and concurrently with reduced doses of cyclosporine and with corticosteroids. TDM of everolimus and cyclosporine is recommended for all patients receiving these products. [see Dosage and Administration (2.2, 2.3)]
1.2 Prophylaxis of Organ Rejection in Liver Transplantation
Everolimus is indicated for the prophylaxis of allograft rejection in adult patients receiving a liver transplant. Everolimus is to be administered no earlier than 30 days post-transplant concurrently in combination with reduced doses of tacrolimus and with corticosteroids [See Warnings and Precautions (5.5) and Clinical Studies (14.2)]. Therapeutic drug monitoring (TDM) of everolimus and tacrolimus is recommended for all patients receiving these products. [See Dosage and Administration (2.3, 2.5)]
1.3 Limitations of Use
The safety and efficacy of everolimus has not been established in the following populations:
- Kidney transplant patients at high immunologic risk
- Recipients of transplanted organs other than kidney or liver [See Warnings and Precautions (5.7)]
- Pediatric patients (less than 18 years).
2 DOSAGE AND ADMINISTRATION
Patients receiving everolimus may require dose adjustments based on everolimus blood concentrations achieved, tolerability, individual response, change in concomitant medications and the clinical situation. Optimally, dose adjustments of everolimus should be based on trough concentrations obtained 4 or 5 days after a previous dosing change. Dose adjustment is required if the trough concentration is below 3ng/mL. The total daily dose of everolimus should be doubled using the available tablet strengths (0.25 mg, 0.5 mg, 0.75 mg or 1 mg). Dose adjustment is also required if the trough concentration is greater than 8 ng/mL on 2 consecutive measures; the dose of everolimus should be decreased by 0.25 mg twice daily [See Dosage and Administration (2.3), Clinical Pharmacology (12.3)]
2.1 Dosage in Adult Kidney Transplant Patients
An initial everolimus dose of 0.75 mg orally twice daily (1.5 mg per day) is recommended for adult kidney transplant patients in combination with reduced dose cyclosporine, administered as soon as possible after transplantation. [see Dosage and Administration (2.3, 2.4), Clinical Studies (14.1)].
Oral prednisone should be initiated once oral medication is tolerated. Steroid doses may be further tapered on an individualized basis depending on the clinical status of patient and function of graft.
2.2 Dosage in Adult Liver Transplant Patients
Start everolimus at least 30 days post-transplant. An initial dose of 1 mg orally twice daily (2 mg per day) is recommended for adult liver transplant patients in combination with reduced dose tacrolimus. [See Dosage and Administration (2.3, 2.5), Clinical Studies (14.2)]
Steroid doses may be further tapered on an individualized basis depending on the clinical status of patient and function of graft.
2.3 Therapeutic Drug Monitoring (TDM) - Everolimus
Routine everolimus whole blood therapeutic drug concentration monitoring is recommended for all patients. The recommended everolimus therapeutic range is 3 to 8 ng/mL. [See Clinical Pharmacology (12.7)] Careful attention should be made to clinical signs and symptoms, tissue biopsies, and laboratory parameters. It is important to monitor everolimus blood concentrations, in patients with hepatic impairment, during concomitant administration of CYP3A4 inducers or inhibitors, when switching cyclosporine formulations and/or when cyclosporine dosing is reduced according to recommended target concentrations [see Clinical Pharmacology (12.7, 12.8)].
There is an interaction of cyclosporine on everolimus, and consequently, everolimus concentrations may decrease if cyclosporine exposure is reduced. There is little to no pharmacokinetic interaction of tacrolimus on everolimus, and thus, everolimus concentrations do not decrease if the tacrolimus exposure is reduced. [See Drug Interactions (7.12)]
The everolimus recommended therapeutic range of 3 to 8 ng/mL is based on an LC/MS/MS assay method. Currently in clinical practice, everolimus whole blood trough concentrations may be measured by chromatographic or immunoassay methodologies. Because the measured everolimus whole blood trough concentrations depend on the assay used, individual patient sample concentration values from different assays may not be interchangeable. Consideration of assay results must be made with knowledge of the specific assay used. Therefore, communication should be maintained with the laboratory performing the assay.
2.4 Therapeutic Drug Monitoring (TDM) - Cyclosporine in Kidney Transplant Patients
Both cyclosporine doses and the target range for whole blood trough concentrations should be reduced, when given in a regimen with everolimus, in order to minimize the risk of nephrotoxicity [see Warnings and Precautions (5.6), Drug Interactions (7.2), Clinical Pharmacology (12.8)].
The recommended cyclosporine therapeutic ranges when administered with everolimus are 100 to 200 ng/mL through Month 1 post-transplant, 75 to 150 ng/mL at Months 2 and 3 post-transplant, 50 to 100 ng/mL at Month 4 post-transplant, and 25 to 50 ng/mL from Month 6 through Month 12 post-transplant. The median trough concentrations observed in the clinical trial ranged between 161 to 185 ng/mL through Month 1 post-transplant and between 111 to 140 ng/mL at Months 2 and 3 post-transplant. The median trough concentration was 99 ng/mL at Month 4 post-transplant and ranged between 46 to 75 ng/mL from Months 6 through Month 12 post-transplant [see Clinical Pharmacology (12.8), Clinical Studies (14.1)].
Cyclosporine, USP Modified is to be administered as oral capsules twice daily unless cyclosporine oral solution or intravenous administration of cyclosporine cannot be avoided. Cyclosporine, USP Modified should be initiated as soon as possible - and no later than 48 hours - after reperfusion of the graft and dose adjusted to target concentrations from Day 5 onwards.
If impairment of renal function is progressive the treatment regimen should be adjusted. In renal transplant patients, the cyclosporine dose should be based on cyclosporine whole blood trough concentrations [see Clinical Pharmacology (12.8)].
In renal transplantation, there are limited data regarding dosing everolimus with reduced cyclosporine trough concentrations of 25 to 50 ng/mL after 12 months. Everolimus has not been evaluated in clinical trials with other formulations of cyclosporine. Prior to dose reduction of cyclosporine it should be ascertained that steady-state everolimus whole blood trough concentration is at least 3 ng/mL. There is an interaction of cyclosporine on everolimus, and consequently, everolimus concentrations may decrease if cyclosporine exposure is reduced [see Drug Interactions (7.2)].
2.5 Therapeutic Drug Monitoring (TDM) - Tacrolimus in Liver Transplant Patients
Both tacrolimus doses and the target range for whole blood trough concentrations should be reduced, when given in a regimen with everolimus, in order to minimize the potential risk of nephrotoxicity. [See Warnings and Precautions (5.6), Clinical Pharmacology (12.9)]
The recommended tacrolimus therapeutic range when administered with everolimus are whole blood trough (C-0h) concentrations of 3 to 5 ng/mL by three weeks after the first dose of everolimus (approximately Month 2) and through Month 12 post-transplant.
The median tacrolimus trough concentrations observed in the clinical trial ranged between 8.6 to 9.5 ng/mL at Weeks 2 and 4 post-transplant (prior to initiation of everolimus). The median tacrolimus trough concentrations ranged between 7 to 8.1 ng/mL at Weeks 5 and 6 post-transplant, between 5.2 to 5.6 ng/mL at Months 2 and 3 post-transplant, and between 4.3 to 4.9 ng/mL between Months 4 and 12 post-transplant. [See Clinical Pharmacology (12.9), Clinical Studies (14.2)]
Tacrolimus is to be administered as oral capsules twice daily unless intravenous administration of tacrolimus cannot be avoided.
In liver transplant patients, the tacrolimus dose should be based on tacrolimus whole blood trough concentrations. [SeeClinical Pharmacology (12.9)]
In liver transplantation, there are limited data regarding dosing everolimus with reduced tacrolimus trough concentrations of 3 to 5 ng/mL after 12 months. Prior to dose reduction of tacrolimus it should be ascertained that the steady-state everolimus whole blood trough concentration is at least 3 ng/mL. Unlike the interaction between cyclosporine and everolimus, tacrolimus does not affect everolimus trough concentrations, and consequently, everolimus concentrations do not decrease if the tacrolimus exposure is reduced.
2.6 Administration
Everolimus tablets should be swallowed whole with a glass of water and not crushed before use.
Administer everolimus consistently approximately 12 hours apart with or without food to minimize variability in absorption and at the same time as cyclosporine or tacrolimus. [See Clinical Pharmacology (12.3)]
2.7 Hepatic Impairment
Whole blood trough concentrations of everolimus should be closely monitored in patients with impaired hepatic function. For patients with mild hepatic impairment (Child-Pugh Class A), the initial daily dose should be reduced by approximately one-third of the normally recommended daily dose. For patients with moderate or severe hepatic impairment (Child-Pugh B or C), the initial daily dose should be reduced to approximately one-half of the normally recommended daily dose. Further dose adjustment and/or dose titration should be made if a patient’s whole blood trough concentration of everolimus, as measured by an LC/MS/MS assay, is not within the target trough concentration range of 3 to 8 ng/mL. [See Clinical Pharmacology (12.6)]
3 DOSAGE FORMS AND STRENGTHS
Everolimus tablets are available as 0.25 mg, 0.5 mg, 0.75 mg and 1 mg tablets.
Table 1. Description of Everolimus Tablets
| Dosage Strength | 0.25 mg | 0.5 mg | 0.75 mg | 1mg |
| Appearance | White to off white, round flat faced bevel edge tablets | |||
| Imprint |
“P” on one side and “158” on the other |
“P” on one side and “159” on the other |
“P” on one side and “160” on the other | “P” on one side and “283” on the other |
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Management of Immunosuppression
Only physicians experienced in management of systemic immunosuppressant therapy in transplantation should prescribe everolimus. Patients receiving the drug should be managed in facilities equipped and staffed with adequate laboratory and supportive medical resources. The physician responsible for the maintenance therapy should have complete information requisite for the follow-up of the patient. In limited data with the complete elimination of CNI (calcineurin inhibition), there was an increased risk of acute rejection.
5.2 Lymphomas and Other Malignancies
Patients receiving immunosuppressants, including everolimus, are at increased risk of developing lymphomas and other malignancies, particularly of the skin. The risk appears to be related to the intensity and duration of immunosuppression rather than to the use of any specific agent.
As usual for patients with increased risk for skin cancer, exposure to sunlight and ultraviolet light should be limited by wearing protective clothing and using a sunscreen with a high protection factor.
5.3 Serious Infections
Patients receiving immunosuppressants, including everolimus, are at increased risk of developing bacterial, viral, fungal, and protozoal infections, including opportunistic infections. [See Warnings and Precautions (5.13), Adverse Reactions (6.1, 6.2)] These infections may lead to serious, including fatal, outcomes. Because of the danger of over immunosuppression, which can cause increased susceptibility to infection, combination immunosuppressant therapy should be used with caution.
Antimicrobial prophylaxis for Pneumocystis jiroveci (carinii) pneumonia and prophylaxis for cytomegalovirus (CMV) is recommended in transplant recipients.
5.4 Kidney Graft Thrombosis
An increased risk of kidney arterial and venous thrombosis, resulting in graft loss, has been reported, usually within the first 30 days post-transplantation [see Boxed Warning].
5.5 Hepatic Artery Thrombosis
Mammalian target of rapamycin (mTOR) inhibitors are associated with an increase in hepatic artery thrombosis (HAT). Reported cases mostly have occurred within the first 30 days post-transplant and most also lead to graft loss or death. Therefore, everolimus should not be administered earlier than 30 days after liver transplant.
5.6 Everolimus and Calcineurin Inhibitor-Induced Nephrotoxicity
In kidney transplant recipients, Everolimus with standard dose cyclosporine increases the risk of nephrotoxicity resulting in a lower glomerular filtration rate. Reduced doses of cyclosporine are required for use in combination with everolimus in order to reduce renal dysfunction [see Boxed Warning, Indications and Usage (1.1), Clinical Pharmacology (12.8)].
In liver transplant recipients, everolimus has not been studied with standard dose tacrolimus. Reduced doses of tacrolimus should be used in combination with everolimus in order to minimize the potential risk of nephrotoxicity. [See Indications and Usage (1.2), Clinical Pharmacology (12.9)].
Renal function should be monitored during the administration of everolimus tablets. Consider switching to other immunosuppressive therapies if renal function does not improve after dose adjustments or if the dysfunction is thought to be drug related. Caution should be exercised when using other drugs which are known to impair renal function.
5.7 Heart Transplantation
In a clinical trial of de novo heart transplant patients, everolimus in an immunosuppressive regimen with or without induction therapy, resulted in an increased mortality often associated with serious infections within the first three months post- transplantation compared to the control regimen. Use of everolimus in heart transplantation is not recommended.
5.8 Angioedema
Everolimus has been associated with the development of angioedema. The concomitant use of everolimus with other drugs known to cause angioedema, such as angiotensin converting enzyme (ACE) inhibitors may increase the risk of developing angioedema.
5.9 Wound Healing and Fluid Accumulation
Everolimus increases the risk of delayed wound healing and increases the occurrence of wound-related complications like wound dehiscence, wound infection, incisional hernia, lymphocele and seroma. These wound-related complications may require more surgical intervention. Generalized fluid accumulation, including peripheral edema (e.g., lymphoedema) and other types of localized fluid collection, such as pericardial and pleural effusions and ascites have also been reported.
5.10 Interstitial Lung Disease (ILD)/Non-Infectious Pneumonitis
A diagnosis of interstitial lung disease (ILD) should be considered in patients presenting with symptoms consistent with infectious pneumonia but not responding to antibiotic therapy and in whom infectious, neoplastic and other non-drug causes have been ruled-out through appropriate investigations. Cases of ILD, implying lung intraparenchymal inflammation (pneumonitis) and/or fibrosis of non-infectious etiology, some reported with pulmonary hypertension (including pulmonary arterial hypertension (PAH)) as a secondary event, have occurred in patients receiving rapamycins and their derivatives, including everolimus. Most cases generally resolve on drug interruption with or without glucocorticoid therapy. However, fatal cases have also occurred.
5.11 Hyperlipidemia
Increased serum cholesterol and triglycerides, requiring the need for anti-lipid therapy, have been reported to occur following initiation of everolimus and the risk of hyperlipidemia is increased with higher everolimus whole blood trough concentrations. [See Adverse Reactions (6.2)] Use of anti-lipid therapy may not normalize lipid levels in patients receiving everolimus.
Any patient who is administered everolimus should be monitored for hyperlipidemia. If detected, interventions, such as diet, exercise, and lipid-lowering agents should be initiated as outlined by the National Cholesterol Education Program guidelines. The risk/benefit should be considered in patients with established hyperlipidemia before initiating an immunosuppressive regimen containing everolimus. Similarly, the risk/benefit of continued everolimus therapy should be re evaluated in patients with severe refractory hyperlipidemia. Everolimus has not been studied in patients with baseline cholesterol levels greater than 350 mg/dL.
Due to an interaction with cyclosporine, clinical trials of everolimus and cyclosporine in kidney transplant patients strongly discouraged patients from receiving the HMG-CoA reductase inhibitors simvastatin and lovastatin. During everolimus therapy with cyclosporine, patients administered an HMG-CoA reductase inhibitor and/or fibrate should be monitored for the possible development of rhabdomyolysis and other adverse effects, as described in the respective labeling for these agents [see Drug Interactions (7.7)].
5.12 Proteinuria
The use of everolimus in transplant patients has been associated with increased proteinuria. The risk of proteinuria increased with higher everolimus whole blood trough concentrations. Patients receiving everolimus should be monitored for proteinuria. [See Adverse Reactions (6.2)]
5.13 Polyoma Virus Infections
Patients receiving immunosuppressants, including Everolimus, are at increased risk for opportunistic infections; including polyoma virus infections. Polyoma virus infections in transplant patients may have serious, and sometimes fatal, outcomes. These include polyoma virus-associated nephropathy (PVAN), mostly due to BK virus infection, and JC virus associated progressive multiple leukoencephalopathy (PML). PVAN has been observed in patients receiving immunosuppressants, including Everolimus. PVAN is associated with serious outcomes; including deteriorating renal function and kidney graft loss. [See Adverse Reactions (6.2)]. Patient monitoring may help detect patients at risk for PVAN. Reductions in immunosuppression should be considered for patients who develop evidence of PVAN or PML. Physicians should also consider the risk that reduced innumosuppression represents to the functioning allograft.
5.14 Interaction with Strong Inhibitors and Inducers of CYP3A4
Coadministration of everolimus with strong CYP3A4-inhibitors (e.g., ketoconazole, itraconazole, voriconazole, clarithromycin, telithromycin, ritonavir, boceprevir, telaprevir) and strong CYP3A4 inducers (e.g., rifampin, rifabutin) is not recommended without close monitoring of everolimus whole blood trough concentrations. [See Drug Interactions (7)]
5.15 Thrombotic Microangiopathy/Thrombotic Thrombocytopenic Purpura/Hemolytic Uremic Syndrome (TMA/TTP/HUS)
The concomitant use of everolimus with cyclosporine may increase the risk of thrombotic microangiopathy/thrombotic thrombocytopenic purpura/hemolytic uremic syndrome. Monitor hematologic parameters [see Adverse Reactions (6.2)].
5.16 New Onset Diabetes after Transplant
Everolimus has been shown to increase the risk of new onset diabetes mellitus after transplant. Blood glucose concentrations should be monitored closely in patients using everolimus.
5.17 Embryo-Fetal Toxicity
Based on animal studies and the mechanism of action [see Clinical Pharmacology (12.1)], Everolimus may cause fetal harm when administered to a pregnant woman. In animal studies, eveolimus caused embryo-fetal toxicity when administered during the period of organogenesis at maternal exposures that were equal to or less than human exposures at the recommended lowest starting dose. Advise pregnant women of the potential risk to a fetus. Advise female patients of reproductive potential to avoid becoming pregnant and to use effective contraception while using everolimus and for 8 weeks after ending treatment. [see Use in Specific Populations (8.1, 8.3)]
5.18 Male Infertility
Azospermia or oligospermia may be observed. [See Adverse Reactions (6.2), Nonclinical Toxicology (13.1)] Everolimus is an anti-proliferative drug and affects rapidly dividing cells like the germ cells.
5.19 Immunizations
The use of live vaccines should be avoided during treatment with everolimus; examples include (not limited to) the following: intranasal influenza, measles, mumps, rubella, oral polio, BCG, yellow fever, varicella, and TY21a typhoid vaccines.
6 ADVERSE REACTIONS
6.1 Serious and Otherwise Important Adverse Reactions
The following adverse reactions are discussed in greater detail in other sections of the label.
- Hypersensitivity reactions [See Contraindications (4.1)]
- Lymphomas and Other Malignancies [See Boxed Warning, Warnings and Precautions (5.2)]
- Serious Infections [See Warnings and Precautions (5.3)]
- Kidney Graft Thrombosis [see Warnings and Precautions (5.4)]
- Hepatic Artery Thrombosis [See Warnings and Precautions (5.5)]
- Everolimus and Calcineurin Inhibitor-Induced Nephrotoxicity [See Warnings and Precautions (5.6)]
- Heart Transplantation [See Warnings and Precautions (5.7)]
- Angioedema [See Warnings and Precautions (5.8)]
- Wound Healing and Fluid Accumulation [See Warnings and Precautions (5.9)]
- Interstitial Lung Disease/Non-Infectious Pneumonitis [See Warnings and Precautions (5.10)]
- Hyperlipidemia [See Warnings and Precautions (5.11)]
- Proteinuria [See Warnings and Precautions (5.12)]
- Polyoma Virus Infections [See Warnings and Precautions (5.13)]
- Thrombotic Microangiopathy/Thrombotic Thrombocytopenic Purpura/Hemolytic Uremic Syndrome (TMA/TTP/HUS) [See Warnings and Precautions (5.15)]
- New Onset Diabetes After Transplant [See Warnings and Precautions (5.16)]
- Male Infertility [See Warnings and Precautions (5.18)]
6.2 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, the adverse reaction rates observed cannot be directly compared to rates in other trials and may not reflect the rates observed in clinical practice.
Kidney transplantation
The data described below reflect exposure to everolimus in an open-label, randomized trial of de novo kidney transplant patients of concentration-controlled everolimus at an initial everolimus starting dose of 1.5 mg per day [target trough concentrations 3 to 8 ng/mL with reduced exposure cyclosporine (N=274) compared to mycophenolic acid (N=273) with standard exposure cyclosporine]. All patients received basiliximab induction therapy and corticosteroids. The population was between 18 and 70 years, more than 43% were 50 years of age or older (mean age was 46 years in the everolimus group, 47 years control group); a majority of recipients were male (64% in the everolimus group, 69% control group); and a majority of patients were Caucasian (70% in the everolimus group, 69% control group). Demographic characteristics were comparable between treatment groups. The most frequent diseases leading to transplantation were balanced between groups and included hypertension/nephrosclerosis, glomerulonephritis/glomerular disease and diabetes mellitus. Significantly more patients discontinued everolimus 1.5 mg per day treatment (83/277, 30%) than discontinued the control regimen (60/277, 22%). Of those patients who prematurely discontinued treatment, most discontinuations were due to adverse reactions: 18% in the everolimus group compared to 9% in the control group (p-value = 0.004). This difference was more prominent between treatment groups among female patients. In those patients discontinuing study medication, adverse reactions were collected up to 7 days after study medication discontinuation and serious adverse reactions up to 30 days after study medication discontinuation.
Discontinuation of everolimus at a higher dose (3 mg per day) was 95/279, 34%, including 20% due to adverse reactions, and this regimen is not recommended (see below).
The overall incidences of serious adverse reactions were 57% (159/278) in the everolimus group and 52% (141/273) in the mycophenolic acid group. Infections and infestations reported as serious adverse reactions had the highest incidence in both groups [20% (54/274) in the everolimus group and 25% (69/273) in the control group]. The difference was mainly due to the higher incidence of viral infections in the mycophenolic acid group, mainly CMV and BK virus infections. Injury, poisoning and procedural complications reported as serious adverse reactions had the second highest incidence in both groups [14% (39/274) in the everolimus group and 12% (32/273) in the control group] followed by renal and urinary disorders [10% (28/274) in the everolimus group and 13% (36/273) in the control group] and vascular disorders [10% (26/274) in the everolimus group and 7% (20/273) in the control group].
A total of 13 patients died during the first 12 months of study; 7 (3%) in the everolimus group and 6 (2%) in the control group. The most common causes of death across the study groups were related to cardiac conditions and infections.
There were 12 (4%) graft losses in the everolimus group and 8 (3%) in the control group over the 12 month study period. Of the graft losses, 4 were due to renal artery and two due to renal vein thrombosis in the everolimus group (2%) compared to two renal artery thromboses in the control group (1%) [see Boxed Warning and Warnings and Precautions (5.4)].
The most common (greater than or equal to 20%) adverse reactions observed in the everolimus group were: peripheral edema, constipation, hypertension, nausea, anemia, urinary tract infection, and hyperlipidemia.
Infections
The overall incidence of bacterial, fungal and viral infections reported as adverse reactions was higher in the control group (68%) compared to the everolimus group (64%) and was primarily due to an increased number of viral infections (21% in the control group and 10% in the everolimus group). The incidence of CMV infections reported as adverse reactions was 8% in the control group compared to 1% in the everolimus group; and 3% of the serious CMV infections in the control group versus 0% in the everolimus group were considered serious [see Warnings and Precautions (5.3)].
BK Virus
BK virus infections were lower in incidence in the everolimus group (2 patients, 1%) compared to the control group (11 patients, 4%). One of the two BK virus infections in the everolimus group, and two of the 11 BK virus infections in the control group were also reported as serious adverse reactions. BK virus infections did not result in graft loss in any of the groups in the clinical trial.
Wound Healing and Fluid Collections
Wound healing-related reactions were identified through a retrospective search and request for additional data. The overall incidence of wound-related reactions, including lymphocele, seroma, hematoma, dehiscence, incisional hernia, and infections was 35% in the everolimus group compared to 26% in the control group. More patients required intraoperative repair debridement or drainage of incisional wound complications and more required drainage of lymphoceles and seromas in the everolimus group compared to control.
Adverse reactions due to major fluid collections such as edema and other types of fluid collections was 45% in the everolimus group and 40% in the control group [see Warnings and Precautions (5.9)].
Neoplasms
Adverse reactions due to malignant and benign neoplasms were reported in 3% of patients in the everolimus group and 6% in the control group. The most frequently reported neoplasms in the control group were basal cell carcinoma, squamous cell carcinoma, skin papilloma and seborrheic keratosis. One patient in the everolimus group who underwent a melanoma excision prior to transplantation died due to metastatic melanoma [see Boxed Warning and Warnings and Precautions (5.2)].
New Onset Diabetes Mellitus (NODM)
NODM reported based on adverse reactions and random serum glucose values, was 9% in the everolimus group compared to 7% in the control group.
Endocrine Effects in Males
In the everolimus group, serum testosterone levels significantly decreased while the FSH levels significantly increased without significant changes being observed in the control group. In both the everolimus and the control groups mean testosterone and FSH levels remained within the normal range with the mean FSH level in the everolimus group being at the upper limit of the normal range (11.1 U/L). More patients were reported with erectile dysfunction in the everolimus treatment group compared to the control group (5% compared to 2%, respectively).
Table 2 compares the incidence of treatment-emergent adverse reactions reported with an incidence of greater than or equal to 10% for patients receiving everolimus with reduced dose cyclosporine or mycophenolic acid with standard dose cyclosporine. Within each MedDRA system organ class, the adverse reactions are presented in order of decreasing frequency.
Table 2. Incidence Rates of Frequent (Greater than or Equal to 10% in Any Treatment Group) Adverse Reactions by Primary System Organ Class and Preferred Term after Kidney Transplantation (Safety Population*)
|
Primary System Organ Class Preferred Term |
Everolimus 1.5 mg With Reduced Exposure Cyclosporine N=274 n (%) |
Mycophenolic acid 1.44 g With Standard Exposure Cyclosporine N=273 n (%) |
|
Any Adverse Reactions* |
271 (99) |
270 (99) |
|
Blood Lymphatic System Disorders |
93 (34) |
111 (41) |
|
Anemia |
70 (26) |
68 (25) |
|
Leukopenia |
8 (3) |
33 (12) |
|
Gastrointestinal Disorders |
196 (72) |
207 (76) |
|
Constipation |
105 (38) |
117 (43) |
|
Nausea |
79 (29) |
85 (31) |
|
Diarrhea |
51 (19) |
54 (20) |
|
Vomiting |
40 (15) |
60 (22) |
|
Abdominal pain |
36 (13) |
42 (15) |
|
Dyspepsia |
12 (4) |
31 (11) |
|
Abdominal pain upper |
9 (3) |
30 (11) |
|
General Disorders and Administrative |
181 (66) |
160 (59) |
|
site conditions | ||
|
Edema Peripheral |
123 (45) |
108 (40) |
|
Pyrexia |
51 (19) |
40 (15) |
|
Fatigue |
25 (9) |
28 (10) |
|
Infections and Infestations |
169 (62) |
185 (68) |
|
Urinary Tract Infection |
60 (22) |
63 (23) |
|
Upper Respiratory Tract Infection |
44 (16) |
49 (18) |
|
Injury, Poisoning and Procedural Complications |
163 (60) |
163 (60) |
|
Incision site pain |
45 (16) |
47 (17) |
|
Procedural pain |
40 (15) |
37 (14) |
|
Investigations |
137 (50) |
133 (49) |
|
Blood Creatinine Increased |
48 (18) |
59 (22) |
|
Metabolism and Nutrition Disorders |
222 (81) |
199 (73) |
|
Hyperlipidemia |
57 (21) |
43 (16) |
|
Hyperkalemia |
49 (18) |
48 (18) |
|
Hypercholesterolemia |
47 (17) |
34 (13) |
|
Dyslipidemia |
41 (15) |
24 (9) |
|
Hypomagnesemia |
37 (14) |
40 (15) |
|
Hypophosphatemia |
35 (13) |
35 (13) |
|
Hyperglycemia |
34 (12) |
38 (14) |
|
Hypokalemia |
32 (12) |
32 (12) |
|
Musculoskeletal and Connective Tissue Disorders |
112 (41) |
105 (39) |
|
Pain in Extremity |
32 (12) |
29 (11) |
|
Back Pain |
30 (11) |
28 (10) |
|
Nervous System Disorders |
92 (34) |
109 (40) |
|
Headache |
49 (18) |
40 (15) |
|
Tremor |
23 (8) |
38 (14) |
|
Psychiatric Disorders |
90 (33) |
72 (26) |
|
Insomnia |
47 (17) |
43 (16) |
|
Renal and Urinary Disorders |
112 (41) |
124 (45) |
|
Hematuria |
33 (12) |
33 (12) |
|
Dysuria |
29 (11) |
28 (10) |
|
Respiratory, Thoracic and Mediastinal Disorders |
86 (31) |
93 (34) |
|
Cough |
20 (7) |
30 (11) |
|
Vascular disorders |
122 (45) |
124 (45) |
|
Hypertension |
81 (30) |
82 (30) |
* The safety analysis population defined as all randomized kidney transplant patients who received at least one dose of treatment and had at least one post-baseline safety assessment.
Adverse reaction that occurred with at least a 5% higher frequency in the everolimus 1.5 mg group compared to the control group were: peripheral edema (45% compared to 40%), hyperlipidemia (21% compared to 16%), dyslipidemia (15% compared to 9%), and stomatitis/mouth ulceration (8% compared to 3%).
A third treatment group of everolimus 3 mg per day (1.5 mg twice daily; target trough concentrations 6 to 12 ng/mL) with reduced exposure cyclosporine was included in the study described above. Although as effective as the lower dose everolimus group, the overall safety was worse and consequently higher doses of everolimus cannot be recommended. Out of 279 patients, 95 (34%) discontinued the study medication with 57 (20%) doing so because of adverse reactions. The most frequent adverse reactions leading to discontinuation of everolimus when used at this higher dose were injury, poisoning and procedural complications (everolimus 1.5 mg: 5%, everolimus 3 mg: 7%, and control: 2%), infections (2%, 6%, and 3%, respectively), renal and urinary disorders (4%, 7%, and 4%, respectively) and gastrointestinal disorders (1%, 3%, and 2%).
The combination of fixed dose everolimus and standard doses of cyclosporine in previous kidney clinical trials resulted in frequent elevations of serum creatinine with higher mean and median serum creatinine values observed than in the current study with reduced exposure cyclosporine. These results indicate that everolimus increases the cyclosporine-induced nephrotoxicity; and therefore should only be used in a concentration-controlled regimen with reduced exposure cyclosporine [see Boxed Warnings, Indications and Usage (1.1), Warnings and Precautions (5.6)].
Liver transplantation
The data described below reflect exposure to everolimus starting 30 days after transplantation in an open-label, randomized trial of liver transplant patients. Seven hundred and nineteen (719) patients who fulfilled the inclusion/exclusion criteria [see Clinical Studies (14.2)] were randomized into one of the three treatment groups of the study. During the first 30 days prior to randomization patients received tacrolimus and corticosteroids, with or without mycophenolate mofetil (about 70 to 80% received MMF). No induction antibody was administered. At randomization, MMF was discontinued and patients were randomized to everolimus initial dose of 1 mg twice per day (2 mg daily) and adjusted to protocol specified target trough concentrations of 3 to 8 ng/mL with reduced exposure tacrolimus [protocol specified target troughs 3 to 5 ng/mL] (N=245) [See Clinical Pharmacology (12.7, 12.9)] or to a control group of standard exposure tacrolimus [protocol specified target troughs 8 to 12 ng/mL up to Month 4 post-transplant, then 6 to 10 ng/mL Month 4 through Month 12 post-transplant] (N=241). A third randomized group was discontinued prematurely [See Clinical Studies (14.2)] and is not described in this section.
The population was between 18 and 70 years, more than 50% were 50 years of age (mean age was 54 years in the everolimus group, 55 years in the tacrolimus control group); 74% were male in both everolimus and control groups, respectively, and a majority were Caucasian (86% everolimus group, 80% control group). Demographic characteristics were comparable between treatment groups. The most frequent diseases leading to transplantation were balanced between groups. The most frequent causes of end-stage liver disease (ESLD) were alcoholic cirrhosis, hepatitis C, and hepatocellular carcinoma and were balanced between groups.
Twenty-seven percent (27%) discontinued study drug in the everolimus group compared with 22% for the tacrolimus control group during the first 12 months of study. The most common reason for discontinuation of study medication was due to adverse reactions (19% and 11%, respectively), including proteinuria, recurrent hepatitis C, and pancytopenia in the everolimus group. At 24 months, the rate of discontinuation of study medication in liver transplant patients was greater for the everolimus group (42%) compared to tacrolimus control group (33%).
The overall incidences of serious adverse reactions were 50% (122/245) in the everolimus group and 43% (104/241) in the control group at 12 months and similar at 24 months (56% and 54% respectively). Infections and infestations were reported as serious adverse reactions with the highest incidence followed by Gastrointestinal disorders and Hepatobiliary disorders.
During the first 12 months of study, 13 deaths were reported in the everolimus group (one patient never took everolimus). In the same 12 month period, 7 deaths were reported in the tacrolimus control group. Deaths occurred in both groups for a variety of reasons and were mostly associated with liver-related issues, infections and sepsis. In the following 12 months of study, four additional deaths were reported in each treatment group.
The most common adverse reactions (reported for greater or equal to 10% patients in any group) in the everolimus group were: diarrhea, headache, peripheral edema, hypertension, nausea, pyrexia, abdominal pain, and leukopenia (see Table 3).
Infections
The overall incidence of infections reported as adverse reactions was 50% for everolimus and 44% in the control group and similar at 24 months (56% and 52% respectively). The types of infections were reported as follows: bacterial 16% vs 12%, viral 17% vs 13%; and fungal infections 2% vs 5% for everolimus and control, respectively. [See Warnings and Precautions (5.3)]
Wound Healing and Fluid Collections
Wound healing complications were reported as adverse reactions for 11% of patients in the everolimus group compared to 8% of patients in the control group up to 24 months. Pleural effusions were reported in 5% in both groups, and ascites in 4% of patients in the everolimus group and 3% in the control arm.
Neoplasms
Malignant and benign neoplasms were reported as adverse reactions in 4% of patients in the everolimus group and 7% in the control group at 12 months. In the everolimus group 3 malignant tumors were reported compared to 9 cases in the control group. For the everolimus group this included lymphoma, lymphoproliferative disorder and a hepatocellular carcinoma, and for the control group included Kaposi’s sarcoma (2), metastatic colorectal cancer, glioblastoma, malignant hepatic neoplasm, pancreatic neuroendocrine tumor, hemophagocytic histiocytosis, and squamous cell carcinomas. At 24 months the rates of malignancies were similar (10% and 11% respectively) [See Boxed Warning and Warnings and Precautions (5.2)]
Lipid abnormalities
Hyperlipidemia adverse reactions (including the preferred terms: hyperlipidemia, hypercholesterolemia, blood cholesterol increased, blood triglycerides increased, hypertriglyceridemia lipids increased, total cholesterol/HDL ratio increased, and dyslipidemia) were reported for 24% everolimus patients, and 10% control patients at 12 months. Results were similar at 24 months (28% and 12%, respectively).
New Onset of Diabetes After Transplant (NODAT)
Of the patients without diabetes mellitus at randomization, NODAT was reported in 32% in the everolimus group compared to 29% in the control group at 12 months and similar at 24 months.
Table 3 compares the incidence of treatment-emergent adverse reactions reported with an incidence of greater than or equal to 10% for patients receiving everolimus with reduced exposure tacrolimus or standard dose tacrolimus from randomization to 24 months. Within each MedDRA system organ class, the adverse reactions are presented in order of decreasing frequency.
Table 3. Incidence Rates of most Frequent (Greater than or Equal to 10% in Any Treatment Group) Adverse Reactions by Primary System Organ Class and Preferred Term and Treatment at 12 Months and 24 Months after Liver Transplantation (Safety population)
|
Preffered System Organ Class Preffered Term |
12 month |
24 month |
||
|
Everolimus with reduced exposure tacrolimus N=245 n (%) |
Tacrolimus standard exposure N=241 n (%) |
Everolimus with reduced exposure tacrolimus N=245 n (%) |
Tacrolimus standard exposure N=242 n (%) |
|
|
Any Adverse Reaction/Infection |
232 (95) |
229 (95) |
236 (96) |
237 (98) |
|
Blood & lymphatic system disorders |
66 (27) |
47(20) |
79 (32) |
58 (24) |
|
29 (12) |
12 (5) |
31 (13) |
12 (5) |
|
Gastrointestinal disorders |
136 (56) |
121 (50) |
148 (60) |
138 (57) |
|
47 (19) |
50 (21) |
59 (24) |
61 (25) |
|
33 (14) |
28 (12) |
36 (15) |
33 (14) |
|
32 (13) |
22 (9) |
37 (15) |
31 (13) |
|
General disorders and administration site conditions |
94 (38) |
85 (35) |
113 (46) |
98 (41) |
|
43 (18) |
26 (11) |
49 (20) |
31 (13) |
|
32 (13) |
25 (10) |
43 (18) |
28 (12) |
|
22 (9) |
26 (11) |
27 (11) |
28 (12) |
|
Infections and infestations |
123 (50) |
105 (44) |
135 (56) |
125 (52) |
|
28 (11) |
19 (8) |
33 (14) |
24 (10) |
|
Investigations |
81 (33) |
78 (32) |
92 (38) |
98 (41) |
|
16 (7) |
24 (10) |
19 (8) |
25 (10) |
|
Metabolism and nutrition disorders |
111 (45) |
92 (38) |
134 (55) |
106 (44) |
|
23 (9) |
6 (3) |
27 (11) |
9 (4) |
|
Nervous system disorders |
89 (36) |
85 (35) |
99 (40) |
101 (42) |
|
47 (19) |
46 (19) |
53 (22) |
54 (22) |
|
23 (9) |
29 (12) |
25 (10) |
37 (15) |
|
14 (6) |
19 (8) |
17 (7) |
24 (10) |
|
Renal and urinary disorder |
49 (20) |
53 (22) |
67 (27) |
73 (30) |
|
13 (5) |
17 (7) |
24 (10) |
37 (15) |
|
Vascular disorders |
56 (23) |
57 (24) |
72 (29) |
68 (28) |
|
42 (17) |
38 (16) |
52 (21) |
44 (18) |
Primary system organ classes are presented alphabetically.
* The safety analysis population is defined as all randomized liver transplant patients who received at least one dose of treatment and had at least one post-baseline safety assessment.
** No de novo hepatitis C cases were reported
Less common adverse reactions, occurring overall in greater than or equal to 1% to less than 10% of either kidney or liver transplant patients treated with
everolimus include:
Blood and Lymphatic System Disorders: anemia, leukocytosis, lymphadenopathy, neutropenia, pancytopenia, thrombocythemia, thrombocytopenia
Cardiac and Vascular Disorders: angina pectoris, atrial fibrillation, cardiac failure congestive, palpitations, tachycardia, hypertension including hypertensive crisis, hypotension, deep vein thrombosis
Endocrine Disorders: Cushingoid, hyperparathyroidism, hypothyroidism
Eye Disorders: cataract, conjunctivitis, vision blurred
Gastrointestinal Disorders: abdominal distention, abdominal hernia, ascites, constipation, dyspepsia, dysphagia, epigastric discomfort, flatulence, gastritis, gastroesophageal reflux disease, gingival hypertrophy, hematemesis, hemorrhoids, ileus, mouth ulceration, peritonitis, stomatitis
General Disorders and Administrative Site Conditions: chest discomfort, chest pain, chills, fatigue, incisional hernia, inguinal hernia, malaise, edema including generalized edema, pain
Hepatobiliary Disorders: hepatic enzyme increased, bile duct stenosis, bilirubin increased, cholangitis, cholestatis, hepatitis (non-infectious)
Infections and Infestations: BK virus infection [See Warnings and Precautions (5.13)], bacteremia, bronchitis, candidiasis, cellulitis, CMV, folliculitis, gastroenteritis, herpes infections, influenza, lower respiratory tract, nasopharyngitis, onychomycosis, oral candidiasis, oral herpes, osteomyelitis, pneumonia, pyelonephritis, sepsis, sinusitis, tinea pedis, upper respiratory tract infection, urethritis, urinary tract infection, wound infection [See Boxed Warning and Warnings and Precautions (5.3)]
Injury Poisoning and Procedural Complications: incision site complications including infections, perinephric collection, seroma, wound dehiscence, incisional hernia, perinephric hematoma, localized intraabdominal fluid collection, impaired healing, lymophocele, lymphorrhea
Investigations: blood alkaline phosphatase increased, blood creatinine increased, blood glucose increased, hemoglobin decreased, white blood cell count decreased, transaminases increased
Metabolism and Nutrition Disorders: blood urea increased, acidosis, anorexia, dehydration, diabetes mellitus [See Warnings and Precautions (5.16)], decreased appetite, fluid retention, gout, hypercalcemia, hypertriglyceridemia, hyperuricemia, hypocalcemia, hypokalemia, hypoglycemia, hypomagnesemia, hyponatremia, iron deficiency, new onset diabetes mellitus, vitamin B12 deficiency
Musculoskeletal and Connective Tissues Disorders: arthralgia, joint swelling, muscle spasms, muscular weakness, musculoskeletal pain, myalgia, osteoarthritis, osteonecrosis, osteopenia, osteoporosis, spondylitis
Nervous System Disorders: dizziness, hemiparesis, hypoesthesia, lethargy, migraine, neuralgia, paresthesia, somnolence, syncope, tremor
Psychiatric Disorders: agitation, anxiety, depression, hallucination
Renal and Urinary Disorders: bladder spasm, hydronephrosis, micturation urgency, nephritis interstitial, nocturia, pollakiuria, polyuria, proteinuria [See Warnings and Precautions (5.12)], pyuria, renal artery thrombosis [See Boxed Warning and Warnings and Precautions (5.4)], acute renal failure, renal impairment [See Warnings and Precautions (5.6)], renal tubular necrosis, urinary retention
Reproductive System and Breast Disorders: amenorrhea, benign prostatic hyperplasia,erectile dysfunction, ovarian cyst, scrotal edema
Respiratory, Thoracic, Mediastinal Disorders: atelectasis, bronchitis, dyspnea, cough, epistaxis, lower respiratory tract infection, nasal congestion, oropharyngeal pain, pleural effusions, pulmonary edema, rhinorrhea, sinus congestion, wheezing
Skin and Subcutaneous Tissue Disorders: acne, alopecia, dermatitis acneiform, ecchymosis, hirsutism, hyperhydrosis, hypertrichosis, night sweats, pruritus, rash
Vascular Disorders: venous thromboembolism (including deep vein thrombosis), phlebitis, pulmonary embolism
Less common, serious adverse reactions occurring overall in less than 1% of kidney or liver transplant patients treated with everolimus include:
6.3 Post Marketing Experience
Adverse reactions identified from the postmarketing use of the combination regimen of everolimus and cyclosporine that are not specific to any one transplant indication include angioedema [see Warnings and Precautions (5.8)], erythroderma, leukocytoclastic vasculitis, pancreatitis, pulmonary alveolar proteinosis, and pulmonary embolism. There have also been reports of male infertility with mTOR inhibitors including everolimus. [See Warnings and Precautions (5.18)]
7 DRUG INTERACTIONS
7.1 Interactions with Strong Inhibitors or Inducers of CYP3A4 and P-glycoprotein
Everolimus is mainly metabolized by CYP3A4 in the liver and to some extent in the intestinal wall and is a substrate for the multidrug efflux pump, P-glycoprotein (P-gp). Therefore, absorption and subsequent elimination of systemically absorbed everolimus may be influenced by medicinal products that affect CYP3A4 and/or P-gp. Concurrent treatment with strong inhibitors (e.g., ketoconazole, itraconazole, voriconazole, clarithromycin, telithromycin, ritonavir, boceprevir, telaprevir) and inducers (e.g., rifampin, rifabutin) of CYP3A4 is not recommended. Inhibitors of P-gp (e.g., digoxin, cyclosporine) may decrease the efflux of everolimus from intestinal cells and increase everolimus blood concentrations. In vitro, everolimus was a competitive inhibitor of CYP3A4 and of CYP2D6, potentially increasing the concentrations of medicinal products eliminated by these enzymes. Thus, caution should be exercised when coadministering everolimus with CYP3A4 and CYP2D6 substrates with a narrow therapeutic index. [See Dosage and Administration (2.3)]
All in vivo interaction studies were conducted without concomitant cyclosporine. Pharmacokinetic interactions between everolimus and concomitantly administered drugs are discussed below. Drug interaction studies have not been conducted with drugs other than those described below.
7.2 Cyclosporine (CYP3A4/P-gp Inhibitor and CYP3A4 Substrate)
The steady-state Cmax and area under the curve (AUC) estimates of everolimus were significantly increased by coadministration of single dose cyclosporine. [see Clinical Pharmacology (12.5)] Dose adjustment of everolimus might be needed if the cyclosporine dose is altered. [see Dosage and Administration (2.3)] Everolimus had a clinically minor influence on cyclosporine pharmacokinetics in transplant patients receiving cyclosporine (Neoral).
7.3 Ketoconazole and Other Strong CYP3A4 Inhibitors
Multiple-dose ketoconazole administration to healthy volunteers significantly increased single dose estimates of everolimus Cmax, AUC, and half-life. It is recommended that strong inhibitors of CYP3A4 (e.g., ketoconazole, itraconazole, voriconazole, clarithromycin, telithromycin, ritonavir, boceprevir, telaprevir) should not be coadministered with everolimus. [See Warnings and Precautions (5.14), and Clinical Pharmacology (12.5)]
7.4 Erythromycin (Moderate CYP3A4 Inhibitor)
Multiple-dose erythromycin administration to healthy volunteers significantly increased single dose estimates of everolimus Cmax, AUC, and half-life. If erythromycin is coadministered, everolimus blood concentrations should be monitored and a dose adjustment made as necessary. [See Clinical Pharmacology (12.5)]
7.5 Verapamil (CYP3A4 and P-gp Substrate)
Multiple-dose verapamil administration to healthy volunteers significantly increased single dose estimates of everolimus Cmax and AUC. Everolimus half-life was not changed. If verapamil is coadministered, everolimus blood concentrations should be monitored and a dose adjustment made as necessary. [See Clinical Pharmacology (12.5)]
7.6 Atorvastatin (CYP3A4 substrate) and Pravastatin (P-gp substrate)
Single-dose administration of everolimus with either atorvastatin or pravastatin to healthy subjects did not influence the pharmacokinetics of atorvastatin, pravastatin and everolimus, as well as total HMG-CoA reductase bioreactivity in plasma to a clinically relevant extent. However, these results cannot be extrapolated to other HMG-CoA reductase inhibitors. Patients should be monitored for the development of rhabdomyolysis and other adverse reactions as described in the respective labeling for these products.
7.7 Simvastatin and Lovastatin
Due to an interaction with cyclosporine, clinical studies of everolimus with cyclosporine conducted in kidney transplant patients strongly discouraged patients with receiving HMG-CoA reductase inhibitors such as simvastatin and lovastatin [see Warnings and Precautions (5.11)].
7.8 Rifampin (Strong CYP3A4/P-gp Inducers)
Pretreatment of healthy subjects with multiple-dose rifampin followed by a single dose of everolimus increased everolimus clearance and decreased the everolimus Cmax and AUC estimates. Combination with rifampin is not recommended. [See Warnings and Precautions (5.14) and Clinical Pharmacology (12.5)]
7.9 Midazolam (CYP3A4/5 substrate)
Single-dose administration of midazolam to healthy volunteers following administration of multiple-dose everolimus indicated that everolimus is a weak inhibitor of CYP3A4/5. Dose adjustment of midazolam or other CYP3A4/5 substrates is not necessary when everolimus is coadministered with midazolam or other CYP3A4/5 substrates. [See Clinical Pharmacology (12.5)]
7.10 Other Possible Interactions
Moderate inhibitors of CYP3A4 and P-gp may increase everolimus blood concentrations (e.g., fluconazole; macrolide antibiotics; nicardipine, diltiazem; nelfinavir, indinavir, amprenavir). Inducers of CYP3A4 may increase the metabolism of everolimus and decrease everolimus blood concentrations (e.g., St. John’s Wort [Hypericum perforatum]; anticonvulsants: carbamazepine, phenobarbital, phenytoin; efavirenz, nevirapine).
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
Based on animal studies and the mechanism of action [see Clinical Pharmacology (12.1)], Everolimus can cause fetal harm when administered to a pregnant woman. There are limited case reports of everolimus use in pregnant women; however, these reports are insufficient to inform a drug associated risk of adverse developmental outcomes. Reproductive studies in animals have demonstrated that everolimus was maternally toxic in rabbits, and caused embryo-fetal toxicities in rats and rabbits, at exposures near or below those achieved in human transplant patients. Advise pregnant women of the potential risk to a fetus.
The background risk of major birth defects and miscarriage for the indicated population is unknown; however, in the U.S. general population, the estimated background risk of major birth defects is 2 to 4% and of miscarriage is 15 to 20% of clinically recognized pregnancies.
Data
Animal Data
Everolimus crossed the placenta and was toxic to the conceptus.
Everolimus administered daily to pregnant rats by oral gavage at 0.1 mg/kg (approximately one tenth the exposure in humans administered the lowest starting dose of 0.75 mg twice daily), from before mating through organogenesis, resulted in increased preimplantation loss and embryonic resorptions. These effects occurred in the absence of maternal toxicities.
Everolimus administered daily by oral gavage to pregnant rabbits during organogenesis resulted in abortions, maternal toxicity and lethality, and increased fetal resorptions. At these doses, exposure to everolimus (AUC) was approximately one-tenth, one-half, and one and one-half fold the exposures in humans administered the starting clinical dose, respectively.
In a pre- and post-natal development study in rats, animals were dosed from implantation through lactation. At a dose of
0.1 mg/kg (0.6 mg/m2), there were no adverse effects on delivery and lactation or signs of maternal toxicity; however, there were reductions in body weight (up to 9% reduction) and in survival of offspring (~5%). There were no drug-related effects on the developmental parameters (morphological development, motor activity, learning, or fertility assessment) in the offspring.
8.2 Lactation
Risk Summary
There is no data regarding the presence of everolimus in human milk, the effects on breastfed infants, or the effects on milk production. Everolimus and/or its metabolites are readily transferred into milk of lactating rats at a concentration 3.5 times higher than in maternal rat serum. In pre-post-natal and juvenile studies in rats, exposure to everolimus during the postnatal period caused developmental toxicity [see Pregnancy (8.1) and Nonclinical Toxicology (13.2)]. Advise lactating women not to breastfeed because of the potential for serious adverse reactions in infants exposed to everolimus.
8.3 Females and Males of Reproductive Potential
Contraception
Females should not be pregnant or become pregnant while receiving everolimus. Advise females of reproductive potential that animal studies have been performed showing everolimus to be harmful to the mother and developing fetus [see Pregnancy (8.1)]. Females of reproductive potential are recommended to use highly effective contraception methods while receiving everolimus and up to 8 weeks after treatment has been stopped.
Infertility
Females
Amenorrhea occurred in female patients taking everolimus [see Adverse Reactions (6.2)]. Everolimus may cause pre-implantation loss in females based on animal data [see Nonclinical Toxicology (13.1)].
Female fertility may be compromised by treatment with everolimus.
Males
Everolimus treatment may impair fertility in males based on human [see Warnings and Precautions (5.18), Adverse Reactions (6.2, 6.3)] and animal findings [see Nonclinical Toxicology (13.1)].
8.4 Pediatric Use
The safe and effective use of everolimus in kidney or liver transplant patients younger than 18 years of age has not been established.
8.5 Geriatric Use
There is limited clinical experience on the use of everolimus in patients of age 65 years or older. There is no evidence to suggest that elderly patients will require a different dosage recommendation from younger adult patients. [See Clinical Pharmacology (12.5)]
8.6 Hepatic Impairment
Everolimus whole blood trough concentrations should be closely monitored in patients with impaired hepatic function. For patients with mild hepatic impairment (Child-Pugh Class A), the dose should be reduced by approximately one-third of the normally recommended daily dose. For patients with moderate or severe hepatic impairment (Child-Pugh B or C), the initial daily dose should be reduced to approximately half of the normally recommended daily dose. Further dose adjustment and/or dose titration should be made if a patient’s whole blood trough concentration of everolimus, as measured by an LC/MS/MS assay, is not within the target trough concentration range of 3 to 8 ng/mL. [See Clinical Pharmacology (12.6)]
8.7 Renal Impairment
No dose adjustment is needed in patients with renal impairment. [See Clinical Pharmacology (12.6)]
10 OVERDOSAGE
Reported experience with overdose in humans is very limited. There is a single case of an accidental ingestion of 1.5 mg everolimus in a 2-year-old child where no adverse reactions were observed. Single doses up to 25 mg have been administered to transplant patients with acceptable acute tolerability. Single doses up to 70 mg (without cyclosporine) have been given with acceptable acute tolerability. General supportive measures should be followed in all cases of overdose. Everolimus is not considered dialyzable to any relevant degree (less than 10% of everolimus removed within 6 hours of hemodialysis). In animal studies, everolimus showed a low acute toxic potential. No lethality or severe toxicity was observed after single oral doses of 2000 mg/kg (limit test) in either mice or rats.
11 DESCRIPTION
Everolimus is a macrolide immunosuppressant.
The chemical name of everolimus is
(1R, 9S, 12S, 15R, 16E, 18R, 19R, 21R, 23S, 24E, 26E, 28E, 30S, 32S, 35R)-1, 18-dihydroxy-12 -{(1R)-2-[(1S,3R,4R)-4-(2-hydroxyethoxy)-3-methoxycyclohexyl]-1-methylethyl}-19,30-dimethoxy-15, 17, 21, 23, 29, 35-hexamethyl-11, 36-dioxa-4-aza-tricyclo[30.3.1.04,9] hexatriaconta-16,24,26,28-tetraene-2, 3,10,14,20-pentaone.
The molecular formula is C53H83NO14 and the molecular weight is 958.25. The structural formula is:

Everolimus tablets are supplied as tablets for oral administration containing 0.25 mg, 0.5 mg, 0.75 mg and 1 mg of everolimus together with butylated hydroxytoluene, lactose monohydrate, hypromellose, lactose anhydrous, crospovidone and magnesium stearate as inactive ingredients.
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Everolimus inhibits antigenic and interleukin (IL-2 and IL-15) stimulated activation and proliferation of T and B lymphocytes.
In cells, everolimus binds to a cytoplasmic protein, the FK506 Binding Protein-12 (FKBP-12), to form an immunosuppressive complex (everolimus: FKBP-12) that binds to and inhibits the mammalian Target Of Rapamycin (mTOR), a key regulatory kinase. In the presence of everolimus phosphorylation of p70 S6 ribosomal protein kinase (p70S6K), a substrate of mTOR, is inhibited. Consequently, phosphorylation of the ribosomal S6 protein and subsequent protein synthesis and cell proliferation are inhibited. The everolimus: FKBP-12 complex has no effect on calcineurin activity.
In rats and nonhuman primate models, everolimus effectively reduces kidney allograft rejection resulting in prolonged graft survival.
12.3 Pharmacokinetics
Everolimus pharmacokinetics have been characterized after oral administration of single and multiple doses to adult kidney transplant patients, hepatically-impaired patients and healthy subjects.
Absorption
After oral dosing, peak everolimus concentrations occur 1 to 2 hours post dose. Over the dose range of 0.5 mg to 2 mg twice daily, everolimus Cmax and AUC are dose proportional in transplant patients at steady-state.
Food Effect
In 24 healthy subjects, a high-fat breakfast (44.5 g fat) reduced everolimus Cmax by 60%, delayed Tmax by a median 1.3 hours, and reduced AUC by 16% compared with a fasting administration. To minimize variability, everolimus should be taken consistently with or without food. [See Dosage and Administration (2.6)]
Distribution
The blood-to-plasma ratio of everolimus is concentration dependent ranging from 17% to 73% over the range of 5 ng/mL to 5000 ng/mL. Plasma protein binding is approximately 74% in healthy subjects and in patients with moderate hepatic impairment. The apparent distribution volume associated with the terminal phase (Vz/F) from a single-dose pharmacokinetic study in maintenance kidney transplant patients is 342 to 107 L (range 128 to 589 L).
Elimination
Metabolism
Everolimus is a substrate of CYP3A4 and P-gp. Following oral administration, everolimus is the main circulating component in human blood. Six main metabolites of everolimus have been detected in human blood, including three monohydroxylated metabolites, two hydrolytic ring-opened products, and a phosphatidycholine conjugate of everolimus. These metabolites were also identified in animal species used in toxicity studies, and showed approximately 100-times less activity than everolimus itself.
Excretion
After a single dose of radiolabeled everolimus was given to transplant patients receiving cyclosporine, the majority (80%) of radioactivity was recovered from the feces and only a minor amount (5%) was excreted in urine. Parent drug was not detected in urine and feces.
Pharmacokinetics in Kidney Transplant Patients
Steady-state is reached by Day 4 with an accumulation in blood concentrations of 2- to 3-fold compared with the exposure after the first dose. Table 4 below provides a summary of the steady-state pharmacokinetic parameters.
Table 4. Steady-State Pharmacokinetic Parameters (mean +/-SD) Following the Administration of 0.75 mg Twice Daily
|
Cmax |
Tmax |
AUC |
CL/F1 |
Vc/F1 |
Half-life (T1/2) |
|
11.1 + 4.6 ng/mL |
1-2 h |
75 + 31 ngh/mL |
8.8 L/h |
110 L |
30 ± 11h |
1 population pharmacokinetic analysis
The half-life estimates from 12 maintenance renal transplant patients who received single doses of everolimus at 0.75 mg or 2.5 mg with their maintenance cyclosporine regimen indicate that the pharmacokinetics of everolimus are linear over the clinically-relevant dose range. Results indicate the half-life of everolimus in maintenance renal transplant patients receiving single doses of 0.75 mg or 2.5 mg everolimus during steady-state cyclosporine treatment was 30 ± 11 hours (range 19 to 53 hours).
12.5 Drug-Drug Interactions
Everolimus is known to be a substrate for both cytochrome CYP3A4 and P-gp. The pharmacokinetic interaction between everolimus and concomitantly administered drugs is discussed below. Drug interaction studies have not been conducted with drugs other than those described below. [See Warnings and Precautions (5.14), and Drug Interactions (7)]
Cyclosporine (CYP3A4/P-gp Inhibitor and CYP3A4 Substrate): Everolimus should be taken concomitantly with cyclosporine in kidney transplant patients. Everolimus concentrations may decrease when doses of cyclosporine are reduced, unless the everolimus dose is increased [see Dosage and Administration (2.1), Drug Interactions (7.2)].
In a single-dose study in healthy subjects, cyclosporine (Neoral) administered at a dose of 175 mg increased everolimus AUC by 168% (range, 46% to 365%) and Cmax by 82% (range, 25% to 158%) when administered with 2 mg everolimus compared with administration of everolimus alone [see Drug Interactions (7.2)].
Ketoconazole and Other Strong CYP3A4 Inhibitors: Multiple-dose administration of 200 mg ketoconazole twice daily for 5 days to 12 healthy volunteers significantly increased everolimus Cmax, AUC, and half-life by 3.9-fold, 15-fold, and 89%, respectively, when coadministered with 2 mg everolimus. It is recommended that strong inhibitors of CYP3A4 (e.g., ketoconazole, itraconazole, voriconazole, clarithromycin, telithromycin, ritonavir, boceprevir, telaprevir) should not be coadministered with everolimus. [See Warnings and Precautions (5.14) and Drug Interactions (7.3)]
Erythromycin (Moderate CYP3A4 Inhibitor): Multiple-dose administration of 500 mg erythromycin 3 times daily for 5 days to 16 healthy volunteers significantly increased everolimus Cmax, AUC, and half-life by 2.0-fold, 4.4-fold, and 39%, respectively, when coadministered with 2 mg everolimus. If erythromycin is coadministered, everolimus blood concentrations should be monitored and a dose adjustment made as necessary. [See Drug Interactions (7.4)]
Verapamil (CYP3A4 Inhibitor and P-gp Substrate): Multiple-dose administration of 80 mg verapamil 3 times daily for 5 days to 16 healthy volunteers significantly increased everolimus Cmax and AUC by 2.3-fold and 3.5-fold, respectively, when coadministered with 2 mg everolimus. Everolimus half-life was not changed. If verapamil is coadministered, everolimus blood concentrations should be monitored and a dose adjustment made as necessary. [See Drug Interactions (7.5)]
Atorvastatin (CYP3A4 Substrate) and Pravastatin (P-gp Substrate): Following administration of a single dose of 2 mg everolimus to 12 healthy subjects, the concomitant administration of a single oral dose administration of atorvastatin 20 mg or pravastatin 20 mg only slightly decreased everolimus Cmax and AUC by 9% and 10%, respectively. There was no apparent change in the mean T1/2 or median Tmax. In the same study, the concomitant everolimus dose slightly increased the mean Cmax of atorvastatin by 11% and slightly decreased the AUC by 7%. The concomitant everolimus dose decreased the mean Cmax and AUC of pravastatin by 10% and 5%, respectively. No dosage adjustments are needed for concomitant administration of everolimus and atorvastatin and pravastatin. [See Drug Interactions (7.6)]
Midazolam (CYP3A4/5 Substrate): In 25 healthy male subjects, coadministration of a single dose of midazolam 4 mg oral solution with steady-state everolimus (10 mg daily dose for 5 days) resulted in a 25% increase in midazolam Cmax and a 30% increase in midazolam AUC; whereas, the terminal half-life of midazolam and the metabolic AUC-ratio (1 hydroxymidazolam/midazolam) were not affected. [See Drug Interactions (7.9)]
Rifampin (Strong CYP3A4 and P-gp Inducer): Pretreatment of 12 healthy subjects with multiple-dose rifampin (600 mg once-daily for 8 days) followed by a single dose of 4 mg everolimus increased everolimus clearance nearly 3-fold, and decreased Cmax by 58% and AUC by 63%. Combination with rifampin is not recommended. [See Drug Interactions (7.8)]
12.6 Specific Populations
Hepatic Impairment
Relative to the AUC of everolimus in subjects with normal hepatic function, the average AUC in 6 patients with mild hepatic impairment (Child-Pugh Class A) was 1.6-fold higher following administration of a 10 mg single-dose. In 2 independently studied groups of 8 and 9 patients with moderate hepatic impairment (Child-Pugh Class B) the average AUC was 2.1-fold and 3.3-fold higher following administration of a 2 mg or a 10 mg single-dose, respectively; and in 6 patients with severe hepatic impairment (Child-Pugh Class C) the average AUC was 3.6-fold higher following administration of a 10 mg single-dose. For patients with mild hepatic impairment (Child-Pugh Class A), the dose should be reduced by approximately one-third of the normally recommended daily dose. For patients with moderate or severe hepatic impairment (Child-Pugh B or C), the initial daily dose should be reduced to approximately one-half of the normally recommended daily dose. Further dose adjustment and/or dose titration should be made if a patient’s whole blood trough concentration of everolimus, as measured by an LC/MS/MS assay, is not within the target trough concentration range of 3 to 8 ng/mL. [See Dosage and Administration (2.7)]
Renal Impairment
No pharmacokinetic studies in patients with renal impairment were conducted. Post-transplant renal function (creatinine clearance range 11 to 107 mL/min) did not affect the pharmacokinetics of everolimus, therefore, no dosage adjustments are needed in patients with renal impairment.
Geriatrics
A limited reduction in everolimus oral CL of 0.33% per year was estimated in adults (age range studied was 16 to 70 years). There is no evidence to suggest that elderly patients will require a different dosage recommendation from younger adult patients.
Race
Based on analysis of population pharmacokinetics, oral clearance (CL/F) is, on average, 20% higher in black transplant patients.
12.7 Everolimus Whole Blood Concentrations Observed in Kidney and in Liver Transplant Patients
Everolimus in Kidney Transplantation
Based on exposure-efficacy and exposure-safety analyses of clinical trials and using an LC/MS/MS assay method, kidney transplant patients achieving everolimus whole blood trough concentrations greater than or equal to 3 ng/mL have been found to have a lower incidence of treated biopsy-proven acute rejection compared with patients whose trough concentrations were below 3 ng/mL. Patients who attained everolimus trough concentrations within the range of 6 to 12 ng/mL had similar efficacy and more adverse reactions than patients who attained lower trough concentrations between 3 to 8 ng/mL [see Dosage and Administration (2.3)].
In the kidney clinical trial [see Clinical Studies (14.1)], everolimus whole blood trough concentrations were measured at Days 3, 7, and 14 and Months 1, 2, 3, 4, 6, 7, 9, and 12. The proportion of patients receiving 0.75 mg twice daily everolimus treatment regimen who had everolimus whole blood trough concentrations within the protocol specified target range of 3 to 8 ng/mL at Days 3, 7, and 14 were 55%, 71% and 69%, respectively. Approximately 80% of patients had everolimus whole blood trough concentrations within the 3 to 8 ng/mL target range by Month 1 and remained stable within range through Month 12 post-transplant. The median everolimus trough concentration for the 0.75 mg twice daily treatment group was between 3 and 8 ng/mL throughout the study duration.
Everolimus in Liver Transplantation
In the liver clinical trial [See Clinical Studies (14.2)] everolimus dosing was initiated after 30 days following transplantation. Whole blood trough everolimus concentrations were measured within 5 days after first dose, followed by weekly intervals for 3 to 4 weeks, and then monthly thereafter. Approximately 49%, 37%, and 18% of patients, respectively, were below 3 ng/mL at 1, 2, and 4 weeks after initiation of everolimus dosing. The majority of patients (approximately 70% to 80%) had everolimus trough blood concentrations within the target range of 3-8 ng/mL after Month 2 through Month 24 post-transplant.
12.8 Cyclosporine Concentrations Observed in Kidney Transplant Patients
In the kidney transplant clinical trial [see Clinical Studies (14.1)], the target cyclosporine whole blood trough concentration for the everolimus treatment arm of 0.75 mg twice daily were 100 to 200 ng/mL through Month 1 post- transplant, 75 to 150 ng/mL at Months 2 and 3 post-transplant, 50 to 100 ng/mL at Month 4 post-transplant, and 25 to 50 ng/mL from Month 6 through Month 12 post-transplant. Table 5 below provides a summary of the observed cyclosporine whole blood trough concentrations during the study.
Table 5. Cyclosporine Trough Concentrations Over 12 Months Post-transplant – Kidney Study Median Values (ng/mL) with 10th and 90th Percentiles
|
Treatment group |
Visit |
N |
Target (ng/mL) |
Median |
10th Percentile |
90th Percentile |
|
Everolimus 0.75 mg twice daily |
Day 3 Day 7 |
242 265 |
100-200 100-200 |
172 185 |
46 75 |
388 337 |
|
Day 14 |
243 |
100-200 |
182 |
97 |
309 |
|
|
Month 1 |
245 |
100-200 |
161 |
85 |
274 |
|
|
Month 2 |
232 |
75-150 |
140 |
84 |
213 |
|
|
Month 3 |
220 |
75-150 |
111 |
68 |
187 |
|
|
Month 4 |
208 |
50-100 |
99 |
56 |
156 |
|
|
Month 6 |
200 |
25-50 |
75 |
43 |
142 |
|
|
Month 7 |
199 |
25-50 |
59 |
36 |
117 |
|
|
Month 9 |
194 |
25-50 |
49 |
28 |
91 |
|
|
Month 12 |
186 |
25-50 |
46 |
25 |
100 |
12.9 Tacrolimus Concentrations in Liver Transplant
In the liver transplant clinical trial [See Clinical Studies (14.2)], the target tacrolimus whole blood trough concentrations were greater than or equal to 8 ng/mL in the first 30 days post-transplant. The protocol required that patients had a tacrolimus trough concentration of at least 8 ng/mL in the week prior to initiation of everolimus. Everolimus was initiated after 30 days post-transplant. At that time, the target tacrolimus trough concentrations were reduced to 3 to 5 ng/mL. Table 6 below provides a summary of the tacrolimus whole blood trough concentrations observed during the study through Month 24 post-transplant.
Table 6. Tacrolimus Trough Concentrations Over 24 Months Post-Transplant – Liver Study Median Values (ng/mL) with 10th and 90th Percentiles
|
Treatment group |
Visit |
N |
Target (ng/mL) |
Median |
10th Percentile |
90th Percentile |
|
Predose group Everolimus 1 mg twice daily (initiated at Month 1) |
Week 4 |
234 |
3-5 |
9.5 |
5.8 |
14.6 |
|
Week 5 |
219 |
3-5 |
8.1 |
4.5 |
13.8 |
|
|
Week 6 |
233 |
3-5 |
7.0 |
4.1 |
12.0 |
|
|
Month 2 |
219 |
3-5 |
5.6 |
3.4 |
10.3 |
|
|
Month 3 |
218 |
3-5 |
5.2 |
3.1 |
9.7 |
|
|
Month 4 |
196 |
3-5 |
4.9 |
2.9 |
7.7 |
|
|
Month 5 |
195 |
3-5 |
4.8 |
2.7 |
7.3 |
|
|
Month 6 |
200 |
3-5 |
4.6 |
3.0 |
7.5 |
|
|
Month 9 |
186 |
3-5 |
4.4 |
2.9 |
8.0 |
|
|
Month 12 |
175 |
3-5 |
4.3 |
2.6 |
7.3 |
|
|
Month 24 |
109 |
3-5 |
3.8 |
2.3 |
5.5 |
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis and Mutagenesis and Impairment of Fertility
Everolimus was not carcinogenic in mice or rats when administered daily by oral gavage for 2 years at doses up to 0.9 mg/kg, the highest dose tested. In these studies, AUCs in mice were much higher (at least 20 times) than those in humans receiving 0.75 mg twice daily, and AUCs in rats were in the same range as those in humans receiving 0.75 mg twice daily.
Everolimus was not mutagenic in the bacterial reverse mutation, the mouse lymphoma thymidine kinase assay, or the chromosome aberration assay using V79 Chinese hamster cells, or in vivo following two daily doses of 500 mg/kg in the mouse micronucleus assay.
In a 13-week male fertility oral gavage study in rats, testicular morphology was affected at 0.5 mg/kg and above, and sperm motility, sperm head count and plasma testosterone concentrations were diminished at 5 mg/kg which caused a decrease in male fertility. There was evidence of reversibility of these findings in animals examined after 13 weeks post- dosing. The 0.5 mg/kg dose in male rats resulted in AUCs in the range of clinical exposures, and the 5 mg/kg dose resulted in AUCs approximately 5 times the AUCs in humans receiving 0.75 mg twice daily.
Oral doses of everolimus in female rats greater or equal to 0.1 mg/kg (approximately 0.13-fold the estimated AUC0-24h in patients receiving the starting dose 0.75 mg twice daily) resulted in increased incidence of pre-implantation loss.
13.2 Animal Toxicology and/or Pharmacology
In an oral neonatal and juvenile development study in rats, oral administration of everolimus from postnatal Day 7 to 70 produced dose-related delayed attainment of developmental landmarks, including delayed eye-opening, delayed reproductive development in males and females, and increased latency time during the learning and memory phases were observed at doses as low as 0.15 mg/kg/day. Exposures in the rat at these doses were equal to or less than those obtained in adult human transplant patients.
14 CLINICAL STUDIES
14.1 Prevention of Organ Rejection after Kidney Transplantation
A 24-month, multi-national, open-label, randomized (1:1:1) trial was conducted comparing two concentration-controlled everolimus regimens of 1.5 mg per day starting dose (targeting 3 to 8 ng/mL using an LC/MS/MS assay method and 3 mg per day starting dose (targeting 6 to 12 ng/mL using an LC/MS/MS assay method) with reduced exposure cyclosporine and corticosteroids, to 1.44 g per day of mycophenolic acid with standard exposure cyclosporine and corticosteroids. The mean cyclosporine starting dose was 5.2, 5.0 and 5.7 mg/kg body weight/day in the everolimus 1.5 mg, 3 mg and in mycophenolic acid groups, respectively. The cyclosporine dose in the everolimus group was then adjusted to the blood trough concentration ranges indicated in Table 5, whereas in the mycophenolic acid group the target ranges were 200 to 300 ng/mL starting Day 5: 200 to 300 ng/mL, and 100 to 250 ng/mL from Month 2 to Month 12.
All patients received basiliximab induction therapy. The study population consisted of 18 to 70 year old male and female low to moderate risk renal transplant recipients undergoing their first transplant. Low to moderate immunologic risk was defined in the study as an ABO blood type compatible first organ or tissue transplant recipient with anti-human leukocyte antigen (HLA) Class I panel reactive antibody (PRA) less than 20% by a complement dependent cytotoxicity-based assay, or less than 50% by a flow cytometry or ELISA-based assay, and with a negative T-cell cross match. Eight hundred thirty-three (833) patients were randomized after transplantation; 277 randomized to the everolimus 1.5 mg per day group, 279 to the everolimus 3 mg per day group and 277 to the mycophenolic acid 1.44 g per day group. The study was conducted at 79 renal transplant centers across Europe, South Africa, North and South America, and Asia-Pacific. There were no major baseline differences between treatment groups with regard to recipient or donor disease characteristics. The majority of transplant recipients in all groups (70% to 76%) had three or more HLA mismatches; mean percentage of panel reactive antibodies ranged from 1% to 2%. The rate of premature treatment discontinuation at 12 months was 30% and 22% in the everolimus 1.5 mg and control groups, respectively, (p=0.03, Fisher’s exact test) and was more prominent between groups among female patients. Results at 12 months indicated that everolimus 1.5 mg per day is comparable to control with respect to efficacy failure, defined as treated biopsy-proven acute rejection*, graft loss, death or loss to follow-up. The percentage of patients experiencing this endpoint and each individual variable in the everolimus and control groups is shown in Table 7.
Table 7. Efficacy Failure by Treatment Group (ITT Population) at 12 Months after Kidney Transplantation
|
Everolimus 1.5 mg per day With reduced exposure CsA N=277 n (%) |
Mycophenolic Acid 1.44 g per day With standard exposure CsA N=277 n (%) |
|
|
Efficacy Endpoints1 |
||
|
Efficacy Failure Endpoint2 |
70 (25.3) |
67 (24.2) |
|
Treated Biopsy Proven Acute Rejection |
45 (16.2) |
47 (17.0) |
|
Death |
7 (2.5) |
6 (2.2) |
|
Graft Loss |
12 (4.3) |
9 (3.2) |
|
Loss to Follow-up |
12 (4.3) |
9 (3.2) |
|
Graft Loss or Death or Loss to Follow-up3 |
32 (11.6) |
26 (9.4) |
|
Graft Loss or Death |
18 (6.5) |
15 (5.4) |
|
Loss to Follow-up3 |
14 (5.1) |
11 (4.0) |
* Treated biopsy-proven acute rejection (tBPAR) was defined as a histologically confirmed acute rejection with a biopsy graded as IA, IB, IIA, IIB, or III according to 1997 Banff criteria that were treated with anti-rejection medication.
1 The difference in rates (Everolimus –mycophenolic acid) with 95% CI for primary efficacy failure endpoint is 1.1% (-6.1%, 8.3%); and for the graft loss, death or loss to follow-up endpoint is 2.2% (-2.9%, 7.3%).
2 Includes treated BPAR, graft loss, death or loss to follow-up by Month 12 where loss to follow-up represents patient who did not experience treated BPAR, graft loss or death and whose last contact date is prior to 12 month visit
3 Loss to follow-up (for Graft Loss, Death, or Loss to Follow-up) represents patient who did not experience death or graft loss and whose last contact date is prior to 12 month visit
The estimated mean glomerular filtration rate (using the MDRD equation) for everolimus 1.5 mg (target trough concentrations 3 to 8 ng/mL) and mycophenolic acid groups were comparable at Month 12 in the ITT population (Table 8).
Table 8. Estimated Glomerular Filtration Rates (mL/min/1.73m2) by MDRD at 12 Months after Kidney Transplantation *
|
Month 12 GFR (MDRD) |
Everolimus 1.5 mg per day with reduced exposure CsA N=276 |
Mycophenolic Acid 1.44 g per day with standard exposure CsA N=277 |
|
Mean (SD)** |
54.6 (21.7) |
52.3 (26.5) |
|
Median (Range) |
55.0 (0-140.9) |
50.1 (0.0-366.4) |
* Analysis based on using a subject’s last observation carried forward for missing data at 12 months due to death or lost to follow-up data, a value of zero is used for subjects who experienced a graft loss.
** SD=standard deviation
Two earlier studies compared fixed doses of everolimus 1.5 mg per day and 3 mg per day, without TDM, combined with standard exposure cyclosporine and corticosteroids to mycophenolate mofetil 2 g per day and corticosteroids. Antilymphocyte antibody induction was prohibited in both studies. Both were multicenter, double-blind (for first 12 months), randomized trials (1:1:1) of 588 and 583 de novo renal transplant patients, respectively. The 12-month analysis of GFR showed increased rates of renal impairment in both the everolimus groups compared to the mycophenolate mofetil group in both studies. Therefore, reduced exposure cyclosporine should be used in combination with everolimus in order to avoid renal dysfunction and everolimus trough concentrations should be adjusted using TDM to maintain trough concentrations between 3 to 8 ng/mL [see Boxed Warning, Dosage and Administration (2.4), Warnings and Precautions (5.6)].
14.2 Prevention of Organ Rejection after Liver Transplantation
A 24-month, multinational, open-label, randomized (1:1:1) trial was conducted in liver transplant patients starting 30 days post-transplant. During the first 30 days, after transplant and prior to randomization, patients received tacrolimus and corticosteriods, with or without mycophenolate mofetil. No induction antibody was administered. Approximately 70% to 80% of patients received at least one dose of mycophenolate mofetil at a median total daily dose of 1.5 g during the first 30 days. For eligibility, patients had to have a tacrolimus trough concentration of at least 8 ng/mL in the week prior to randomization.
At randomization, mycophenolate mofetil was discontinued and patients were randomized to one of two everolimus treatment groups [initial dose of 1 mg twice per day (2 mg daily) and adjusted to target trough concentrations using an LC/MS/MS assay of 3 to 8 ng/mL] either with reduced exposure of tacrolimus (target trough whole blood concentrations of 3 to 5 ng/mL) or tacrolimus elimination. In the tacrolimus elimination group, at Month 4 post-transplant, once the everolimus trough concentrations were within the target range of 6 to 10 ng/mL, reduced exposure tacrolimus was eliminated. The everolimus with tacrolimus elimination group was discontinued early due to higher incidence of acute rejection. In the control group, patients received standard exposure tacrolimus (target trough whole blood concentrations of 8 to 12 ng/mL tapered to 6 to 10 ng/mL by month 4 post-transplant). All patients received corticosteroids during the trial.
The study population consisted of 18 to 70 year old male and female liver transplant recipients undergoing their first transplant, mean age was approximately 54 years, more than 70% of patients were male, and the majority of patients were Caucasian, with approximately 89% of patients per treatment group completing the study. Key stratification parameters of HCV status (31 to 32% HCV positive across groups) and renal function (mean baseline eGFR range 79 to 83 mL/min/1.73 m2) were also balanced between groups.
A total of 1147 patients were enrolled into the run-in period of this trial. At 30 days post-transplant a total 719 patients, who were eligible according to study inclusion/exclusion criteria, were randomized into one of three treatment groups: everolimus with reduced exposure tacrolimus; N=245, everolimus with tacrolimus elimination (tacrolimus elimination group); N=231, or standard dose/exposure tacrolimus (tacrolimus control); N=243. The study was conducted at 89 liver transplant centers across Europe, including the United Kingdom and Ireland, North and South America, and Australia.
Key inclusion criteria were recipients 18 to 70 years of age, eGFR greater or equal to 30 mL/min/1.73 m2, tacrolimus trough level of greater or equal to 8 ng/mL in the week prior to randomization, and the ability to take oral medication.
Key exclusion criteria were recipients of multiple solid organ transplants, history of malignancy (except hepatocellular carcinoma within Milan criteria), human immunodeficiency virus, and any surgical or medical condition which significantly alter the absorption, distribution, metabolism and excretion of study drug.
There were no major baseline differences between treatment groups with regard to recipient or donor disease characteristics. Mean MELD scores at time of transplantation, cold ischemia times (CIT), and ABO matching were similar across groups. Overall the treatment groups were comparable with respect to the key determinants of liver transplantation.
The tacrolimus elimination group was stopped prematurely due to a higher incidence of acute rejection and adverse reactions leading to treatment discontinuation reported during the elimination phase of tacrolimus. Therefore, a treatment regimen of everolimus with tacrolimus elimination is not recommended.
Results up to 24 months are presented indicating that everolimus with reduced exposure tacrolimus is comparable to standard exposure tacrolimus with respect to efficacy failure, defined as treated biopsy-proven acute rejection, graft loss, death or loss to follow-up throughout 12-24 months of treatment. The percentage of patients experiencing this endpoint and each individual variable in the everolimus and control group for each time interval is shown in Table 9.
Table 9. Efficacy Failure by Treatment Group (ITT Population) at 12 and 24 Months after Liver Transplantation
|
Everolimus With reduced Exposure Tacrolimus N=245 n (%) |
Tacrolimus (standard exposure) N=243 n (%) |
|
|
Efficacy Endpoints1 at 12 months |
||
|
Composite Efficacy Failure Endpoint1,2 |
22 (9.0) |
33 (13.6) |
|
Treated Biopsy Proven Acute Rejection* |
7 (2.9) |
17 (7.0) |
|
Death |
13 (5.3) |
7 (2.9) |
|
Graft Loss |
6 (2.4) |
3 (1.2) |
|
Loss to Follow-up2 |
4 (1.6) |
9 (3.7) |
|
Graft Loss or Death or Loss to Follow-up |
18 (7.3) |
18 (7.4) |
|
Graft Loss or Death |
14 (5.7) |
8 (3.3) |
|
Loss to Follow-up |
4 (1.6) |
10 (4.1) |
|
Efficacy Endpoints at 24 months |
||
|
Composite Efficacy Failure Endpoint2 |
45 (18.4) |
53 (21.8) |
|
Treated Biopsy Proven Acute Rejection |
11 (4.5) |
18 (7.4) |
|
Death |
17 (6.9) |
11 (4.5) |
|
Graft loss |
9 (3.7) |
7 (2.9) |
|
Loss to follw-up2 |
18 (7.3) |
23 (9.5) |
|
Graft loss or Death or Loss to follow-up3 |
38 (15.5) |
36 (16.0) |
|
Graft loss or Death |
20 (8.2) |
15 (6.2) |
|
Loss to follow-up3 |
18 (7.3) |
24 (9.9) |
* Treated biopsy-proven acute rejection (tBPAR) was defined as histologically confirmed acute rejection with a rejection activity index (RAI) greater than or equal to RAI score 3 that received anti-rejection treatment.
1 The difference in rates (everolimus – control) at 12 months with 97.5% CI for efficacy failure endpoint based on normal approximation with Yates continuity correction is -4.6% (-11.4%, 2.2%); and for the graft loss, death or loss to follow-up endpoint is -0.1% (-5.4%, 5.3%).
2 Loss to follow-up (for treated BPAR, graft loss, death or loss to follow-up) represents patients who did not experience treated BPAR, graft loss or death and whose last contact date is prior to 12- or 24-month visit.
3 Loss to follow-up (for Graft Loss, Death, or Loss to Follow-up) represents patients who did not experience death or graft loss and whose last contact date is prior to 12- or 24-month visit.
At 12 months, the estimated mean glomerular filtration rate (eGFR) using the MDRD equation for the everolimus group was 80.9 mL/min/1.73 m2 and the tacrolimus control was 70.3 mL/min/1.73 m2 in the ITT population. At Month 24, the eGFR using the MDRD equation for the everolimus group was 74.7 mL/min/1.73 m2 and the tacrolimus control the eGFR was 67.8 mL/min/1.73 m2 (Table 10).
Table 10. Estimated Glomerular Filtration Rates (mL/min/1.73 m2) by MDRD at 12 and 24 Months after Liver Transplantation
|
eGFR (MDRD) |
Everolimus with reduced exposure Tacrolimus |
Tacrolimus (standard exposure) |
|
Month 12 |
N=215 |
N=209 |
|
Mean (SD) |
80.9 (27.3) |
70.3 (23.1) |
|
Median* (Range) |
78.3 (28.4-153.1) |
66.4 (27.9-155.8) |
|
Month 24 |
N=184 |
N=186 |
|
Mean (SD) |
74.7 (26.1) |
67.8 (21.0) |
|
Median* (Range) |
72.9 (20.3-151.6) |
65.2 (27.0-148.9) |
Figure 1. Mean and 95% CI of eGFR (MDRD 4) [mL/min/1.73m2] by Visit Window and Treatment after Liver Transplantation (ITT population - 24 Month Analysis)*
![Mean and 95% CI of eGFR (MDRD 4) [mL/min/1.73m2] by Visit Window and Treatment (ITT population 24 Month Analysis)*](https://hellopharmacist.nyc3.digitaloceanspaces.com/spl/20220422_59cb86d5-8706-4619-b074-af253d3e0b68/everolimus-tabs-2.jpg)
* Everolimus dosing was initiated 30 days after transplantation
Although the initial protocol was designed for 24 months, the study was subsequently extended to 36 months. One hundred six patients (43%) in the everolimus group and 125 patients (51%) in the control group participated in the extenstion study from Month 24 to Month 36 after transplantation. The results for the everolimus group at 36 months were consistent with the results at 24 months in terms of tBPAR, graft loss, death and eGFR.
16 HOW SUPPLIED
Everolimus Tablets are packed in child-resistant blisters.
Table 11. Description of Everolimus Tablets
| Dosage Strength | 0.25 mg | 0.5 mg | 0.75 mg | 1mg |
| Appearance | White to off white, round flat faced bevel edge tablets | |||
| Imprint |
“P” on one side and “158” on the other |
“P” on one side and “159” on the other |
“P” on one side and “160” on the other |
“P” on one side and “283” on the other |
| NDC Number | 49884-158-02 | 49884-159-02 | 49884-160-02 | 49884-283-02 |
Each strength is available in boxes of 60 tablets (6 blister strips of 10 tablets each).
Storage
Store at 25°C (77°F); excursions permitted to 15 to 30°C (59 to 86°F). [see USP Controlled Room Temperature]
Protect from light and moisture.
17 PATIENT COUNSELING INFORMATION
Administration
Inform patients that everolimus should be taken orally twice a day approximately 12 hours apart consistently either with or without food.
Inform patients to avoid grapefruit and grapefruit juice which increase blood drug concentrations of everolimus. [See Warnings and Precautions (5.20)]
Advise patients that everolimus should be used concurrently with reduced doses of cyclosporine and that any change in doses of these medications should be made under physician supervision. A change in the cyclosporine dose may also require a change in the dosage of everolimus.
Inform patients of the necessity of repeated laboratory tests according to physician recommendations while they are taking everolimus.
Development of Lymphomas and Other Malignancies
Inform patients they are at risk of developing lymphomas and other malignancies, particularly of the skin, due to immunosuppression. Advise patients to limit exposure to sunlight and ultraviolet (UV) light by wearing protective clothing and using a sunscreen with a high protection factor. [See Warnings and Precautions (5.2)]
Increased Risk of Infection
Inform patients they are at increased risk of developing a variety of infections, including opportunistic infections, due to immunosuppression. Advise patients to contact their physician if they develop any symptoms of infection. [See Warnings and Precautions (5.3, 5.13)]
Kidney Graft Thrombosis
Inform patients that everolimus has been associated with an increased risk of kidney arterial and venous thrombosis, resulting in graft loss, usually within the first 30 days post-transplantation. [see Warnings and Precautions (5.4)].
Everolimus and Calcineurin Inhibitor-Induced Nephrotoxicity
Advise patients of the risks of impaired kidney function with the combination of everolimus and cyclosporine as well as the need for routine blood concentration monitoring for both drugs. Advise patients of the importance of serum creatinine monitoring. [See Warnings and Precautions (5.6)]
Angioedema
Inform patients of the risk of angioedema and that concomitant use of ACE inhibitors may increase this risk. Advise patients to seek prompt medical attention if symptoms occur. [See Warnings and Precautions (5.8)]
Wound Healing Complications and Fluid Accumulation
Inform patients the use of everolimus has been associated with impaired or delayed wound healing, fluid accumulation and the need for careful observation of their incision site. [See Warnings and Precautions (5.9)]
Interstitial Lung Disease/Non-Infectious Pneumonitis
Inform patients the use of everolimus may increase the risk of non-infectious pneumonitis. Advise patients to seek medical attention if they develop clinical symptoms consistent with pneumonia. [See Warnings and Precautions (5.10)]
Hyperlipidemia
Inform patients the use of everolimus has been associated with increased serum cholesterol and triglycerides that may require treatment and the need for monitoring of blood lipid concentrations. [See Warnings and Precautions (5.11)]
Proteinuria
Inform patients the use of everolimus has been associated with an increased risk of proteinuria. [See Warnings and Precautions (5.12)]
Pregnancy and Lactation
Advise women of childbearing age to avoid becoming pregnant throughout treatment and for 8 weeks after everolimus therapy has stopped. Everolimus can cause fetal harm if taken during pregnancy. Advise a pregnant woman of the potential risk to a fetus. Also advise not to breastfeed while taking everolimus [Use in Specific Population (8.1, 8.2)].
Male and Female Fertility
Inform male and female patients that everolimus may impair fertility [see Warnings and Precautions (5.18), Use in Specific Populations (8.1, 8.3), Non-Clinical Toxicology (13.1)].
Medications that Interfere with Everolimus
Some medications can increase or decrease blood concentrations of everolimus. Advise patients to inform their physician if they are taking any of the following: antifungals, antibiotics, antivirals, anti-epileptic medicines including carbamazepine, phenytoin and barbiturates, herbal/dietary supplements (St. John’s Wort), and/or rifampin. [See Warnings and Precautions (5.14)]
New Onset Diabetes
Inform patients the use of everolimus may increase the risk of diabetes mellitus and to contact their physician if they develop symptoms. [See Warnings and Precautions (5.16)]
Immunizations
Inform patients that vaccinations may be less effective while they are being treated with everolimus. Advise patients live vaccines should be avoided. [See Warnings and Precautions (5.19)]
Patient with Hereditary Disorders
Advise patients to inform their physicians that if they have hereditary disorders of galactose intolerance (Lapp-lactase deficiency or glucose-galactose malabsorption) not to take everolimus. [See Warnings and Precautions (5.21)]
Medication Guide
MEDICATION GUIDE
Everolimus Tablets
(e ver OH li mus)
What is the most important information I should know about everolimus tablet? Everolimus tablet can cause serious side effects, including:
- Increased risk of getting certain cancers. People who take everolimus tablet have a higher chance of getting lymphoma and other cancers, especially skin cancer. Talk to your doctor about your risk for cancer.
- Increased risk of serious infections. Everolimus tablet weakens the body’s immune system and affects your ability to fight infections. Serious infections can happen with everolimus tablet that may lead to death. People taking everolimus tablet have a higher chance of getting infections caused by viruses, bacteria, and fungi (yeast).
o Call your doctor if you have symptoms of infection including fever or chills.
- Blood clot in the blood vessels of your transplanted kidney. If this happens, it usually occurs within the first 30 days after your kidney transplant. Tell your doctor right away if you:
o have pain in your groin, lower back, side or stomach (abdomen)
o make less urine or you do not pass any urine
o have blood in your urine or dark colored urine (tea-colored)
o have fever, nausea, or vomiting
-
Serious problems with your transplanted kidney (nephrotoxicity). You will need to start with a lower dose of cyclosporine when you take it with everolimus tablet. Your Doctor should do regular blood tests to check your levels of both everolimus and cyclosporine.
- Increased risk of death that can be related to infection, in people who have had a heart transplant. You should not take everolimus tablet if you have had a heart transplant without talking to your doctor.
See the section “What are the possible side effects of everolimus tablet?” for information about other serious side effects.
What is everolimus tablet?
Everolimus tablet is a prescription medicine used to prevent transplant rejection (antirejection medicine) in people who have received a kidney transplant or liver transplant. Transplant rejection happens when the body’s immune system perceives the new transplanted kidney as “foreign” and attacks it.
Everolimus tablet is used with other medicines called cyclosporine, corticosteroids and certain other transplant medicines to prevent rejection of your transplanted kidney. Everolimus tablet is used with other medicines called tacrolimus and corticosteroids to prevent rejection of your transplanted liver.
It is not known if everolimus tablet is safe and effective in transplanted organs other than kidney and liver.
It is not known if everolimus tablet is safe and effective in children under 18 years of age.
Do not take everolimus tablet if you are allergic to:
- everolimus (Afinitor®) or any of the ingredients in everolimus tablets. See the end of this Medication Guide for a complete list of ingredients in everolimus tablets.
- sirolimus (Rapamune®)
Before taking everolimus tablet, tell your doctor about all of your medical conditions, including if you:
- have liver problems
- have skin cancer or it runs in your family
- have high cholesterol or triglycerides (fat in your blood)
- have Lapp lactase deficiency or glucose-galactose malabsorption. You should not take everolimus tablet if you have this disorder.
- are pregnant or could become pregnant. Everolimus tablet will harm your unborn baby. If you are able to become pregnant you should use effective birth control during treatment and for 8 weeks after your last dose of everolimus tablet. Talk to your doctor about birth control methods that may be right for you during this time. If you become pregnant or think you are pregnant, tell your healthcare provider right away. You should not become pregnant during treatment with everolimus tablet.
- are breastfeeding or plan to breastfeed. It is not known if everolimus passes into your breast milk.
Tell your doctor about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements.
Especially tell your doctor if you take:
- antifungal medicine
- antibiotic medicine
- heart medicine
- high blood pressure medicine
- a medicine to lower cholesterol or triglycerides
- Cyclosporine (Sandimmune, Gengraf, Neoral)
- tuberculosis (TB) medicine
- HIV medicine
- St. John’s Wort
- seizure (anticonvulsant) medicine
How should I take everolimus tablet?
- Take everolimus tablet exactly as your doctor tells you.
- Do not stop taking everolimus tablet or change your dose unless your doctor tells you to.
- Take everolimus tablet at the same time as your dose of cyclosporine medicine.
- Do not stop taking or change your dose of cyclosporine or tacrolimus medicine unless your doctor tells you to.
- If your doctor changes your dose of cyclosporine your dose of everolimus tablet may change.
- Take everolimus tablet 2 times a day about 12 hours apart.
- Swallow everolimus tablet whole with a glass of water. Do not crush or chew everolimus tablet.
- Take everolimus tablet with or without food. If you take everolimus tablet with food, always take
- everolimus tablet with food. If you take everolimus tablet without food, always take everolimus tablet without food.
- Your doctor will do regular blood tests to check your kidney function while you take everolimus tablet. It is important that you get these tests done when your doctor tells you to. Blood tests will monitor how your kidneys are working and make sure you are getting the right dose of everolimus tablet and other transplant medications they may be on (cyclosporine and tacrolimus).
- If you take too much everolimus tablet, call your doctor or go to the nearest hospital emergency room right away.
What should I avoid while taking everolimus tablet?
- Avoid receiving any live vaccines while taking everolimus tablet. Some vaccines may not work as well while you are taking everolimus tablet.
- Do not eat grapefruit or drink grapefruit juice while you are taking everolimus tablet. Grapefruit may increase your blood level of everolimus.
- Limit the amount of time you spend in the sunlight. Avoid using tanning beds or sunlamps. People who take everolimus tablet have a higher risk of getting skin cancer. See the section “What is the most important information I should know about everolimus tablet?” Wear protective clothing when you are in the sun and use a sunscreen with a high protection factor (SPF 30 and above). This is especially important if you have fair skin or if you have a family history of skin cancer.
- Avoid becoming pregnant. See the section “What should I tell my doctor before taking everolimus tablet?”
What are possible side effects of Everolimus tablet?
Everolimus tablet can cause serious side effects, including:
- See “What is the most important information I should know about Everolimus tablet?”
- swelling under your skin especially around your mouth, eyes and in your throat (angioedema). Your chance of having swelling under your skin is higher if you take everolimus tablet along with certain other medicines. Tell your doctor right away or go to the nearest emergency room if you have any of these symptoms of angioedema:
· sudden swelling of your face, mouth, throat, tongue or hands
· hives or welts
· itchy or painful swollen skin
· trouble breathing
- delayed wound healing. Everolimus tablet can cause your incision to heal slowly or not heal well. Call your doctor right away if you have any of the following symptoms:
· your incision is red, warm or painful
· blood, fluid, or pus in your incision
· your incision opens up
· swelling of your incision
- lung or breathing problems. Tell your doctor right away if you have new or worsening cough, shortness of breath, difficulty breathing or wheezing. In some patients lung or breathing problems have been severe, and can even lead to death. Your doctor may need to stop everolimus tablet or lower your dose.
- increased cholesterol and triglycerides (fat in your blood). If your cholesterol and triglyceride levels are high your doctor may want to lower them with diet, exercise and certain medicines.
- protein in your urine (proteinuria).
- change in kidney function. Everolimus tablet may cause kidney problems when taken along with a standard dose of cyclosporine medicine instead of a lower dose.
Your doctor should do blood and urine tests to monitor your cholesterol, triglycerides and kidney function.
- viral infections. Certain viruses can live in your body and cause active infections when your immune system is weak. Viral infections that can happen with everolimus tablet include BK virus-associated nephropathy. BK virus can affect how your kidney works and cause your transplanted kidney to fail.
- blood clotting problems.
- diabetes. Tell your doctor if you have frequent urination, increased thirst or hunger.
-
infertility, male. Everolimus tablet can affect fertility in males and may affect your ability to father a child. Talk with your doctor if this is a concern for you.
-
infertility, female. Everolimus tablet can affect fertility in females and may affect your ability to become pregnant. Talk to your doctor if this is a concern for you.
The most common side effects of everolimus tablet in people who have had a kidney or liver transplant include:
These common side effects have been reported in both kidney and liver transplant patients:
- nausea
- swelling of the lower legs, ankles and feet
- high blood pressure
The most common side effects of everolimus tablet in people who have had a kidney transplant include:
- constipation
- low red blood cell count (anemia)
- urinary tract infection
- increased fat in the blood (cholesterol and triglycerides)
The most common side effects of everolimus tablet in people who have had a liver transplant include:
- diarrhea
- headache
- fever
- abdominal pain
- low white blood cells
These are not all of the possible side effects of everolimus tablet.
Call your doctor for medical advice about side effects. You may report side effects to the FDA at 1-800-FDA-1088.
How should I store everolimus tablets?
- Store everolimus tablets between 59°F to 86°F (15°C to 30°C).
- Everolimus tablets are packed in child-resistant blisters.
- Keep everolimus tablets out of the light.
- Keep everolimus tablets dry.
Keep Everolimus tablets and all medicines out of the reach of children. General information about the safe and effective use of Everolimus tablets.
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use everolimus tablet for a condition for which it was not prescribed. Do not give everolimus tablet to other people, even if they have the same symptoms you have. It may harm them.
You can ask your doctor or pharmacist for information about everolimus tablet that is written for healthcare professionals. For more information, call 1-800-828-9393 or visit www.parpharm.com
What are the ingredients in everolimus tablet? Active ingredient: everolimus
Inactive ingredients: butylated hydroxytoluene, lactose monohydrate, hypromellose, lactose anhydrous, crospovidone and magnesium stearate as inactive ingredients.
This Medication Guide has been approved by the U.S. Food and Drug Administration.
Any other trademarks in this document are the property of their respective owners.
Manufactured by:
Par Pharmaceutical
Chestnut Ridge, NY 10977
R09/2021
INGREDIENTS AND APPEARANCE
| EVEROLIMUS
everolimus tablet |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| EVEROLIMUS
everolimus tablet |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| EVEROLIMUS
everolimus tablet |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| EVEROLIMUS
everolimus tablet |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Par Pharmaceutical, Inc. (092733690) |