Search by Drug Name or NDC
NDC 50804-0826-03 Pain Relief 500 mg/1 Details
Pain Relief 500 mg/1
Pain Relief is a ORAL TABLET, COATED in the HUMAN OTC DRUG category. It is labeled and distributed by GoodSense. The primary component is ACETAMINOPHEN.
MedlinePlus Drug Summary
Acetaminophen is used to relieve mild to moderate pain from headaches, muscle aches, menstrual periods, colds and sore throats, toothaches, backaches, and reactions to vaccinations (shots), and to reduce fever. Acetaminophen may also be used to relieve the pain of osteoarthritis (arthritis caused by the breakdown of the lining of the joints). Acetaminophen is in a class of medications called analgesics (pain relievers) and antipyretics (fever reducers). It works by changing the way the body senses pain and by cooling the body.
Related Packages: 50804-0826-03Last Updated: 11/30/2022
MedLinePlus Full Drug Details: Acetaminophen
Product Information
| NDC | 50804-0826 |
|---|---|
| Product ID | 50804-826_e7901a04-c98e-fe5e-e053-2a95a90a8ddb |
| Associated GPIs | 64200010000315 |
| GCN Sequence Number | n/a |
| GCN Sequence Number Description | n/a |
| HIC3 | n/a |
| HIC3 Description | n/a |
| GCN | n/a |
| HICL Sequence Number | n/a |
| HICL Sequence Number Description | n/a |
| Brand/Generic | n/a |
| Proprietary Name | Pain Relief |
| Proprietary Name Suffix | Extra Strength |
| Non-Proprietary Name | Acetaminophen |
| Product Type | HUMAN OTC DRUG |
| Dosage Form | TABLET, COATED |
| Route | ORAL |
| Active Ingredient Strength | 500 |
| Active Ingredient Units | mg/1 |
| Substance Name | ACETAMINOPHEN |
| Labeler Name | GoodSense |
| Pharmaceutical Class | n/a |
| DEA Schedule | n/a |
| Marketing Category | OTC MONOGRAPH NOT FINAL |
| Application Number | part343 |
| Listing Certified Through | 2024-12-31 |
Package
Package Images
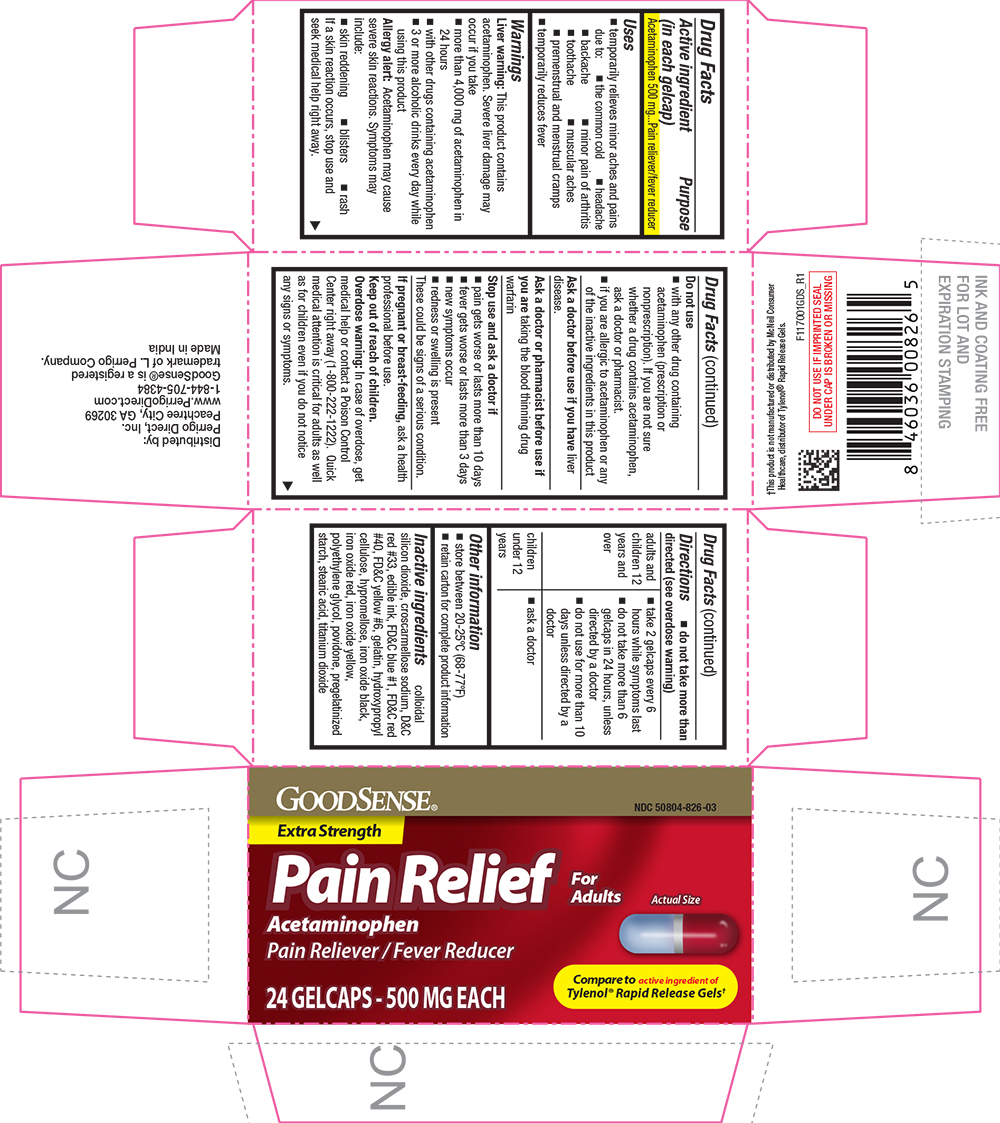
NDC 50804-0826-03 (50804082603)
| NDC Package Code | 50804-826-03 |
|---|---|
| Billing NDC | 50804082603 |
| Package | 1 BOTTLE, PLASTIC in 1 CARTON (50804-826-03) / 24 TABLET, COATED in 1 BOTTLE, PLASTIC |
| Marketing Start Date | 2017-08-01 |
| NDC Exclude Flag | N |
| Pricing Information | N/A |
Standard Product Labeling (SPL)/Prescribing Information SPL da4339c0-1822-41d8-8c20-214c379a5aa7 Details
Uses
Warnings
Liver warning
This product contains acetaminophen. Severe liver damage may occur if you take
- more than 4,000 mg of acetaminophen in 24 hours
- with other drugs containing acetaminophen
- 3 or more alcoholic drinks every day while using this product
Allergy alert
Acetaminophen may cause severe skin reactions. Symptoms may include:
- skin reddening
- blisters
- rash
If a skin reaction occurs, stop use and seek medical help right away.
Do not use
- with any other drug containing acetaminophen (prescription or nonprescription). If you are not sure whether a drug contains acetaminophen, ask a doctor or pharmacist.
- if you are allergic to acetaminophen or any of the inactive ingredients in this product
Directions
- do not take more than directed (see overdose warning)
| adults and children 12 years and over |
|
| children under 12 years |
|
Inactive ingredients
colloidal silicon dioxide, croscarmellose sodium, D&C red #33, edible ink, FD&C blue #1, FD&C red #40, FD&C yellow #6, gelatin, hydroxypropyl cellulose, hypromellose, iron oxide black, iron oxide red, iron oxide yellow, polyethylene glycol, povidone, pregelatinized starch, stearic acid, titanium dioxide
PRINCIPAL DISPLAY PANEL
INGREDIENTS AND APPEARANCE
| PAIN RELIEF
EXTRA STRENGTH
acetaminophen tablet, coated |
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
| Labeler - GoodSense (076059836) |