Search by Drug Name or NDC
NDC 49884-0425-11 ALISKIREN 300 mg/1 Details
ALISKIREN 300 mg/1
ALISKIREN is a ORAL TABLET, FILM COATED in the HUMAN PRESCRIPTION DRUG category. It is labeled and distributed by Par Pharmaceutical, Inc.. The primary component is ALISKIREN HEMIFUMARATE.
MedlinePlus Drug Summary
Aliskiren is used alone or in combination with some medications to treat high blood pressure. Aliskiren is in a class of medications called direct renin inhibitors. It works by decreasing certain natural chemicals that tighten the blood vessels, so blood vessels relax and the heart can pump blood more efficiently. High blood pressure is a common condition and when not treated, can cause damage to the brain, heart, blood vessels, kidneys and other parts of the body. Damage to these organs may cause heart disease, a heart attack, heart failure, stroke, kidney failure, loss of vision, and other problems. In addition to taking medication, making lifestyle changes will also help to control your blood pressure. These changes include eating a diet that is low in fat and salt, maintaining a healthy weight, exercising at least 30 minutes most days, not smoking, and using alcohol in moderation.
Related Packages: 49884-0425-11Last Updated: 11/30/2022
MedLinePlus Full Drug Details: Aliskiren
Product Information
| NDC | 49884-0425 |
|---|---|
| Product ID | 49884-425_c54a494b-ffd9-4502-81bb-96b3e0de7415 |
| Associated GPIs | 36170010100340 |
| GCN Sequence Number | 062288 |
| GCN Sequence Number Description | aliskiren hemifumarate TABLET 300 MG ORAL |
| HIC3 | A4T |
| HIC3 Description | RENIN INHIBITOR, DIRECT |
| GCN | 98076 |
| HICL Sequence Number | 034493 |
| HICL Sequence Number Description | ALISKIREN HEMIFUMARATE |
| Brand/Generic | Generic |
| Proprietary Name | ALISKIREN |
| Proprietary Name Suffix | n/a |
| Non-Proprietary Name | ALISKIREN |
| Product Type | HUMAN PRESCRIPTION DRUG |
| Dosage Form | TABLET, FILM COATED |
| Route | ORAL |
| Active Ingredient Strength | 300 |
| Active Ingredient Units | mg/1 |
| Substance Name | ALISKIREN HEMIFUMARATE |
| Labeler Name | Par Pharmaceutical, Inc. |
| Pharmaceutical Class | Renin Inhibitor [EPC], Renin Inhibitors [MoA] |
| DEA Schedule | n/a |
| Marketing Category | ANDA |
| Application Number | ANDA206665 |
| Listing Certified Through | 2024-12-31 |
Package
Package Images


NDC 49884-0425-11 (49884042511)
| NDC Package Code | 49884-425-11 |
|---|---|
| Billing NDC | 49884042511 |
| Package | 30 TABLET, FILM COATED in 1 BOTTLE (49884-425-11) |
| Marketing Start Date | 2019-03-25 |
| NDC Exclude Flag | N |
| Pricing Information | |
| Price Per Unit | 6.34367 |
| Pricing Unit | EA |
| Effective Date | 2024-02-21 |
| NDC Description | ALISKIREN 300 MG TABLET |
| Pharmacy Type Indicator | C/I |
| OTC | N |
| Explanation Code | 1 |
| Classification for Rate Setting | G |
| As of Date | 2024-02-21 |
Standard Product Labeling (SPL)/Prescribing Information SPL f3fd2503-25ef-4660-a29a-f62c491e28fb Details
HIGHLIGHTS OF PRESCRIBING INFORMATION
ALISKIREN tablets, for oral use
Initial U.S. Approval: 2007
RECENT MAJOR CHANGES
| None. |
INDICATIONS AND USAGE
Aliskiren tablets are a renin inhibitor (RI) indicated for:
- The treatment of hypertension in adults to lower blood pressure (1.1)
Lowering blood pressure reduces the risk of fatal and nonfatal cardiovascular events, primarily strokes and myocardial infarctions.
DOSAGE AND ADMINISTRATION
DOSAGE FORMS AND STRENGTHS
Tablets: 150 mg, 300 mg (3)
CONTRAINDICATIONS
WARNINGS AND PRECAUTIONS
- Avoid concomitant use with ARBs or ACEIs particularly in patients with renal impairment [creatinine clearance (CrCl) <60 mL/min]. (5.2, 5.4)
- Anaphylactic Reactions and Head and Neck Angioedema. (5.3)
- Hypotension: Correct imbalances in volume and/or salt depleted patients. (5.4)
- Impaired Renal Function: Monitor serum creatinine periodically. (5.5)
- Hyperkalemia: Monitor potassium levels periodically. (5.6)
ADVERSE REACTIONS
Most common adverse reaction: diarrhea (incidence 2.3%) (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Par Pharmaceutical at 1-800-828-9393 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS
USE IN SPECIFIC POPULATIONS
Lactation: Breastfeeding not recommended. (8.2)
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 3/2019
Table Of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
WARNING: FETAL TOXICITY
1 INDICATIONS AND USAGE
1.1 Hypertension
2 DOSAGE AND ADMINISTRATION
2.1 Adult Hypertension
2.4 Relationship to Meals
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Fetal Toxicity
5.2 Renal Impairment/Hyperkalemia/Hypotension when Aliskiren Tablets are Given in Combination with ARBs or ACEIs
5.3 Anaphylactic Reactions and Head and Neck Angioedema
5.4 Hypotension
5.5 Impaired Renal Function
5.6 Hyperkalemia
5.7 Cyclosporine or Itraconazole
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Postmarketing Experience
7 DRUG INTERACTIONS
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Renal Impairment
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
13.2 Animal Toxicology and/or Pharmacology
14 CLINICAL STUDIES
14.1 Aliskiren Monotherapy
14.2 Aliskiren in Combination with Other Antihypertensives
14.3 Aliskiren in Patients with Diabetes Treated with ARB or ACEI (ALTITUDE study)
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- *
- Sections or subsections omitted from the full prescribing information are not listed.
WARNING
1 INDICATIONS AND USAGE
1.1 Hypertension
Aliskiren tablets are indicated for the treatment of hypertension in adults to lower blood pressure. Lowering blood pressure reduces the risk of fatal and nonfatal cardiovascular events, primarily strokes and myocardial infarctions. These benefits have been seen in controlled trials of antihypertensive drugs from a wide variety of pharmacologic classes. There are no controlled trials demonstrating risk reduction with aliskiren tablets.
Control of high blood pressure should be part of comprehensive cardiovascular risk management, including, as appropriate, lipid control, diabetes management, antithrombotic therapy, smoking cessation, exercise, and limited sodium intake. Many patients will require more than 1 drug to achieve blood pressure goals. For specific advice on goals and management, see published guidelines, such as those of the National High Blood Pressure Education Program’s Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC).
Numerous antihypertensive drugs, from a variety of pharmacologic classes and with different mechanisms of action, have been shown in randomized controlled trials to reduce cardiovascular morbidity and mortality, and it can be concluded that it is blood pressure reduction, and not some other pharmacologic property of the drugs, that is largely responsible for those benefits. The largest and most consistent cardiovascular outcome benefit has been a reduction in the risk of stroke, but reductions in myocardial infarction and cardiovascular mortality have also been seen regularly.
Elevated systolic or diastolic pressure causes increased cardiovascular risk, and the absolute risk increase per mmHg is greater at higher blood pressures, so that even modest reductions of severe hypertension can provide substantial benefit. Relative risk reduction from blood pressure reduction is similar across populations with varying absolute risk, so the absolute benefit is greater in patients who are at higher risk independent of their hypertension (e.g., patients with diabetes or hyperlipidemia), and such patients would be expected to benefit from more aggressive treatment to a lower blood pressure goal.
Some antihypertensive drugs have smaller blood pressure effects (as monotherapy) in black patients, and many antihypertensive drugs have additional approved indications and effects (e.g., on angina, heart failure, or diabetic kidney disease). These considerations may guide selection of therapy.
2 DOSAGE AND ADMINISTRATION
2.1 Adult Hypertension
The usual recommended starting dose of aliskiren tablets is 150 mg once daily. In patients whose blood pressure is not adequately controlled, the daily dose may be increased to 300 mg. Doses above 300 mg did not give an increased blood pressure response but resulted in an increased rate of diarrhea. The antihypertensive effect of a given dose is substantially attained (85% to 90%) by 2 weeks.
2.4 Relationship to Meals
Patients should establish a routine pattern for taking aliskiren tablets with regard to meals. High-fat meals decrease absorption substantially [see Clinical Pharmacology (12.3)].
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
Do not use aliskiren with ARBs or ACEIs in patients with diabetes [see Warnings and Precautions (5.2) and Clinical Studies (14.3)].
Aliskiren tablets are contraindicated in patients with known hypersensitivity to any of the components [see Warnings and Precautions (5.3)].
5 WARNINGS AND PRECAUTIONS
5.1 Fetal Toxicity
Use of drugs that act on the renin-angiotensin system during the second and third trimesters of pregnancy reduces fetal renal function and increases fetal and neonatal morbidity and death. Resulting oligohydramnios can be associated with fetal lung hypoplasia and skeletal deformations. Potential neonatal adverse effects include skull hypoplasia, anuria, hypotension, renal failure, and death. When pregnancy is detected, discontinue aliskiren tablets as soon as possible [see Use in Specific Populations (8.1)].
5.2 Renal Impairment/Hyperkalemia/Hypotension when Aliskiren Tablets are Given in Combination with ARBs or ACEIs
Aliskiren tablets are contraindicated in patients with diabetes who are receiving ARBs or ACEIs because of the increased risk of renal impairment, hyperkalemia, and hypotension. In general, avoid combined use of aliskiren with ACE inhibitors or ARBs, particularly in patients with creatinine clearance (CrCl) less than 60 mL/min [see Contraindications (4), Drug Interactions (7) and Clinical Studies (14.3)].
5.3 Anaphylactic Reactions and Head and Neck Angioedema
Hypersensitivity reactions such as anaphylactic reactions and angioedema of the face, extremities, lips, tongue, glottis and/or larynx have been reported in patients treated with aliskiren tablets and has necessitated hospitalization and intubation. This may occur at any time during treatment and has occurred in patients with and without a history of angioedema with ACEIs or angiotensin receptor antagonists. Anaphylactic reactions have been reported from postmarketing experience with unknown frequency. If angioedema involves the throat, tongue, glottis or larynx, or if the patient has a history of upper respiratory surgery, airway obstruction may occur and be fatal. Patients who experience these effects, even without respiratory distress, require prolonged observation and appropriate monitoring measures since treatment with antihistamines and corticosteroids may not be sufficient to prevent respiratory involvement. Prompt administration of subcutaneous epinephrine solution 1:1000 (0.3 mL to 0.5 mL) and measures to ensure a patent airway may be necessary.
Discontinue aliskiren tablets immediately in patients who develop anaphylactic reactions or angioedema, and do not readminister [see Dosage and Administration (2.1) and Contraindications (4)].
5.4 Hypotension
Symptomatic hypotension may occur after initiation of treatment with aliskiren tablets in patients with marked volume depletion, patients with salt depletion, or with combined use of aliskiren and other agents acting on the renin-angiotensin-aldosterone system (RAAS). The volume or salt depletion should be corrected prior to administration of aliskiren tablets, or the treatment should start under close medical supervision.
A transient hypotensive response is not a contraindication to further treatment, which usually can be continued without difficulty once the blood pressure has stabilized.
5.5 Impaired Renal Function
Monitor renal function periodically in patients treated with aliskiren tablets. Changes in renal function, including acute renal failure, can be caused by drugs that affect the RAAS. Patients whose renal function may depend in part on the activity of the RAAS (e.g., patients with renal artery stenosis, severe heart failure, post-myocardial infarction or volume depletion) or patients receiving ARB, ACEI or nonsteroidal anti-inflammatory drug (NSAID), including selective Cyclooxygenase-2 inhibitors (COX-2 inhibitors), therapy may be at particular risk for developing acute renal failure on aliskiren tablets [see Warnings and Precautions (5.2), Drug Interactions (7), Use in Specific Populations (8.6), and Clinical Studies (14.3)]. Consider withholding or discontinuing therapy in patients who develop a clinically significant decrease in renal function.
5.6 Hyperkalemia
Monitor serum potassium periodically in patients receiving aliskiren tablets. Drugs that affect the RAAS can cause hyperkalemia. Risk factors for the development of hyperkalemia include renal insufficiency, diabetes, combination use with ARBs or ACEIs [see Contraindications (4), Warnings and Precautions (5.2), and Clinical Studies (14.3)], NSAIDs, or potassium supplements or potassium sparing diuretics.
5.7 Cyclosporine or Itraconazole
When aliskiren was given with cyclosporine or itraconazole, the blood concentrations of aliskiren were significantly increased. Avoid concomitant use of aliskiren with cyclosporine or itraconazole [see Drug Interactions (7)].
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
The following serious adverse reactions are discussed in greater detail in other sections of the label:
- Fetal Toxicity [see Warnings and Precautions (5.1)]
- Anaphylactic Reactions and Head and Neck Angioedema [see Warnings and Precautions (5.3)]
- Hypotension [see Warnings and Precautions (5.4)]
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in clinical trials of another drug and may not reflect the rates observed in practice.
Adult Hypertension
Data described below reflect the evaluation of the safety of aliskiren tablets in more than 6,460 patients, including over 1,740 treated for longer than 6 months, and more than 1,250 patients for longer than 1 year. In placebo-controlled clinical trials, discontinuation of therapy due to a clinical adverse event, including uncontrolled hypertension, occurred in 2.2% of patients treated with aliskiren tablets versus 3.5% of patients given placebo. These data do not include information from the ALTITUDE study which evaluated the use of aliskiren in combination with ARBs or ACEIs [see Contraindications (4), Warnings and Precautions (5.2), and Clinical Studies (14.3)].
Angioedema: Two cases of angioedema with respiratory symptoms were reported with aliskiren tablets use in the clinical studies. Two other cases of periorbital edema without respiratory symptoms were reported as possible angioedema and resulted in discontinuation. The rate of these angioedema cases in the completed studies was 0.06%.
In addition, 26 other cases of edema involving the face, hands, or whole body were reported with aliskiren tablets use including 4 leading to discontinuation.
In the placebo-controlled studies, however, the incidence of edema involving the face, hands, or whole body was 0.4% with aliskiren tablets compared with 0.5% with placebo. In a long-term active-control study with aliskiren tablets and hydrochlorothiazide (HCTZ) arms, the incidence of edema involving the face, hand or whole body was 0.4% in both treatment arms [see Warnings and Precautions (5.2)].
Gastrointestinal: Aliskiren tablets produces dose-related gastrointestinal (GI) adverse reactions. Diarrhea was reported by 2.3% of patients at 300 mg, compared to 1.2% in placebo patients. In women and the elderly (age 65 years and older) increases in diarrhea rates were evident starting at a dose of 150 mg daily, with rates for these subgroups at 150 mg comparable to those seen at 300 mg for men or younger patients (all rates about 2.0% to 2.3%). Other GI symptoms included abdominal pain, dyspepsia, and gastroesophageal reflux, although increased rates for abdominal pain and dyspepsia were distinguished from placebo only at 600 mg daily. Diarrhea and other GI symptoms were typically mild and rarely led to discontinuation.
Cough: Aliskiren tablets was associated with a slight increase in cough in the placebo-controlled studies (1.1% for any aliskiren tablets use versus 0.6% for placebo). In active-controlled trials with ACE inhibitor (ramipril, lisinopril) arms, the rates of cough for the aliskiren tablets arms were about one-third to one-half the rates in the ACE inhibitor arms.
Seizures: Single episodes of tonic-clonic seizures with loss of consciousness were reported in 2 patients treated with aliskiren tablets in the clinical trials. One of these patients did have predisposing causes for seizures and had a negative electroencephalogram (EEG) and cerebral imaging following the seizures (for the other patient EEG and imaging results were not reported). Aliskiren tablets was discontinued and there was no rechallenge.
Other adverse effects with increased rates for aliskiren tablets compared to placebo included rash (1% versus 0.3%), elevated uric acid (0.4% versus 0.1%), gout (0.2% versus 0.1%) and renal stones (0.2% versus 0%).
Aliskiren’s effect on ECG intervals was studied in a randomized, double-blind, placebo and active-controlled (moxifloxacin), 7-day repeat dosing study with Holter-monitoring and 12 lead ECGs throughout the interdosing interval. No effect of aliskiren on QT interval was seen.
Clinical Laboratory Findings
In controlled clinical trials, clinically relevant changes in standard laboratory parameters were rarely associated with the administration of aliskiren tablets in patients with hypertension not concomitantly treated with an ARB or ACEI. In multiple-dose studies in hypertensive patients, aliskiren tablets had no clinically important effects on total cholesterol, HDL, fasting triglycerides, or fasting glucose.
Blood Urea Nitrogen, Creatinine: In patients with hypertension not concomitantly treated with an ARB or ACEI, minor increases in blood urea nitrogen (BUN) or serum creatinine were observed in less than 7% of patients treated with aliskiren tablets alone versus 6% on placebo [see Warnings and Precautions (5.2)].
Hemoglobin and Hematocrit: Small decreases in hemoglobin and hematocrit (mean decreases of approximately 0.08 g/dL and 0.16 volume percent, respectively, for all aliskiren monotherapy) were observed. The decreases were dose-related and were 0.24 g/dL and 0.79 volume percent for 600 mg daily. This effect is also seen with other agents acting on the renin angiotensin system, such as angiotensin inhibitors and ARBs, and may be mediated by reduction of angiotensin II which stimulates erythropoietin production via the AT1 receptor. These decreases led to slight increases in rates of anemia with aliskiren compared to placebo were observed (0.1% for any aliskiren use, 0.3% for aliskiren 600 mg daily, versus 0% for placebo). No patients discontinued therapy due to anemia.
Serum Potassium: In patients with hypertension not concomitantly treated with an ARB or ACEI, increases in serum potassium greater than 5.5 mEq/L were infrequent (0.9% compared to 0.6% with placebo) [see Contraindications (4) and Warnings and Precautions (5.6)].
Serum Uric Acid: Aliskiren monotherapy produced small median increases in serum uric acid levels (about 6 micromol/L) while HCTZ produced larger increases (about 30 micromol/L). The combination of aliskiren with HCTZ appears to be additive (about 40 micromol/L increase). The increases in uric acid appear to lead to slight increases in uric acid-related AEs: elevated uric acid (0.4% versus 0.1%), gout (0.2% versus. 0.1%), and renal stones (0.2% versus 0%).
Creatine Kinase: Increases in creatine kinase of greater than 300% were recorded in about 1% of aliskiren monotherapy patients versus 0.5% of placebo patients. Five cases of creatine kinase rises, 3 leading to discontinuation and 1 diagnosed as subclinical rhabdomyolysis, and another as myositis, were reported as adverse events with aliskiren use in the clinical trials. No cases were associated with renal dysfunction.
6.2 Postmarketing Experience
The following adverse reactions have been reported in aliskiren postmarketing experience. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to estimate their frequency or establish a causal relationship to drug exposure.
Hypersensitivity: anaphylactic reactions and angioedema requiring airway management and hospitalization
Urticaria
Peripheral edema
Hepatic enzyme increase with clinical symptoms of hepatic dysfunction
Severe cutaneous adverse reactions, including Stevens-Johnson syndrome and toxic epidermal necrolysis
Pruritus
Erythema
Hyponatremia
Nausea, Vomiting
7 DRUG INTERACTIONS
Cyclosporine: Avoid coadministration of cyclosporine with aliskiren [see Warnings and Precautions (5.7) and Clinical Pharmacology (12.3)].
Itraconazole: Avoid coadministration of itraconazole with aliskiren [see Warnings and Precautions (5.7) and Clinical Pharmacology (12.3)].
Nonsteroidal Anti-Inflammatory Drugs (NSAIDs) including selective Cyclooxygenase-2 inhibitors (COX-2 inhibitors): In patients who are elderly, volume-depleted (including those on diuretic therapy), or with compromised renal function, coadministration of NSAIDs, including selective COX-2 inhibitors with agents that affect the RAAS, including aliskiren, may result in deterioration of renal function, including possible acute renal failure. These effects are usually reversible. Monitor renal function periodically in patients receiving aliskiren and NSAID therapy.
The antihypertensive effect of aliskiren may be attenuated by NSAIDs.
Dual Blockade of the Renin-Angiotensin-Aldosterone System (RAAS): The concomitant use of aliskiren with other agents acting on the RAAS such as ACEIs or ARBs is associated with an increased risk of hypotension, hyperkalemia, and changes in renal function (including acute renal failure) compared to monotherapy. Most patients receiving the combination of two drugs that inhibit the renin-angiotensin system do not obtain any additional benefit compared to monotherapy. In general, avoid combined use of aliskiren with ACE inhibitors or ARBs, particularly in patients with CrCl less than 60 mL/min. Monitor blood pressure, renal function, and electrolytes in patients taking aliskiren and other agents that affect the RAAS [see Warnings and Precautions (5.4, 5.5, 5.6)].
The concomitant use of aliskiren with an ARB or an ACEI in diabetic patients is contraindicated [see Contraindications (4)].
Furosemide: Oral coadministration of aliskiren and furosemide reduced exposure to furosemide. Monitor diuretic effects when furosemide is coadministered with aliskiren.
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
Aliskiren can cause fetal harm when administered to a pregnant woman. Use of drugs that act on the renin-angiotensin system during the second and third trimesters of pregnancy reduces fetal renal function and increases fetal and neonatal morbidity and death [see Clinical Considerations]. Most epidemiologic studies examining fetal abnormalities after exposure to antihypertensive use in the first trimester have not distinguished drugs affecting the renin-angiotensin system from other antihypertensive agents. When pregnancy is detected, discontinue aliskiren as soon as possible.
The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major malformations and miscarriage in clinically recognized pregnancies is 2 to 4%, and 15 to 20%, respectively.
Clinical Considerations
Disease-associated maternal and/or embryo/fetal risk
Hypertension in pregnancy increases the maternal risk for pre-eclampsia, gestational diabetes, premature delivery, and delivery complications (e.g., need for cesarean section, and post-partum hemorrhage). Hypertension increases the fetal risk for intrauterine growth restriction and intrauterine death. Pregnant women with hypertension should be carefully monitored and managed accordingly.
Fetal/Neonatal adverse reactions
Use of drugs that act on the renin-angiotensin system in the second and third trimesters of pregnancy can result in the following: reduced fetal renal function leading to anuria and renal failure, oligohydramnios, fetal lung hypoplasia and skeletal deformations, including skull hypoplasia, hypotension, and death. In the unusual case that there is no appropriate alternative to therapy with drugs affecting the renin-angiotensin system for a particular patient, apprise the mother of the potential risk to the fetus.
In patients taking aliskiren during pregnancy, perform serial ultrasound examinations to assess the intra- amniotic environment. Fetal testing may be appropriate, based on the week of gestation. Patients and physicians should be aware, however, that oligohydramnios may not appear until after the fetus has sustained irreversible injury. Closely observe infants with histories of in utero exposure to aliskiren for hypotension, oliguria, and hyperkalemia. If oliguria or hypotension occur in neonates with a history of in utero exposure to aliskiren, support blood pressure and renal perfusion. Exchange transfusions or dialysis may be required as a means of reversing hypotension and substituting for disordered renal function.
Data
Animal Data
In developmental toxicity studies, pregnant rats and rabbits received oral aliskiren hemifumarate during organogenesis at doses up to 20 and 7 times the maximum recommended human dose (MRHD) based on body surface area (mg/m2), respectively, in rats and rabbits. (Actual animal doses were up to 600 mg/kg/day in rats and up to 100 mg/kg/day in rabbits.) No teratogenicity was observed; however, fetal birth weight was decreased in rabbits at doses 3.2 times the MRHD based on body surface area (mg/m2). Aliskiren was present in placentas, amniotic fluid and fetuses of pregnant rabbits.
8.2 Lactation
There is no information regarding the presence of aliskiren in human milk, the effects on the breastfed infant, or the effects on milk production. Because of the potential for serious adverse reactions, including hypotension, hyperkalemia and renal impairment in nursing infants, advise a nursing woman that breastfeeding is not recommended during treatment with aliskiren tablets.
8.4 Pediatric Use
Safety and effectiveness have not been established in pediatric patients younger than 18 years old.
Preclinical studies indicate a potential for substantial increase in exposure to aliskiren in pediatric patients [see Nonclinical Toxicology (13.2)].
Neonates with a history of in utero exposure to aliskiren tablets
If oliguria or hypotension occurs, direct attention toward support of blood pressure and renal perfusion. Exchange transfusions or dialysis may be required as a means of reversing hypotension and/or substituting for disordered renal function.
8.5 Geriatric Use
Of the total number of patients receiving aliskiren in clinical studies, 1,275 (19%) were 65 years or older and 231 (3.4%) were 75 years or older. No overall differences in safety or effectiveness were observed between these subjects and younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients, but greater sensitivity of some older individuals cannot be ruled out.
8.6 Renal Impairment
Safety and effectiveness of aliskiren tablets in patients with severe renal impairment [creatinine clearance (CrCl) less than 30 mL/min] have not been established as these patients were excluded in clinical trials [see Clinical Studies (14)].
10 OVERDOSAGE
Limited data are available related to overdosage in humans. The most likely manifestation of overdosage would be hypotension. If symptomatic hypotension occurs, supportive treatment should be initiated.
Aliskiren is poorly dialyzed. Therefore, hemodialysis is not adequate to treat aliskiren overexposure [see Clinical Pharmacology (12.3)].
11 DESCRIPTION
Aliskiren tablets contain aliskiren hemifumarate, adirect renin inhibitor. Aliskiren hemifumarate is chemically described as (2S,4S,5S,7S)-N-(2-carbamoyl-2-methylpropyl)-5-amino-4-hydroxy-2,7-diisopropyl-8-[4-methoxy-3-(3-methoxypropoxy)phenyl]-octanamide hemifumarate and its structural formula is:
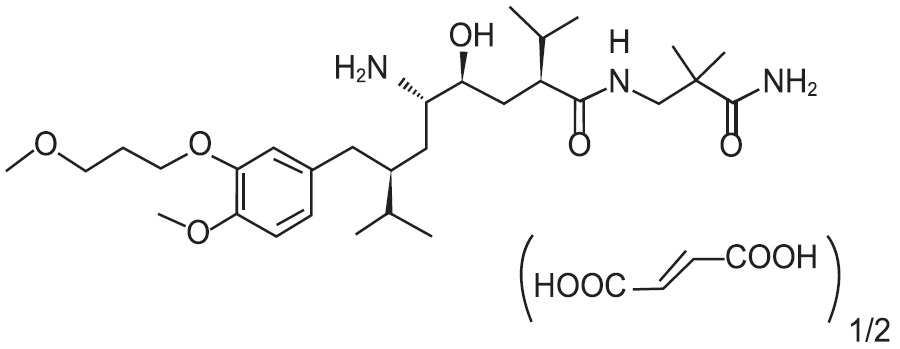
Molecular formula: C30H53N3O6 • 0.5 C4H4O4
Aliskiren hemifumarate is a white to slightly yellowish crystalline powder with a molecular weight of 609.8 (free base 551.8). It is soluble in phosphate buffer, n-octanol, and highly soluble in water.
Aliskiren is available as film-coated tablets, which contains 165.765 mg or 331.530 mg aliskiren hemifumarate (equivalent to 150 mg or 300 mg aliskiren) and the following excipients: calcium stearate, colloidal silicon dioxide, crospovidone, hypromellose, iron oxide red, microcrystalline cellulose, polyethylene glycol, talc and titanium dioxide. In addition, the 150 mg strength contains iron oxide black.
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Renin is secreted by the kidney in response to decreases in blood volume and renal perfusion. Renin cleaves angiotensinogen to form the inactive decapeptide angiotensin I (Ang I). Ang I is converted to the active octapeptide angiotensin II (Ang II) by ACE and non-ACE pathways. Ang II is a powerful vasoconstrictor and leads to the release of catecholamines from the adrenal medulla and prejunctional nerve endings. It also promotes aldosterone secretion and sodium reabsorption. Together, these effects increase blood pressure. Ang II also inhibits renin release, thus providing a negative feedback to the system. This cycle, from renin through angiotensin to aldosterone and its associated negative feedback loop, is known as the renin-angiotensin-aldosterone system (RAAS). Aliskiren is a direct renin inhibitor, decreasing plasma renin activity (PRA) and inhibiting the conversion of angiotensinogen to Ang I. Whether aliskiren affects other RAAS components, e.g., ACE or non-ACE pathways, is not known.
All agents that inhibit the RAAS, including renin inhibitors, suppress the negative feedback loop, leading to a compensatory rise in plasma renin concentration. When this rise occurs during treatment with ACEIs and ARBs, the result is increased levels of PRA. During treatment with aliskiren, however, the effect of increased renin levels is blocked so that PRA, Ang I and Ang II are all reduced, whether aliskiren is used as monotherapy or in combination with other antihypertensive agents.
12.2 Pharmacodynamics
In placebo-controlled clinical trials, PRA was decreased in a range of 50% to 80%. This reduction in PRA was not dose-related and did not correlate with blood pressure reductions. The clinical implications of the differences in effect on PRA are not known.
12.3 Pharmacokinetics
Aliskiren is poorly absorbed (bioavailability about 2.5%) with an approximate accumulation half-life of 24 hours. Steady state blood levels are reached in about 7 to 8 days.
Absorption and Distribution
Following oral administration, peak plasma concentrations of aliskiren are reached within 1 to 3 hours. When taken with a high-fat meal, mean AUC and Cmax of aliskiren are decreased by 71% and 85% respectively. In the clinical trials of aliskiren, it was administered without requiring a fixed relation of administration to meals.
Metabolism and Elimination
About one-fourth of the absorbed dose appears in the urine as parent drug. How much of the absorbed dose is metabolized is unknown. Based on the in vitro studies, the major enzyme responsible for aliskiren metabolism appears to be CYP3A4. Aliskiren does not inhibit the CYP450 isoenzymes (CYP 1A2, 2C8, 2C9, 2C19, 2D6, 2E1, and 3A) or induce CYP3A4.
Transporters: Pgp (MDR1/Mdr1a/1b) was found to be the major efflux system involved in intestinal absorption and elimination via biliary excretion of aliskiren in preclinical studies. The potential for drug interactions at the Pgp site will likely depend on the degree of inhibition of this transporter.
Drug Interactions
The effect of coadministered drugs on the pharmacokinetics of aliskiren and vice versa, were studied in several single- and multiple-dose studies. Pharmacokinetic measures indicating the magnitude of these interactions are presented in Figure 1 (impact of coadministered drugs on aliskiren) and Figure 2 (impact of aliskiren on coadministered drugs).
Figure 1: The Impact of Coadministered Drugs on the Pharmacokinetics of Aliskiren

*Ketoconazole: A 400 mg once daily dose was not studied, but would be expected to increase aliskiren blood levels further.
**Ramipril, valsartan, irbesartan: In general, avoid combined use of aliskiren with ACE inhibitors or ARBs, particularly in patients with CrCl less than 60 mL/min [see Drug Interactions (7)].
Warfarin: There was no clinically significant effect of a single dose of warfarin 25 mg on the pharmacokinetics of aliskiren.
Figure 2: The Impact of Aliskiren on the Pharmacokinetics of Coadministered Drugs

*Furosemide: Patients receiving furosemide could find its effects diminished after starting aliskiren. In patients with heart failure, coadministration of aliskiren (300 mg/day) reduced plasma AUC and Cmax of oral furosemide (60 mg/day) by 17% and 27%, respectively, and reduced 24-hour urinary furosemide excretion by 29%. This change in exposure did not result in statistically significant difference in total urine volume and urinary sodium excretion over 24 hours. However, a transient decrease in urinary sodium excretion and urine volume effects up to 12 hours were observed when furosemide was coadministered with aliskiren 300 mg/day.
**Ramipril, valsartan: In general, avoid combined use of aliskiren with ACE inhibitors or ARBs, particularly in patients with CrCl less than 60 mL/min [see Drug Interactions (7)].
Special Populations
Renally Impaired Patients: Aliskiren was evaluated in patients with varying degrees of renal insufficiency. The rate and extent of exposure (AUC and Cmax) of aliskiren in subjects with renal impairment did not show a consistent correlation with the severity of renal impairment. Adjustment of the starting dose is not required in these patients [see Warnings and Precautions (5.2)].
The pharmacokinetics of aliskiren following administration of a single oral dose of 300 mg was evaluated in patients with End Stage Renal Disease (ESRD) undergoing hemodialysis. When compared to matched healthy subjects, changes in the rate and extent of aliskiren exposure (Cmax and AUC) in ESRD patients undergoing hemodialysis were not clinically significant.
Timing of hemodialysis did not significantly alter the pharmacokinetics of aliskiren in ESRD patients. Therefore, no dose adjustment is warranted in ESRD patients receiving hemodialysis.
Hepatically Impaired Patients: The pharmacokinetics of aliskiren were not significantly affected in patients with mild to severe liver disease. Consequently, adjustment of the starting dose is not required in these patients.
Geriatric Patients: Exposure (measured by AUC) is increased in elderly patients 65 years and older. Adjustment of the starting dose is not required in these patients.
Race: The pharmacokinetic differences between blacks, Caucasians, and Japanese are minimal.
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenic potential was assessed in a 2-year rat study and a 6-month transgenic (rasH2) mouse study with aliskiren hemifumarate at oral doses of up to 1500 mg aliskiren/kg/day. Although there were no statistically significant increases in tumor incidence associated with exposure to aliskiren, mucosal epithelial hyperplasia (with or without erosion/ulceration) was observed in the lower gastrointestinal tract at doses of greater than or equal to 750 mg/kg/day in both species, with a colonic adenoma identified in 1 rat and a cecal adenocarcinoma identified in another, rare tumors in the strain of rat studied. On a systemic exposure (AUC0-24hr) basis, 1500 mg/kg/day in the rat is about 4 times and in the mouse about 1.5 times the maximum recommended human dose (MRHD) (300 mg aliskiren/day). Mucosal hyperplasia in the cecum or colon of rats was also observed at doses of 250 mg/kg/day (the lowest tested dose) as well as at higher doses in 4- and 13-week studies.
Aliskiren hemifumarate was devoid of genotoxic potential in the Ames reverse mutation assay with S. typhimurium and E. coli, the in vitro Chinese hamster ovary cell chromosomal aberration assay, the in vitro Chinese hamster V79 cell gene mutation test and the in vivo mouse bone marrow micronucleus assay.
Fertility of male and female rats was unaffected at doses of up to 250 mg aliskiren/kg/day (8 times the MRHD of 300 mg aliskiren tablets/60 kg on a mg/m2 basis.)
13.2 Animal Toxicology and/or Pharmacology
Reproductive Toxicology Studies: Reproductive toxicity studies of aliskiren hemifumarate did not reveal any evidence of teratogenicity at oral doses up to 600 mg aliskiren/kg/day (20 times the MRHD of 300 mg/day on a mg/m2 basis) in pregnant rats or up to 100 mg aliskiren/kg/day (7 times the MRHD on a mg/m2 basis) in pregnant rabbits. Fetal birth weight was adversely affected in rabbits at 50 mg/kg/day (3.2 times the MRHD on a mg/m2 basis). Aliskiren was present in placenta, amniotic fluid and fetuses of pregnant rabbits.
Juvenile Animal Studies: Juvenile toxicity studies indicated increased systemic exposure to aliskiren 85- to 385-fold in 14-day and 8-day old rats respectively, compared with adult rats. The mdr1 gene expression in juvenile rats was also significantly lower when compared to adult rats. The increased aliskiren exposure in juvenile rats appears to be mainly attributed to lack of maturation of P-gp. The overexposure in juvenile rats was associated with high mortality. [see Use in Specific Populations (8.4)].
14 CLINICAL STUDIES
14.1 Aliskiren Monotherapy
The antihypertensive effects of aliskiren tablets have been demonstrated in 6 randomized, double-blind, placebo-controlled 8-week clinical trials in patients with mild-to-moderate hypertension. The placebo response and placebo-subtracted changes from baseline in seated trough cuff blood pressure are shown in Table 2.
Table 2: Reductions in Seated Trough Cuff Blood Pressure (mmHg systolic/diastolic) in the Placebo-Controlled Studies
|
Aliskiren daily dose, mg |
|||||
|
Study |
Placebo mean change |
75 |
150 |
300 |
600 |
|
Placebo-subtracted |
Placebo-subtracted |
Placebo-subtracted |
Placebo-subtracted |
||
|
1 |
2.9/3.3 |
5.7/4* |
5.9/4.5* |
11.2/7.5* |
-- |
|
2 |
5.3/6.3 |
-- |
6.1/2.9* |
10.5/5.4* |
10.4/5.2* |
|
3 |
10/8.6 |
2.2/1.7 |
2.1/1.7 |
5.1/3.7* |
-- |
|
4 |
7.5/6.9 |
1.9/1.8 |
4.8/2* |
8.3/3.3* |
-- |
|
5 |
3.8/4.9 |
-- |
9.3/5.4* |
10.9/6.2* |
12.1/7.6* |
|
6 |
4.6/4.1 |
-- |
-- |
8.4/4.9† |
-- |
*p value less than 0.05 versus placebo by ANCOVA with Dunnett’s procedure for multiple comparisons
†p value less than 0.05 versus placebo by ANCOVA for the pairwise comparison.
The studies included approximately 2,730 patients given doses of 75 to 600 mg of aliskiren and 1,231 patients given placebo. As shown, there is some increase in response with administered dose in all studies, with reasonable effects seen at 150 mg to 300 mg, and no clear further increases at 600 mg. A substantial proportion (85% to 90%) of the blood pressure-lowering effect was observed within 2 weeks of treatment. Studies with ambulatory blood pressure monitoring showed reasonable control throughout the interdosing interval; the ratios of mean daytime to mean nighttime ambulatory BP range from 0.6 to 0.9.
Patients in the placebo-controlled trials continued open-label aliskiren for up to 1 year. A persistent blood pressure-lowering effect was demonstrated by a randomized withdrawal study (patients randomized to continue drug or placebo), which showed a statistically significant difference between patients kept on aliskiren and those randomized to placebo. With cessation of treatment, blood pressure gradually returned toward baseline levels over a period of several weeks. There was no evidence of rebound hypertension after abrupt cessation of therapy.
Aliskiren lowered blood pressure in all demographic subgroups, although black patients tended to have smaller reduction than Caucasians and Asians, as has been seen with ACEIs and ARBs.
There are no studies of aliskiren tablets or members of the direct renin inhibitors demonstrating reductions in cardiovascular risk in patients with hypertension.
14.2 Aliskiren in Combination with Other Antihypertensives
Hydrochlorothiazide (HCTZ)
Aliskiren 75, 150, and 300 mg and HCTZ 6.25, 12.5, and 25 mg were studied alone and in combination in an 8-week, 2,776-patient, randomized, double-blind, placebo-controlled, parallel-group, 15-arm factorial study. Blood pressure reductions with the combinations were greater than the reductions with the monotherapies as shown in Table 3.
Table 3: Placebo-Subtracted Reductions in Seated Trough Cuff Blood Pressure (mmHg systolic/diastolic) in Combination with Hydrochlorothiazide
|
Hydrochlorothiazide, mg |
|||||
|
Aliskiren, mg |
Placebo mean change |
0 |
6.25 |
12.5 |
25 |
|
Placebo-subtracted |
Placebo-subtracted |
Placebo-subtracted |
Placebo-subtracted |
||
|
0 |
7.5/6.9 |
-- |
3.5/2.1 |
6.4/3.2 |
6.8/2.4 |
|
75 |
-- |
1.9/1.8 |
6.8/3.8 |
8.2/4.2 |
9.8/4.5 |
|
150 |
-- |
4.8/2 |
7.8/3.4 |
10.1/5 |
12/5.7 |
|
300 |
-- |
8.3/3.3 |
-- |
12.3/7 |
13.7/7.3 |
Valsartan
Aliskiren 150 mg and 300 mg and valsartan 160 mg and 320 mg were studied alone and in combination in an 8-week, 1,797-patient, randomized, double-blind, placebo-controlled, parallel-group, 4-arm, dose-escalation study. The dosages of aliskiren and valsartan were started at 150 mg and 160 mg, respectively, and increased at 4 weeks to 300 mg and 320 mg, respectively. Seated trough cuff blood pressure was measured at baseline, 4, and 8 weeks. Blood pressure reductions with the combinations were greater than the reductions with the monotherapies as shown in Table 4. In general, the combination of aliskiren and angiotensin receptor blocker should be avoided [see Contraindications (4), Warnings and Precautions (5), and Drug Interactions (7)].
Table 4: Placebo-Subtracted Reductions in Seated Trough Cuff Blood Pressure (mmHg systolic/diastolic) in Combination with Valsartan
|
Aliskiren, mg |
Placebo mean change |
Valsartan, mg |
||
|
0 |
160 |
320 |
||
|
0 |
4.6/4.1* |
-- |
5.6/3.9 |
8.2/5.6 |
|
150 |
-- |
5.4/2.7 |
10.0/5.7 |
-- |
|
300 |
-- |
8.4/4.9 |
-- |
12.6/8.1 |
* The placebo change is 5.2/4.8 for week 4 endpoint which was used for the dose groups containing aliskiren 150 mg or valsartan 160 mg.
Amlodipine
Aliskiren 150 mg and 300 mg and amlodipine besylate 5 mg and 10 mg were studied alone and in combination in an 8-week, 1,685-patient, randomized, double-blind, placebo-controlled, multifactorial study. Treatment with aliskiren and amlodipine resulted overall in significantly greater reductions in diastolic and systolic blood pressure compared to the respective monotherapy components as shown in Table 5.
Table 5: Placebo-Subtracted Reductions in Seated Trough Cuff Blood Pressure (mmHg systolic/diastolic) in Combination with Amlodipine
|
Aliskiren, mg |
Placebo mean change |
Amlodipine, mg |
||
|
0 |
5 |
10 |
||
|
0 |
6.8/5.4 |
-- |
9.0/5.6 |
14.3/8.5 |
|
150 |
-- |
3.9/2.6 |
13.9/8.6 |
17.1/10.8 |
|
300 |
-- |
8.6/4.9 |
15.0/9.6 |
16.4/11.1 |
14.3 Aliskiren in Patients with Diabetes Treated with ARB or ACEI (ALTITUDE study)
Patients with diabetes with renal disease (defined either by the presence of albuminuria or reduced GFR) were randomized to aliskiren 300 mg daily (n=4296) or placebo (n=4310). All patients were receiving background therapy with an ARB or ACEI. The primary efficacy outcome was the time to the first event of the primary composite endpoint consisting of cardiovascular death, resuscitated sudden death, nonfatal myocardial infarction, nonfatal stroke, unplanned hospitalization for heart failure, onset of end stage renal disease, renal death, and doubling of serum creatinine concentration from baseline sustained for at least 1 month. After a median follow up of about 32 months, the trial was terminated early for lack of efficacy. Higher risk of renal impairment, hypotension and hyperkalemia was observed in aliskiren compared to placebo-treated patients, as shown in Table 6.
Table 6: Incidence of Selected Adverse Events During the Treatment Phase in ALTITUDE
|
Aliskiren N=4272 |
Placebo N=4285 |
|||
|
Serious Adverse Events* (%) |
Adverse Events (%) |
Serious Adverse Events* (%) |
Adverse Events (%) |
|
|
Renal impairment † |
5.7 |
14.5 |
4.3 |
12.4 |
|
Hypotension †† |
2.3 |
19.9 |
1.9 |
16.3 |
|
Hyperkalemia ††† |
1.0 |
38.9 |
0.5 |
28.8 |
†renal failure, renal failure acute, renal failure chronic, renal impairment
††dizziness, dizziness postural, hypotension, orthostatic hypotension, presyncope, syncope
††† Given the variable baseline potassium levels of patients with renal insufficiency on dual RAAS therapy, the reporting of adverse event of hyperkalemia was at the discretion of the investigator.
* A Serious Adverse Event (SAE) is defined as: an event which is fatal or life-threatening, results in persistent or significant disability/incapacity, constitutes a congenital anomaly/birth defect, requires inpatient hospitalization or prolongation of existing hospitalization, or is medically significant (i.e., defined as an event that jeopardizes the patient or may require medical or surgical intervention to prevent one of the outcomes previously listed).
The risk of stroke (3.4% aliskiren versus 2.7% placebo) and death (8.4% aliskiren versus 8.0% placebo) were also numerically higher in aliskiren treated patients.
16 HOW SUPPLIED/STORAGE AND HANDLING
Aliskiren tablets are supplied as a pink colored, film-coated, biconvex round tablet containing 150 mg of aliskiren. Tablet is debossed with “E” on one side and “590” on the other side.
Aliskiren tablets are supplied as a brown colored, film-coated, ovaloid shaped biconvex tablet containing 300 mg of aliskiren. Tablet is debossed with “E” on one side and “591” on the other side.
All strengths are packaged in bottles as described below.
150 mg tablets: Bottles of 30 (NDC 49884-424-11)
300 mg tablets: Bottles of 30 (NDC 49884-425-11)
Store at 20° to 25°C (68° to 77°F); excursions permitted to 15° to 30°C (59° to 86°F) [See USP Controlled Room Temperature]. Protect from moisture.
Dispense in original container.
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Patient Information).
Information for Patients
Pregnancy: Advise female patients of child bearing age about the consequences of exposure to aliskiren tablets during pregnancy. Discuss treatment options with women planning to become pregnant. Advise patients to report pregnancies to their physicians as soon as possible.
Lactation
Advise nursing women that breastfeeding is not recommended during treatment with aliskiren tablets [see Use in Specific Populations (8.2)].
Anaphylactic Reactions and Angioedema: Advise patients to immediately report any signs or symptoms suggesting a severe allergic reaction (difficulty breathing or swallowing, tightness of the chest, hives, general rash, swelling, itching, dizziness, vomiting, or abdominal pain) or angioedema (swelling of face, extremities, eyes, lips, tongue, difficulty in swallowing or breathing) and to take no more drug until they have consulted with the prescribing physicians. Angioedema, including laryngeal edema, may occur at any time during treatment with aliskiren tablets.
Symptomatic Hypotension: Advise patients that lightheadedness can occur, especially during the first days of aliskiren tablets therapy, and that it should be reported to the prescribing physician. Advise patients that if syncope occurs, aliskiren tablets should be discontinued until the physician has been consulted.
Caution patients that inadequate fluid intake, excessive perspiration, diarrhea, or vomiting can lead to an excessive fall in blood pressure, with the same consequences of lightheadedness and possible syncope.
Potassium Supplements: Advise patients receiving aliskiren tablets not to use potassium supplements or salt substitutes containing potassium without consulting the prescribing physician.
Relationship to Meals: Advise patients to establish a routine pattern for taking aliskiren tablets with regard to meals. High-fat meals decrease absorption substantially.
PATIENT INFORMATION
Aliskiren Tablets
(al'' is kye' ren)
What is the most important information I should know about aliskiren tablets?
Aliskiren tablets can cause harm or death to your unborn baby.
• Talk to your doctor about other ways to lower your blood pressure if you plan to become pregnant.
• If you become pregnant during treatment with aliskiren tablets, stop taking aliskiren tablets and tell your doctor right away.
What are aliskiren tablets?
Aliskiren tablet are a prescription medicine used to treat high blood pressure (hypertension) in adults to lower blood pressure.
It is not known if aliskiren tablets are safe and effective in children under 18 years of age.
Do not take aliskiren tablet if:
• you have diabetes and are taking a kind of medicine called an angiotensin receptor blocker (ARB) or angiotensin-converting enzyme inhibitor (ACEI).
• you are allergic to any of the ingredients in aliskiren tablets. See the end of this leaflet for a complete list of the ingredients in aliskiren tablets.
Before taking aliskiren tablets, tell your doctor about all of your medical conditions, including if you:
• have kidney or heart problems.
• have diabetes.
• have ever had an allergic reaction to another blood pressure medicine.
• are pregnant or plan to become pregnant. See “What is the most important information I should know about aliskiren tablets?”
• are breastfeeding or plan to breastfeed. It is not known if aliskiren passes into your breast milk. You should not breastfeed during treatment with aliskiren tablets
Tell your doctor about all the medicines you take, including prescription and over-the-counter medicines, vitamins and herbal supplements. Especially tell your doctor if you are taking:
• a kind of medicine to control blood pressure called angiotensin receptor blocker (ARB) or angiotensin-converting enzyme inhibitor (ACEI).
• water pills (also called “diuretics”).
• cyclosporine (Gengraf® , Neoral® , Sandimmune®)
• itraconazole (Onmel®, Sporanox®).
• potassium-containing medicines, potassium supplements, or salt substitutes containing potassium.
• nonsteroidal anti-inflammatory drugs (NSAIDs).
Ask your doctor if you are not sure if you are taking one of the medicines listed above.
Know the medicines you take. Keep a list of them to show your doctor or pharmacist when you get a new medicine. Your doctor or pharmacist will know what medicines are safe to take together.
How should I take aliskiren tablets?
• Take aliskiren tablets exactly as your doctor tells you to take them.
• Take aliskiren tablets 1 time a day, about the same time each day.
• Your doctor may change your dose if needed.
What are possible side effects of aliskiren tablets?
Aliskiren tablets may cause serious side effects, including:
• See “What is the most important information I should know about aliskiren tablets?”
• Serious allergic reactions that can be life threatening. Stop taking aliskiren tablets and get emergency medical help right away and if you get any of these symptoms of a serious reactions during treatment with aliskiren tablets:
o trouble breathing or swallowing
o chest tightness
o raised bumps (hives)
o rash
o swelling of the face, lips, tongue, throat, arms, legs or the whole body
o itching
o dizziness
o vomiting
o stomach (abdomen) pain
• Low blood pressure (hypotension). Your blood pressure may get too low if you also take water pills, are on a low-salt diet, sweat more than normal, receive dialysis treatments, have heart problems, or have vomiting or diarrhea. Lie down if you feel faint or dizzy and call your doctor right away.
• Kidney problems, including kidney failure. Tell your doctor if you urinate less.
• Increased potassium blood levels (hyperkalemia). Your doctor will check your potassium blood level during treatment with aliskiren tablets.
The most common side effect of aliskiren tablets is diarrhea.
These are not all of the possible side effects of aliskiren tablets.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
How should I store aliskiren tablets?
• Store aliskiren tablets at room temperature between 68° to 77°F (20° to 25°C).
• Keep aliskiren tablets in the original container it comes in. Do not remove the desiccant (drying agent) from the bottle.
• Protect aliskiren tablets from moisture.
Keep aliskiren tablets and all medicines out of the reach of children.
General information about the safe and effective use of aliskiren tablets.
Medicines are sometimes prescribed for purpose other than those listed in the Patient Information leaflet. Do not take aliskiren tablets for a condition for which it was not prescribed. Do not give aliskiren tablets to other people, even if they have the same symptoms that you have. It may harm them. You can ask your doctor or pharmacist for information that is written for healthcare professionals.
What is high blood pressure (hypertension)?
Blood pressure is the force in your blood vessels when your heart beats and when your heart rests. You have high blood pressure when the force is too great. High blood pressure makes the heart work harder to pump blood through the body and causes damage to the blood vessels. Aliskiren tablets can help your blood vessels relax so your blood pressure is lower. Medicines that lower your blood pressure lower your chance of having a stroke or heart attack.
What are the ingredients in aliskiren tablets?
Aliskiren is available as film-coated tablets, which contains 165.765 mg or 331.530 mg aliskiren hemifumarate (equivalent to 150 mg or 300 mg aliskiren).
Active ingredient: aliskiren hemifumarate
Inactive ingredients: calcium stearate, colloidal silicon dioxide, crospovidone, hypromellose, iron oxide red, microcrystalline cellulose, polyethylene glycol, talc and titanium dioxide. In addition, the 150 mg strength contains iron oxide black.
For more information about aliskiren tablets, call Par Pharmaceutical at 1-800-828-9393.
This Patient Information has been approved by the U.S. Food and Drug Administration.
All brand names listed are the registered trademarks of their respective owners and are not trademarks of Par Pharmaceutical.
Dist. by:
Par Pharmaceutical
Chestnut Ridge, NY 10977 U.S.A.
Mfg. by:
Par Formulations Private Limited,
9/215, Pudupakkam, Kelambakkam - 603 103
Made in India.
Mfg. Lic. No.: TN00002121
OS424-01-74-02
Revised: 02/2019
PACKAGE LABEL PRINCIPLE DISPLAY PANEL
INGREDIENTS AND APPEARANCE
| ALISKIREN
aliskiren tablet, film coated |
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
| ALISKIREN
aliskiren tablet, film coated |
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
| Labeler - Par Pharmaceutical, Inc. (092733690) |