Search by Drug Name or NDC
NDC 50090-5994-00 TROSPIUM CHLORIDE 60 mg/1 Details
TROSPIUM CHLORIDE 60 mg/1
TROSPIUM CHLORIDE is a ORAL CAPSULE, EXTENDED RELEASE in the HUMAN PRESCRIPTION DRUG category. It is labeled and distributed by A-S Medication Solutions. The primary component is TROSPIUM CHLORIDE.
MedlinePlus Drug Summary
Trospium is used to treat an overactive bladder (a condition in which the bladder muscles contract uncontrollably and cause frequent urination, urgent need to urinate, and inability to control urination). Trospium is in a class of medications called antimuscarinics. It works by relaxing the bladder muscles to prevent urgent, frequent, or uncontrolled urination.
Related Packages: 50090-5994-00Last Updated: 11/30/2022
MedLinePlus Full Drug Details: Trospium
Product Information
| NDC | 50090-5994 |
|---|---|
| Product ID | 50090-5994_88490ba7-995b-4bcc-85a0-9a68f31b68a8 |
| Associated GPIs | 54100065207020 |
| GCN Sequence Number | 063466 |
| GCN Sequence Number Description | trospium chloride CAP ER 24H 60 MG ORAL |
| HIC3 | R1A |
| HIC3 Description | URINARY TRACT ANTISPASMODIC/ANTIINCONTINENCE AGENT |
| GCN | 99193 |
| HICL Sequence Number | 017498 |
| HICL Sequence Number Description | TROSPIUM CHLORIDE |
| Brand/Generic | Generic |
| Proprietary Name | TROSPIUM CHLORIDE |
| Proprietary Name Suffix | n/a |
| Non-Proprietary Name | TROSPIUM CHLORIDE |
| Product Type | HUMAN PRESCRIPTION DRUG |
| Dosage Form | CAPSULE, EXTENDED RELEASE |
| Route | ORAL |
| Active Ingredient Strength | 60 |
| Active Ingredient Units | mg/1 |
| Substance Name | TROSPIUM CHLORIDE |
| Labeler Name | A-S Medication Solutions |
| Pharmaceutical Class | Cholinergic Muscarinic Antagonist [EPC], Cholinergic Muscarinic Antagonists [MoA] |
| DEA Schedule | n/a |
| Marketing Category | ANDA |
| Application Number | ANDA214760 |
| Listing Certified Through | 2023-12-31 |
Package
Package Images

NDC 50090-5994-00 (50090599400)
| NDC Package Code | 50090-5994-0 |
|---|---|
| Billing NDC | 50090599400 |
| Package | 90 CAPSULE, EXTENDED RELEASE in 1 BOTTLE (50090-5994-0) |
| Marketing Start Date | 2022-06-10 |
| NDC Exclude Flag | N |
| Pricing Information | N/A |
Standard Product Labeling (SPL)/Prescribing Information SPL ee939d17-4239-4597-b001-6a3f80e5d0f5 Details
HIGHLIGHTS OF PRESCRIBING INFORMATION
TROSPIUM CHLORIDE extended-release capsules, for oral use
Initial U.S. Approval: 2004
INDICATIONS AND USAGE
Trospium Chloride Extended-Release Capsules are a muscarinic antagonist indicated for the treatment of overactive bladder (OAB) with symptoms of urge urinary incontinence, urgency, and urinary frequency. ( 1)
DOSAGE AND ADMINISTRATION
- The recommended dosage of trospium chloride extended-release capsules is one 60 mg capsule daily in the morning. Trospium chloride extended-release capsules should be dosed with water on an empty stomach, at least one hour before a meal. ( 2)
- Trospium chloride extended-release capsules are not recommended for use in patients with severe renal impairment (creatinine clearance less than 30 mL/minute). ( 2)
DOSAGE FORMS AND STRENGTHS
- 60 mg capsules ( 3)
CONTRAINDICATIONS
WARNINGS AND PRECAUTIONS
- Trospium chloride extended-release capsules should be administered with caution to patients with clinically significant bladder outflow obstruction or gastrointestinal obstructive disorders due to risk of urinary or gastric retention. ( 5.1, 5.3)
- Angioedema of the face, lips, tongue and/or larynx has been reported with trospium chloride. ( 5.2)
- In patients with narrow angle glaucoma, trospium chloride extended-release capsules should be used only with careful monitoring. ( 5.4)
- Central Nervous System Effects: Somnolence has been reported with trospium chloride extended-release capsules. Advise patients not to drive or operate heavy machinery until they know how trospium chloride extended-release capsules affect them. ( 5.5)
- Trospium chloride extended-release capsules are not recommended for use in patients with severe renal impairment (creatinine clearance less than 30 mL/minute). ( 5.6)
- Alcohol should not be consumed within 2 hours of trospium chloride extended-release capsules administration. ( 5.7)
ADVERSE REACTIONS
The most common adverse reactions (greater than or equal to 1%) with trospium chloride extended-release capsules are dry mouth (10.7%) and constipation (8.5%). ( 6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Slate Run Pharmaceuticals, LLC at 1-888-341-9214 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS
- Some drugs which are actively secreted by the kidney may interact with trospium chloride extended-release capsules by competing for renal tubular secretion. ( 7)
- Concomitant use with digoxin did not affect the pharmacokinetics of either drug. ( 7.1)
- Exposure to trospium on average was comparable in the presence of and without antacid, however, some individuals demonstrated increases or decreases in trospium exposure in the presence of antacid. The clinical relevance of these findings is not known. ( 7.2)
- Concomitant use with metformin immediate release tablets reduced exposure and peak concentration of trospium. ( 7.3)
USE IN SPECIFIC POPULATIONS
- The safety and effectiveness of trospium chloride extended-release capsules in pediatric patients have not been established. ( 8.4)
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 6/2022
Table Of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Risk of Urinary Retention
5.2 Angioedema
5.3 Decreased Gastrointestinal Motility
5.4 Controlled Narrow-angle Glaucoma
5.5 Central Nervous System Effects
5.6 Patients with Severe Renal Impairment
5.7 Alcohol Interaction
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Post-marketing Experience
7 DRUG INTERACTIONS
7.1 Digoxin
7.2 Antacid
7.3 Metformin
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Labor and Delivery
8.3 Nursing Mothers
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Renal Impairment
8.7 Hepatic Impairment
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
17.1 Angioedema
17.2 When Not to Use
17.3 Administration
17.4 Adverse Reactions
- *
- Sections or subsections omitted from the full prescribing information are not listed.
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
The recommended dosage of trospium chloride extended-release capsules is one 60 mg capsule daily in the morning. Trospium chloride extended-release capsules should be dosed with water on an empty stomach, at least one hour before a meal.
Trospium chloride extended-release capsules are not recommended for use in patients with severe renal impairment (creatinine clearance less than 30 mL/minute) [see Warnings and Precautions ( 5.6), Use in Specific Populations ( 8.6), and Clinical Pharmacology ( 12.3)].
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Risk of Urinary Retention
Trospium chloride extended-release capsules should be administered with caution to patients with clinically significant bladder outflow obstruction because of the risk of urinary retention [see Contraindications ( 4)].
5.2 Angioedema
Angioedema of the face, lips, tongue and/or larynx has been reported with trospium chloride. In one case, angioedema occurred after the first dose of trospium chloride. Angioedema associated with upper airway swelling may be life threatening. If involvement of the tongue, hypopharynx, or larynx occurs, trospium chloride should be promptly discontinued and appropriate therapy and/or measures necessary to ensure a patent airway should be promptly provided.
5.3 Decreased Gastrointestinal Motility
Trospium chloride extended-release capsules should be administered with caution to patients with gastrointestinal obstructive disorders because of the risk of gastric retention [see Contraindications ( 4)] . Trospium chloride extended-release capsules, like other antimuscarinic agents, may decrease gastrointestinal motility and should be used with caution in patients with conditions such as ulcerative colitis, intestinal atony and myasthenia gravis.
5.4 Controlled Narrow-angle Glaucoma
In patients being treated for narrow-angle glaucoma, trospium chloride extended-release capsules should only be used if the potential benefits outweigh the risks, and in that circumstance only with careful monitoring [see Contraindications ( 4)].
5.5 Central Nervous System Effects
Trospium chloride extended-release capsules and trospium chloride immediate release tablets are associated with anticholinergic central nervous system (CNS) effects [see Adverse Reactions ( 6.2)] . A variety of CNS anticholinergic effects have been reported, including dizziness, confusion, hallucinations and somnolence. Patients should be monitored for signs of anticholinergic CNS effects, particularly after beginning treatment or increasing the dose. Advise patients not to drive or operate heavy machinery until they know how trospium chloride extended-release capsules affect them. If a patient experiences anticholinergic CNS effects, dose reduction or drug discontinuation should be considered.
5.6 Patients with Severe Renal Impairment
Trospium chloride extended-release capsules are not recommended for use in patients with severe renal impairment (creatinine clearance less than 30 mL/minute) [see Dosage and Administration ( 2), Use in Specific Populations ( 8.6), and Clinical Pharmacology ( 12.3)].
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, the adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in clinical practice.
The data described below reflect exposure to trospium chloride extended-release capsules in 578 patients for 12 weeks in two Phase 3 double-blind, placebo controlled trials (n=1,165). These studies included overactive bladder patients of ages 21 to 90 years, of which 86% were female and 85% were Caucasian. Patients received 60 mg daily doses of trospium chloride extended-release capsules. Patients in these studies were eligible to continue treatment with trospium chloride extended-release capsules 60 mg for up to one year. From both these controlled trials combined, 769 and 238 patients received treatment with trospium chloride extended-release capsules for at least 24 and 52 weeks, respectively.
There were 157 (27.2%) trospium chloride extended-release capsules patients and 98 (16.7%) placebo patients who experienced one or more double-blind treatment-emergent adverse events (TEAEs) that were assessed by the investigator as at least possibly related to study medication. The most common TEAEs were dry mouth and constipation which, when reported, commonly occurred early in treatment (often within the first week). In the two Phase 3 studies, constipation, dry mouth, and urinary retention led to discontinuation in 1%, 0.7%, and 0.5% of patients treated with trospium chloride extended-release capsules 60 mg daily, respectively. In the placebo group, there were no discontinuations due to dry mouth or urinary retention and one due to constipation.
The incidence of serious adverse events was similar among patients receiving trospium chloride extended-release capsules and patients receiving placebo. No treatment-emergent serious adverse events in either treatment group were judged by the investigators as being possibly related to the study medication.
Table 1 lists those treatment emergent adverse events from the trials that were assessed by the investigator as possibly related to study medication, reported in at least 1% of trospium chloride extended-release capsules patients, and were more common for the trospium chloride extended-release capsules group than for placebo.
Table 1: Incidence of treatment-emergent adverse events reported in at least 1% of patients judged by the investigator as at least possibly related to treatment and more common for the Trospium Chloride Extended-Release Capsules group than for placebo
| MedDRA Preferred term | Number of patients (%) | |
| Placebo N=587 | Trospium Chloride Extended-Release Capsules N=578 | |
| Dry mouth | 22 (3.7) | 62 (10.7) |
| Constipation | 9 (1.5) | 49 (8.5) |
| Dry eye | 1 (0.2) | 9 (1.6) |
| Flatulence | 3 (0.5) | 9 (1.6) |
| Nausea | 2 (0.3) | 8 (1.4) |
| Abdominal pain | 2 (0.3) | 8 (1.4) |
| Dyspepsia | 4 (0.7) | 7 (1.2) |
| Urinary tract infection | 5 (0.9) | 7 (1.2) |
| Constipation aggravated | 3 (0.5) | 7 (1.2) |
| Abdominal distension | 2 (0.3) | 6 (1.0) |
| Nasal dryness | 0 (0.0) | 6 (1.0) |
Additional adverse events reported in less than 1% of trospium chloride extended-release capsules treated patients and more common for trospium chloride extended-release capsules than placebo, judged by the investigator at least possibly related to treatment were: vision blurred, feces hard, back pain, somnolence, urinary retention, and dry skin.
Table 2 lists all treatment-emergent adverse events for the trials reported in at least 2% of all trospium chloride extended-release capsules patients and more common for the trospium chloride extended-release capsules group than for placebo without regard to the investigator’s judgment on drug relatedness.
Table 2: Incidence of treatment-emergent adverse events reported in at least 2% of patients regardless of reported relationship to treatment and more common for the Trospium Chloride Extended-Release Capsules group than for placebo
| MedDRA Preferred term | Number of patients (%) | |
| Placebo N=587 | Trospium Chloride Extended-Release Capsules N=578 | |
| Dry mouth | 22 (3.7) | 64 (11.1) |
| Constipation | 10 (1.7) | 52 (9.0) |
| Urinary tract infection | 29 (4.9) | 42 (7.3) |
| Nasopharyngitis | 10 (1.7) | 17 (2.9) |
| Influenza | 9 (1.5) | 13 (2.2) |
Additional adverse events reported in less than 2% of trospium chloride extended-release capsules treated patients and twice as frequent for trospium chloride extended-release capsules compared to placebo, regardless of reported relationship to treatment were: tachycardia, dry eyes, abdominal pain, dyspepsia, abdominal distension, constipation aggravated, nasal dryness, and rash.
In the open-label treatment phase, the most common TEAEs reported in the 769 patients with at least 6 months exposure to trospium chloride extended-release capsules were: constipation, and dry mouth. Urinary tract infection and rash was also reported in several patients, including one of each judged by the investigator to be possibly related to treatment. Several adverse events were reported as severe in the open-label treatment phase, including one urinary tract infection, two urinary retention events, and one aggravated constipation.
6.2 Post-marketing Experience
The following adverse reactions have been identified during post-approval use of trospium chloride. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Gastrointestinal – gastritis; Cardiovascular – palpitations, supraventricular tachycardia, chest pain, syncope, “hypertensive crisis”; Immunological – Stevens-Johnson syndrome, anaphylactic reaction, angioedema; Nervous System – dizziness, confusion, vision abnormal, hallucinations, somnolence, and delirium; Musculoskeletal – rhabdomyolysis; General – rash.
7 DRUG INTERACTIONS
Trospium is metabolized by ester hydrolysis and excreted by the kidneys through a combination of tubular secretion and glomerular filtration. Based on in vitro data, no clinically relevant metabolic drug-drug interactions are anticipated with trospium chloride extended-release capsules. However, some drugs which are actively secreted by the kidney may interact with trospium chloride extended-release capsules by competing for renal tubular secretion.
The concomitant use of trospium chloride extended-release capsules with other antimuscarinic agents that produce dry mouth, constipation, and other anticholinergic effects may increase the frequency and/or severity of such effects. Trospium chloride extended-release capsules may potentially alter the absorption of some concomitantly administered drugs due to anticholinergic effects on gastrointestinal motility.
7.1 Digoxin
Concomitant use of trospium chloride 20 mg twice daily and digoxin did not affect the pharmacokinetics of either drug [see Clinical Pharmacology ( 12.3)].
7.2 Antacid
While the systemic exposure of trospium on average was comparable with and without antacid containing aluminum hydroxide and magnesium carbonate, 5 out of 11 individuals in a drug interaction study demonstrated either an increase or decrease in trospium exposure, in presence of antacid. The clinical relevance of these findings is not known [see Clinical Pharmacology ( 12.3)].
7.3 Metformin
Co-administration of 500 mg metformin immediate release tablets twice daily reduced the steady-state systemic exposure of trospium by approximately 29% for mean AUC (0-24) and by 34% for mean C max. The effect of a decrease in trospium exposure on the efficacy of trospium chloride extended-release capsules is unknown. The steady-state pharmacokinetics of metformin were comparable when administered with or without 60 mg trospium chloride extended-release capsules once daily under fasted condition. The effect of metformin at higher doses on trospium PK is unknown [see Clinical Pharmacology ( 12.3)].
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Teratogenic Effects
Pregnancy Category C: There are no adequate and well-controlled studies of trospium chloride extended-release capsules in pregnant women. Trospium chloride extended-release capsules should be used during pregnancy only if the potential benefit to the patient outweighs the risk to the patient and fetus. Women who become pregnant during trospium chloride extended-release capsules treatment are encouraged to contact their physician.
Trospium chloride was not teratogenic at statistically significant levels in rats or rabbits administered doses up to 200 mg/kg/day. This corresponds to systemic exposures up to approximately 16 and 32 times, respectively (based on AUC), the clinical exposure at the maximum recommended human dose (MRHD) of 60 mg. However, in rabbits, one fetus in each of the three treated dose groups (1, 1, and 32 times the MRHD) demonstrated multiple malformations, including umbilical hernia and skeletal malformations. A no effect level for maternal and fetal toxicity was observed at levels approximately equivalent to the clinical exposure at the MRHD (20 mg/kg/day in rats and rabbits). No developmental toxicity was observed in the offspring of female rats exposed pre- and post-natally to up to 200 mg/kg/day.
8.2 Labor and Delivery
The effect of trospium chloride extended-release capsules on labor and delivery is unknown.
8.3 Nursing Mothers
Trospium chloride (2 mg/kg orally and 50 mcg/kg intravenously) was excreted, to a limited extent (less than 1%), into the milk of lactating rats (primarily as parent compound). It is not known whether this drug is excreted into human milk. Because many drugs are excreted into human milk, trospium chloride extended-release capsules should be used during lactation only if the potential benefit justifies the potential risk.
8.4 Pediatric Use
The safety and effectiveness of trospium chloride extended-release capsules in pediatric patients have not been established.
8.5 Geriatric Use
Of 1,165 patients in Phase 3 clinical studies of trospium chloride extended-release capsules, 37% (n=428) were ages 65 and over, while 12% (n=143) were ages 75 and over.
No overall differences in effectiveness were observed between those subjects aged 65 and over and younger subjects. In trospium chloride extended-release capsules subjects ages 65 and over compared to younger subjects, the following adverse reactions were reported at a higher incidence: dry mouth, constipation, abdominal pain, dyspepsia, urinary tract infection and urinary retention. In subjects ages 75 and over, three reported a fall and in one of them a relationship to the event could not be excluded.
8.6 Renal Impairment
Severe renal impairment (creatinine clearance less than 30 mL/minute) may significantly alter the disposition of trospium chloride extended-release capsules. In a study of immediate-release trospium chloride, 4.2-fold and 1.8-fold increases in mean AUC (0-∞) and C max, respectively, were detected in patients with severe renal impairment. Use of trospium chloride extended-release capsules is not recommended in patients with severe renal impairment [see Warnings and Precautions ( 5.4) and Clinical Pharmacology ( 12.3)] . The pharmacokinetics of trospium chloride have not been studied in patients with creatinine clearance ranging from 30 to 80 mL/min.
Trospium is known to be substantially excreted by the kidney, and the risk of adverse reactions may be greater in patients with impaired renal function.
8.7 Hepatic Impairment
There is no information regarding the effect of severe hepatic impairment on exposure to trospium chloride extended-release capsules. In a study of patients with mild and with moderate hepatic impairment, given 40 mg of immediate-release trospium chloride, mean C max increased 12% and 63%, respectively, and mean AUC (0-∞) decreased 5% and 15%, respectively, compared to healthy subjects. The clinical significance of these findings is unknown. Caution is advised, however, when administering trospium chloride extended-release capsules to patients with moderate to severe hepatic impairment.
10 OVERDOSAGE
11 DESCRIPTION
Trospium Chloride Extended-Release Capsules are an extended-release formulation of trospium chloride, a quaternary ammonium compound with the chemical name of Spiro [8-azoniabicyclo[3.2.1]octane-8,1'-pyrrolidinium], 3[(hydroxydiphenylacetyl)oxy]-, chloride, (1α, 3β, 5α). The empirical formula of trospium chloride is C 25H 30ClNO 3 and its molecular weight is 427.97. The structural formula of trospium chloride is represented below:

Trospium chloride is a fine, colorless to slightly yellow, crystalline solid. The compound’s solubility in water is approximately 1 g/2 mL.
Trospium Chloride Extended-Release Capsules contain 60 mg of trospium chloride, a muscarinic antagonist, for oral administration. Each capsule also contains the following inactive ingredients: ethylcellulose, FD&C Yellow 6, gelatin, hypromellose, methacrylic acid, methyl acrylate, methyl methacrylate, polyethylene glycol 3350, polysorbate 80, polyvinyl alcohol, sodium lauryl sulfate, sugar spheres, talc, titanium dioxide, and triethyl citrate.
The imprinting ink have the following ingredients: ferrosoferric oxide, potassium hydroxide and shellac.
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Trospium chloride is an antispasmodic, antimuscarinic agent.
Trospium chloride antagonizes the effect of acetylcholine on muscarinic receptors in cholinergically innervated organs including the bladder. Its parasympatholytic action reduces the tonus of smooth muscle in the bladder.
In vitro receptor binding studies have demonstrated the selectivity of trospium chloride for muscarinic over nicotinic receptors, and similar affinity for the M 2 and M 3 muscarinic receptor subtypes. M 2 and M 3 receptors are found in the bladder and may play a role in the pathogenesis of overactive bladder.
12.2 Pharmacodynamics
Placebo-controlled studies assessing the impact on urodynamic variables of an immediate-release formulation of trospium chloride were conducted in patients with conditions characterized by involuntary detrusor contractions. The results demonstrated that trospium chloride increases maximum cystometric bladder capacity and volume at first detrusor contraction.
Electrophysiology
The effect of 20 mg twice daily and up to 100 mg twice daily of an immediate-release formulation of trospium chloride on QT interval was evaluated in a single-blind, randomized, placebo and active (moxifloxacin 400 mg daily) controlled, 5-day parallel trial in 170 male and female healthy volunteer subjects aged 18 to 45 years. The QT interval was measured over a 24-hour period at steady state. Trospium chloride was not associated with an increase in individual corrected (QTcI) or Fridericia corrected (QTcF) QT interval at any time during steady state measurement, while moxifloxacin was associated with a 6.4 msec increase in QTcF.
In this study, asymptomatic, non-specific T-wave inversions were observed more often in subjects receiving trospium chloride than in subjects receiving moxifloxacin or placebo following five days of treatment. The clinical significance of T-wave inversion in this study is unknown. This finding was not observed during routine safety monitoring in overactive bladder patients from 2 placebo-controlled clinical trials in 591 patients treated with 20 mg twice daily of immediate-release trospium chloride, nor was it observed in 2 placebo-controlled clinical trials in 578 patients treated with trospium chloride extended-release capsules.
Also in this study, the immediate-release formulation of trospium chloride was associated with an increase in heart rate that correlated with increasing plasma concentration, with a mean elevation in heart rate compared to placebo of 9 beats per minute for the 20 mg dose and of 18 beats per minute for the 100 mg dose. In the two Phase 3 trospium chloride extended-release capsules trials the mean increase in heart rate compared to placebo was approximately 3 beats per minute in both studies.
12.3 Pharmacokinetics
bsorption: Mean absolute bioavailability of a 20 mg immediate-release dose is 9.6% (range 4.0% to 16.1%). Following a single 60 mg dose of trospium chloride extended-release capsules, peak plasma concentration (C max) of 2.0 ng/mL occurred 5.0 hours post dose. By contrast, following a single 20 mg dose of an immediate-release formulation of trospium chloride, C max was 2.7 ng/mL.
Effect of Food: Administration of trospium chloride extended-release capsules immediately after a high (50%) fat-content meal reduced the oral bioavailability of trospium chloride by 35% for AUC (0-Tlast) and by 60% for C max. Other pharmacokinetic parameters such as T max and t 1/2 were unchanged in the presence of food.
A summary of mean (±standard deviation) pharmacokinetic parameters for a single dose of 60 mg trospium chloride extended-release capsules is provided in Table 3.
| Treatment | AUC
(0-24)
(ng•h/mL) | C
max
(ng/mL) | T
maxa
(h) | t
1/2b
(h) |
| Trospium Chloride Extended-Release Capsules 60 mg | 18.0±13.4 | 2.0±1.5 | 5.0 (3.0 to 7.5) | 36±22 |
a T
max expressed as median (range).
b t
1/2 was determined following multiple (10) doses.
The mean sample concentration-time (+standard deviation) profile for trospium chloride extended-release capsules is shown in Figure 1.
Figure 1: Mean (+SD) Concentration-Time Profile for a Single 60 mg Oral Dose of Trospium Chloride Extended-Release Capsules in Healthy Volunteers

Administration of trospium chloride extended-release capsules immediately after a high (50%) fat-content meal reduced the oral bioavailability of trospium chloride by 35% for AUC (0-Tlast) and by 60% for C max. Other pharmacokinetic parameters such as T max and t 1/2 were unchanged in the presence of food. Coadministration with antacid had inconsistent effects on the oral bioavailability of trospium chloride extended-release capsules.
Distribution: Protein binding ranged from 50 to 85%, depending upon the assessment method used, when a range of concentration levels of trospium chloride (0.5 to 50 mcg/L) were incubated in vitro with human serum.
The ratio of 3H-trospium chloride in plasma to whole blood was 1.6:1. This ratio indicates that the majority of 3H-trospium chloride is distributed in plasma.
Trospium chloride is widely distributed, with an apparent volume of distribution >600 L.
Metabolism: The metabolic pathway of trospium in humans has not been fully defined. Of the dose absorbed following oral administration, metabolites account for approximately 40% of the excreted dose. The major metabolic pathway of trospium is hypothesized as ester hydrolysis with subsequent conjugation of benzylic acid to form azoniaspironortropanol with glucuronic acid. CYP P450 does not contribute significantly to the elimination of trospium. Data taken from in vitro studies of human liver microsomes investigating the inhibitory effect of trospium on seven CYP P450 isoenzyme substrates (CYP1A2, 2A6, 2C9, 2C19, 2D6, 2E1, and 3A4) suggest a lack of inhibition at clinically relevant concentrations.
Excretion: The plasma half-life for trospium following oral administration of trospium chloride extended-release capsules is approximately 35 hours. After oral administration of an immediate-release formulation of 14C-labeled trospium chloride, a majority of the dose (85.2%) was recovered in feces and a smaller amount (5.8% of the dose) was recovered in urine. Of the radioactivity excreted into the urine, 60% was unchanged trospium.
The mean renal clearance for trospium (29.07 L/hour) is 4-fold higher than average glomerular filtration rate, indicating that active tubular secretion is a major route of elimination. There may be competition for elimination with other compounds that are also renally eliminated [see Drug Interactions ( 7)] .
Drug Interactions
Digoxin: Concomitant use of 20 mg trospium chloride immediate release twice daily at steady state and a single dose of 0.5 mg digoxin in a crossover study with 40 male and female subjects did not affect the pharmacokinetics of either drug.
Antacid: A drug interaction study was conducted to evaluate the effect of an antacid containing aluminum hydroxide and magnesium carbonate on the pharmacokinetics of trospium chloride extended-release capsules (n=11). While the systemic exposure of trospium on average was comparable with and without antacid, 5 individuals demonstrated either an increase or decrease in trospium exposure, in presence of antacid.
Metformin: A drug interaction study was conducted in which trospium chloride extended-release capsules 60 mg once daily was co-administered with Glucophage ® (metformin hydrochloride) 500 mg twice daily under steady-state conditions in 44 healthy subjects. Co-administration of 500 mg metformin immediate release tablets twice daily reduced the steady-state systemic exposure of trospium by approximately 29% for mean AUC (0-24) and by 34% for mean C max. The effect of decrease in trospium exposure on the efficacy of trospium chloride extended-release capsules is unknown. The steady-state pharmacokinetics of metformin were comparable when administered with or without 60 mg trospium chloride extended-release capsules once daily under fasted condition. The effect of metformin at higher doses on trospium PK is unknown.
Specific Populations
Age: In a phase 3 clinical trial of trospium chloride extended-release capsules, the observed plasma trospium concentrations were similar in older (greater than or equal to 65 years) and younger (less than 65 years) OAB patients.
Pediatric: The pharmacokinetics of trospium chloride extended-release capsules were not evaluated in pediatric patients.
Race: Pharmacokinetic differences due to race have not been studied.
Gender: Gender differences in pharmacokinetics of trospium chloride extended-release capsules have not been formally assessed. Data from healthy subjects suggests lower exposure in males compared to females.
Hepatic Impairment: There is no information regarding the effect of severe hepatic impairment on exposure to trospium chloride extended-release capsules In a study of patients with mild (Child-Pugh score 5 to 6) and with moderate (Child-Pugh score 7 to 8) hepatic impairment, given 40 mg of immediate-release trospium chloride, mean C max increased 12% and 63% respectively, and mean AUC (0-∞) decreased 5% and 15%, respectively, compared to healthy subjects.
Renal Impairment: The pharmacokinetics of trospium chloride extended-release capsules in patients with severe renal impairment has not been evaluated. In a study of an immediate-release formulation of trospium chloride, 4.2-fold and 1.8-fold increases in mean AUC (0–∞) and C max, respectively, were detected in patients with severe renal impairment (creatinine clearance less than 30 mL/minute), compared with healthy subjects, along with the appearance of an additional elimination phase with a long half-life (~33 hours vs. 18 hours). Use of trospium chloride extended-release capsules is not recommended in patients with severe renal impairment [see Dosage and Administration ( 2)] . The pharmacokinetics of trospium chloride have not been studied in people with creatinine clearance ranging from 30 to 80 mL/min.
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis: Carcinogenicity studies with trospium chloride were conducted in mice and rats for 78 weeks and 104 weeks, respectively, at maximally tolerated doses. No evidence of a carcinogenic effect was found in either mice or rats administered up to 200 mg/kg/day (approximately 1 and 16 times, respectively (based on AUC), the expected clinical exposure levels at the maximum recommended human dose (MRHD) of 60 mg.
Mutagenesis: Trospium chloride was not mutagenic nor genotoxic in tests in vitro in bacteria (Ames test) and mammalian cells (L5178Y mouse lymphoma and CHO cells) or in vivo in the mouse micronucleus test.
Impairment of Fertility: No evidence of impaired fertility was observed in rats administered doses up to 200 mg/kg/day (about 16 times the expected clinical exposure at the MRHD, based on AUC).
14 CLINICAL STUDIES
Trospium chloride extended-release capsules were evaluated for the treatment of patients with overactive bladder who had symptoms of urinary frequency, urgency and urge urinary incontinence in two 12-week, randomized, double-blind, placebo-controlled studies. For both studies, entry criteria required the presence of urge incontinence (predominance of urge), at least one incontinence episode per day, and 10 or more micturitions (voids) per day (assessed by 3-day urinary diary). Medical history and data from the baseline urinary diary confirmed the diagnosis. Approximately 88% of the patients enrolled completed the 12-week studies. The mean age was 60 years, and the majority of patients were female (84%) and Caucasian (86%).
The co-primary endpoints in the trials were the mean change from baseline to Week 12 in number of voids/24 hours (reductions in urinary frequency) and the mean change from baseline to Week 12 in number of incontinence episodes/24 hours. Secondary endpoints included mean change from baseline to Week 12 in volume per void.
Study 1 included 592 patients in both trospium chloride extended-release capsules 60 mg and placebo groups. As illustrated in Table 4 and Figures 2 and 3, trospium chloride extended-release capsules demonstrated statistically significantly (p<0.01) greater reductions in the urinary frequency and incontinence episodes, and increases in void volume when compared to placebo starting at Week 1 and maintained through Weeks 4 and 12.
Table 4: Mean (SE) Change from Baseline in Urinary Frequency, Urge Incontinence Episodes and Void Volume in Study 1
|
Efficacy Endpointa |
Week |
Placebo | Trospium Chloride Extended-Release Capsules |
P-Value |
| Urinary frequency / 24 hours | (N=300) | (N=292) | ||
| Mean Baseline | 0 | 12.7 (0.2) | 12.8 (0.2) | |
| Mean Change from Baseline | 1 | -1.2 (0.1) | -1.7 (0.1) | 0.0092 |
| 4 | -1.6 (0.2) | -2.4 (0.2) | <0.0001 | |
| 12 | -2.0 (0.2) | -2.8 (0.2) | <0.0001 | |
| Urge incontinence episodes / week | (N=300) | (N=292) | ||
| Mean Baseline | 0 | 29.0 (1.3) | 28.8 (1.3) | |
| Mean Change from Baseline | 1 | -8.7 (1.0) | -13.0 (0.9) | 0.0003 |
| 4 | -12.2 (1.1) | -16.5 (1.2) | 0.0054 | |
| 12 | -13.5 (1.1) | -17.3 (1.2) | 0.0024 | |
| Urinary volume / void (mL) | (N=300) | (N=290) | ||
| Mean Baseline | 0 | 155.9 (3.0) | 151.0 (2.9) | |
| Mean Change from Baseline | 1 | 12.1 (2.1) | 21.6 (2.8) | 0.0036 |
| 4 | 17.2 (2.5) | 30.0 (3.1) | 0.0007 | |
| 12 | 18.9 (2.8) | 29.8 (3.2) | 0.0039 |
a treatment differences assessed by rank ANOVA for intent-to-treat population, last observation carried forward (ITT:LOCF) data set
Figure 2: Mean Change from Baseline in Urinary Frequency/24 hours by Visit: Study 1

Figure 3: Mean Change from Baseline in Incontinence Episodes/Week by Visit: Study 1

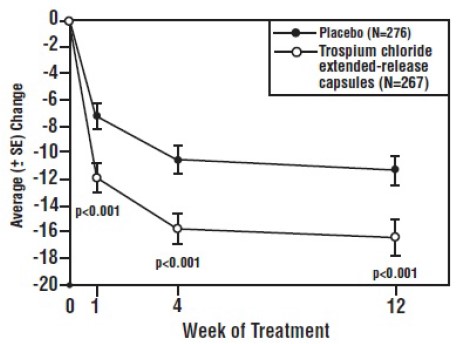
Study 2 included 543 patients in both trospium chloride extended-release capsules 60 mg and placebo groups and was identical in design to Study 1. As illustrated in Table 5 and Figures 4 and 5, trospium chloride extended-release capsules demonstrated statistically significantly (p<0.01) greater reductions in urinary frequency and incontinence episodes, and increases in void volume when compared to placebo at Weeks 4 and 12. However, at Week 1, statistically significant reductions were seen in urinary incontinence episodes and volume void only.
Table 5: Mean (SE) Change from Baseline in Urinary Frequency, Urge Incontinence Episodes and Void Volume in Study 2
|
Efficacy Endpointa | Week | Placebo | Trospium Chloride Extended-Release Capsules | P-
Value |
| Urinary Frequency / 24 hours | (N=276) | (N=267) | ||
| Mean Baseline | 0 | 12.9 (0.2) | 12.8 (0.2) | |
| Mean Change from Baseline | 1 | -1.2 (0.2) | -1.4 (0.2) | 0.0759 |
| 4 | -1.7 (0.2) | -2.3 (0.2) | 0.0047 | |
| 12 | -1.8 (0.2) | -2.5 (0.2) | 0.0009 | |
| Urge incontinence episodes / week | (N=276) | (N=267) | ||
| Mean Baseline | 0 | 28.3 (1.4) | 28.2 (1.2) | |
| Mean Change from Baseline | 1 | -7.3 (1.0) | -11.9 (1.0) | <0.0001 |
| 4 | -10.6 (1.1) | -15.8 (1.1) | <0.0001 | |
| 12 | -11.3 (1.2) | -16.4 (1.3) | <0.0001 | |
| Urinary volume / void (mL) | (N=276) | (N=266) | ||
| Mean Baseline | 0 | 151.8 (2.8) | 149.6 (2.9) | |
| Mean Change from Baseline | 1 | 11.9 (2.5) | 24.1 (2.4) | <0.0001 |
| 4 | 19.6 (3.1) | 29.3 (3.0) | 0.0020 | |
| 12 | 17.8 (3.3) | 31.5 (3.4) | 0.0014 |
a treatment differences assessed by rank ANOVA for intent-to-treat population, last observation carried forward (ITT:LOCF) data set
Figure 4: Mean Change from Baseline in Urinary Frequency/24 hours by Visit: Study 2

Figure 5: Mean Change from Baseline in Incontinence Episodes/Week by Visit: Study 2
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
“See FDA-Approved Patient Labeling (Patient Information)”
17.1 Angioedema
Patients should be informed that trospium chloride extended-release capsules may produce angioedema which could result in life-threatening airway obstruction. Patients should be advised to promptly discontinue trospium chloride extended-release capsules therapy and seek immediate medical attention if they experience edema of the tongue, edema of the laryngopharynx, or difficulty breathing.
17.2 When Not to Use
Prior to treatment, patients should fully understand the risks and benefits of trospium chloride extended-release capsules. In particular, patients should be informed not to take trospium chloride extended-release capsules if they:
• have urinary retention;
• gastric retention;
• uncontrolled narrow-angle glaucoma;
• are allergic to any component of trospium chloride extended-release capsules.
17.3 Administration
Patients should be instructed regarding the recommended dosing and administration of trospium chloride extended-release capsules:
• Take one trospium chloride extended-release capsules daily in the morning with water.
• Take trospium chloride extended-release capsules on an empty stomach or at least 1 hour before a meal.
• Use of alcoholic beverages within 2 hours of dosing with trospium chloride extended-release capsules is not recommended.
17.4 Adverse Reactions
Patients should be informed that the most common side effects with trospium chloride extended-release capsules are dry mouth and constipation and that other less common side effects include trouble emptying the bladder, blurred vision, and heat prostration. Because anticholinergics, such as trospium chloride extended-release capsules, may produce dizziness or blurred vision, patients should be advised to exercise caution in decisions to engage in potentially dangerous activities until the drug’s effects have been determined. Patients should be informed that alcohol may enhance the drowsiness caused by anticholinergic agents.
Manufactured by:
Yichang Humanwell Oral Solid Dosage Plant
Yichang, Hubei, China 443112
Distributed by:
Slate Run Pharmaceuticals, LLC
Columbus, Ohio 43215
Revised: 05/2021
Patient Information
Trospium (TROSE-pee-um) Chloride Extended-Release Capsules
Read the Patient Information that comes with trospium chloride extended-release capsules before you start taking it and each time you get a refill. There may be new information. This leaflet does not take the place of talking with your doctor about your medical condition or your treatment.
What are Trospium Chloride Extended-Release Capsules?
Trospium chloride extended-release capsules are a prescription medicine used to treat adults with overactive bladder who have the following symptoms:
• a strong need to urinate right away;
• leaking or wetting accidents due to a strong need to urinate right away;
• a need to urinate often.
Who should not take Trospium Chloride Extended-Release Capsules?
Do not take trospium chloride extended-release capsules if you:
• have trouble emptying your bladder;
• have delayed or slow emptying of your stomach;
• have an eye problem called “uncontrolled narrow-angle glaucoma”;
• are allergic to trospium chloride extended-release capsules or any of its ingredients. See the end of this leaflet for a complete list of ingredients.
Trospium chloride extended-release capsules have not been studied in children under the age of 18 years.
What should I tell my doctor before starting Trospium Chloride Extended-Release Capsules?
Tell your doctor about all of your medical conditions including if you:
• have any stomach or intestinal problems or problems with constipation;
• have trouble emptying your bladder or have a weak urine stream;
• have an eye problem called narrow-angle glaucoma;
• have kidney problems;
• have liver problems;
• are pregnant or planning to become pregnant. It is not known if trospium chloride extended-release capsules can harm your unborn baby.
• are breastfeeding. It is not known if trospium chloride extended-release capsules pass into breast milk and if it can harm your baby. You should talk to your doctor about the best way to feed your baby if you are taking trospium chloride extended-release capsules.
Tell your doctor about all the medicines you take including prescription and nonprescription medicines, vitamins and herbal supplements. Trospium chloride extended-release capsules and certain other medicines can interact and make some side effects worse. Trospium chloride extended-release capsules can affect how other medicines are handled by the body.
Know all the medicines you take. Keep a list of them with you to show your doctor and pharmacist each time you get a new medicine.
How should I take Trospium Chloride Extended-Release Capsules?
Take trospium chloride extended-release capsules exactly as prescribed.
• Take one trospium chloride extended-release capsules daily in the morning with water.
• Take trospium chloride extended-release capsules on an empty stomach or at least 1 hour before a meal.
• Do not take alcohol within 2 hours of taking trospium chloride extended-release capsules.
• If you take too much trospium chloride extended-release capsules, call your local Poison Control Center or go to an emergency room right away.
What are the possible side effects of Trospium Chloride Extended-Release Capsules?
Trospium chloride extended-release capsules may cause allergic reactions that may be serious. Symptoms of a serious allergic reaction may include swelling of the face, lips, throat or tongue. If you experience these symptoms, you should stop taking trospium chloride extended-release capsules and get emergency medical help right away.
The most common side effects with trospium chloride extended-release capsules are:
• dry mouth;
• constipation.
Trospium chloride extended-release capsules may cause other less common side effects, including:
• trouble emptying the bladder;
• blurred vision and drowsiness. Do not drive or operate heavy machinery until you know how trospium chloride extended-release capsules affect you.
• heat prostration. Due to decreased sweating, heat prostration can occur when drugs such as trospium chloride extended-release capsules are used in a hot environment.
Tell your doctor if you have any side effects that bother you or that do not go away.
These are not all possible side effects of trospium chloride extended-release capsules. For more information, ask your doctor, healthcare professional or pharmacist.
How should I store Trospium Chloride Extended-Release Capsules?
• Keep trospium chloride extended-release capsules and all other medicines out of the reach of children.
• Store trospium chloride extended-release capsules at 20° to 25°C (68° to 77°F), excursions permitted to 15° to 30°C (59° to 86°F) [see USP Controlled Room Temperature]
• Safely dispose of trospium chloride extended-release capsules that are out of date or that you no longer need.
General information about Trospium Chloride Extended-Release Capsules
Medicines are sometimes prescribed for conditions that are not mentioned in patient information leaflets. Do not use trospium chloride extended-release capsules for a condition for which it was not prescribed. Do not give trospium chloride extended-release capsules to other people, even if they have the same symptoms you have. It may harm them.
This leaflet summarizes the most important information about trospium chloride extended-release capsules. If you would like more information, talk with your doctor. You can ask your doctor or pharmacist for information about trospium chloride extended-release capsules that is written for health professionals. For more information, please call 1-888-341-9214.
What are the ingredients in Trospium Chloride Extended-Release Capsules?
Active Ingredient: trospium chloride, USP.
Inactive ingredients: ethylcellulose, FD&C Yellow 6, gelatin, hypromellose, methacrylic acid, methyl acrylate, methyl methacrylate, polyethylene glycol 3350, polysorbate 80, polyvinyl alcohol, sodium lauryl sulfate, sugar spheres, talc, titanium dioxide, and triethyl citrate.
The imprinting ink have the following ingredients: ferrosoferric oxide, potassium hydroxide and shellac.
*Listed trademarks are the property of their respective owners.
Manufactured by:
Yichang Humanwell Oral Solid Dosage Plant
Yichang, Hubei, China 443112
Distributed by:
Slate Run Pharmaceuticals, LLC
Columbus, Ohio 43215
Revised: 05/2021
INGREDIENTS AND APPEARANCE
| TROSPIUM CHLORIDE
trospium chloride capsule, extended release |
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
| Labeler - A-S Medication Solutions (830016429) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| A-S Medication Solutions | 830016429 | RELABEL(50090-5994) , REPACK(50090-5994) | |
 ” and opaque white body printed with “126” in black ink).
” and opaque white body printed with “126” in black ink).