Search by Drug Name or NDC
NDC 50804-0227-12 Aspirin 81 mg/1 Details
Aspirin 81 mg/1
Aspirin is a ORAL TABLET, COATED in the HUMAN OTC DRUG category. It is labeled and distributed by Good Sense (Geiss, Destin & Dunn, Inc.). The primary component is ASPIRIN.
MedlinePlus Drug Summary
Prescription aspirin is used to relieve the symptoms of rheumatoid arthritis (arthritis caused by swelling of the lining of the joints), osteoarthritis (arthritis caused by breakdown of the lining of the joints), systemic lupus erythematosus (condition in which the immune system attacks the joints and organs and causes pain and swelling) and certain other rheumatologic conditions (conditions in which the immune system attacks parts of the body). Nonprescription aspirin is used to reduce fever and to relieve mild to moderate pain from headaches, menstrual periods, arthritis, toothaches, and muscle aches. Nonprescription aspirin is also used to prevent heart attacks in people who have had a heart attack in the past or who have angina (chest pain that occurs when the heart does not get enough oxygen). Nonprescription aspirin is also used to reduce the risk of death in people who are experiencing or who have recently experienced a heart attack. Nonprescription aspirin is also used to prevent ischemic strokes (strokes that occur when a blood clot blocks the flow of blood to the brain) or mini-strokes (strokes that occur when the flow of blood to the brain is blocked for a short time) in people who have had this type of stroke or mini-stroke in the past. Aspirin will not prevent hemorrhagic strokes (strokes caused by bleeding in the brain). Aspirin is in a group of medications called salicylates. It works by stopping the production of certain natural substances that cause fever, pain, swelling, and blood clots. Aspirin is also available in combination with other medications such as antacids, pain relievers, and cough and cold medications. This monograph only includes information about the use of aspirin alone. If you are taking a combination product, read the information on the package or prescription label or ask your doctor or pharmacist for more information.
Related Packages: 50804-0227-12Last Updated: 11/30/2022
MedLinePlus Full Drug Details: Aspirin
Product Information
| NDC | 50804-0227 |
|---|---|
| Product ID | 50804-227_c45f7a4d-d829-478d-a8a0-34720d6c6b22 |
| Associated GPIs | 64100010000601 |
| GCN Sequence Number | 016995 |
| GCN Sequence Number Description | aspirin TABLET DR 81 MG ORAL |
| HIC3 | M9P |
| HIC3 Description | PLATELET AGGREGATION INHIBITORS |
| GCN | 00161 |
| HICL Sequence Number | 001820 |
| HICL Sequence Number Description | ASPIRIN |
| Brand/Generic | Generic |
| Proprietary Name | Aspirin |
| Proprietary Name Suffix | Low Dose Safety Coated |
| Non-Proprietary Name | Aspirin |
| Product Type | HUMAN OTC DRUG |
| Dosage Form | TABLET, COATED |
| Route | ORAL |
| Active Ingredient Strength | 81 |
| Active Ingredient Units | mg/1 |
| Substance Name | ASPIRIN |
| Labeler Name | Good Sense (Geiss, Destin & Dunn, Inc.) |
| Pharmaceutical Class | Anti-Inflammatory Agents, Non-Steroidal [CS], Cyclooxygenase Inhibitors [MoA], Decreased Platelet Aggregation [PE], Decreased Prostaglandin Production [PE], Nonsteroidal Anti-inflammatory Drug [EPC], Platelet Aggregation Inhibitor [EPC] |
| DEA Schedule | n/a |
| Marketing Category | OTC MONOGRAPH NOT FINAL |
| Application Number | part343 |
| Listing Certified Through | n/a |
Package
Package Images
NDC 50804-0227-12 (50804022712)
| NDC Package Code | 50804-227-12 |
|---|---|
| Billing NDC | 50804022712 |
| Package | 1 BOTTLE, PLASTIC in 1 BOX (50804-227-12) / 120 TABLET, COATED in 1 BOTTLE, PLASTIC |
| Marketing Start Date | 2013-03-15 |
| NDC Exclude Flag | N |
| Pricing Information | N/A |
Standard Product Labeling (SPL)/Prescribing Information SPL 4e85dbe9-c4bb-481d-8db9-4b9c0a81aed1 Details
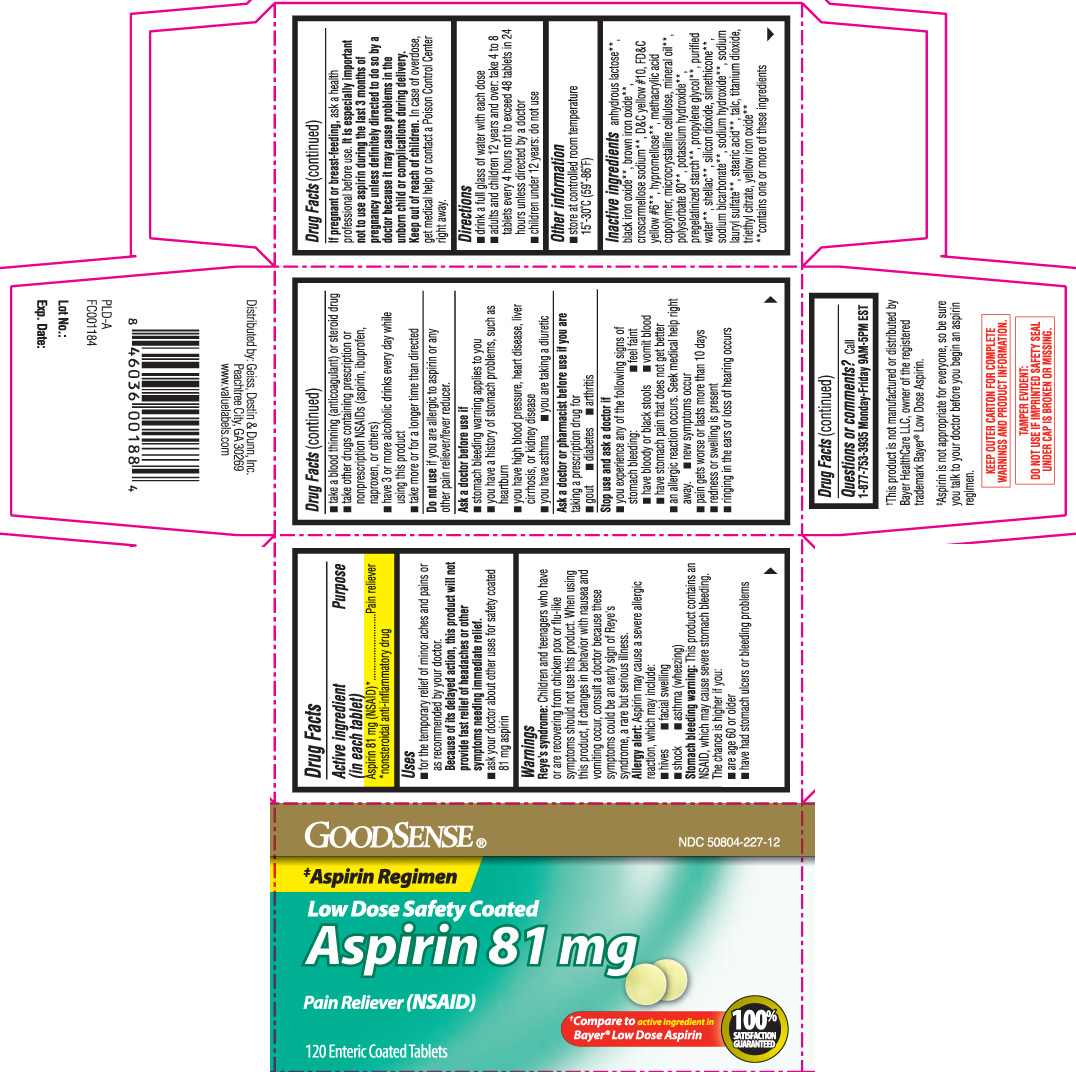
Uses
Warnings
Reye's syndrome: Children and teenagers who have or are recovering from chicken pox or flu-like symptoms should not use this product. When using this product, if changes in behavior with nausea and vomiting occur, consult a doctor because these symptoms could be an early sign of Reye's syndrome, a rare but serious illness.
Allergy alert: Aspirin may cause a severe allergic reaction, which may include:
- hives
- facial swelling
- shock
- asthma (wheezing)
Stomach bleeding warning: This product contains an NSAID, which may cause severe stomach bleeding. The chance is higher if you:
- are age 60 or older
- have had stomach ulcers or bleeding problems
- take a blood thinning (anticoagulant) or steroid drug
- take other drugs containing prescription or nonprescription NSAIDs [aspirin, ibuprofen, naproxen, or others]
- have 3 or more alcoholic drinks every day while using this product
- take more or for a longer time than directed
Ask a doctor before use if
- stomach bleeding warning applies to you
- you have a history of stomach problems, such as heartburn
- you have high blood pressure, heart disease, liver cirrhosis or kidney disease
- you have asthma
- you are taking a diuretic
Ask a doctor or pharmacist before use if you are
taking a prescription drug for
- gout
- diabetes
- arthritis
Stop use and ask a doctor if
- you experience any of the following signs of stomach bleeding:
- feel faint
- have bloody or black stools
- vomit blood
- have stomach pain that does not get better
- an allergic reaction occurs. Seek medical help right away.
- new symptoms occur
- pain gets worse or lasts more than 10 days
- redness or swelling is present
- ringing in the ears or loss of hearing occurs
Directions
Inactive ingredients
anhydrous lactose**, black iron oxide**, brown iron oxide**, croscarmellose sodium**, D&C yellow #10, FD&C yellow #6**, hypromellose**, methacrylic acid copolymer, microcrystalline cellulose, mineral oil**, polysorbate 80**, potassium hydroxide**, pregelatinized starch**, propylene glycol**, purified water**, shellac**, silicon dioxide, simethicone**, sodium bicarbonate**, sodium hydroxide**, sodium lauryl sulfate**, stearic acid**, talc, titanium dioxide, triethyl citrate, yellow iron oxide**
**contains one or more of these ingredients
Principal Display Panel
‡Aspirin Regimen
Low Dose Safety Coated
Aspirin 81 mg
Pain Reliever (NSAID)
†Compare to active ingredient in Bayer® Low Dose Aspirin
Enteric Coated Tablets
†This product is not manufactured or distributed by Bayer HealthCare LLC, owner of the registered trademark Bayer® Low Dose Aspirin.
‡Aspirin is not appropriate for everyone, so be sure you talk to your doctor before you begin an aspirin regimen.
KEEP OUTER CARTON FOR COMPLETE WARNINGS AND PRODUCT INFORMATION.
TAMPER EVIDENT: DO NOT USE IF IMPRINTED SAFETY SEAL UNDER CAP IS BROKEN OR MISSING.
Ditributed by: Geiss, Destin & Dunn, Inc.
Peachtree City, GA 30269
www.valuelabels.com
INGREDIENTS AND APPEARANCE
| ASPIRIN
LOW DOSE SAFETY COATED
aspirin tablet, coated |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Labeler - Good Sense (Geiss, Destin & Dunn, Inc.) (076059836) |
| Registrant - P & L Development, LLC (079765031) |