Search by Drug Name or NDC
NDC 51672-1275-07 Clotrimazole 10 mg/g Details
Clotrimazole 10 mg/g
Clotrimazole is a TOPICAL CREAM in the HUMAN PRESCRIPTION DRUG category. It is labeled and distributed by Taro Pharmaceuticals U.S.A., Inc.. The primary component is CLOTRIMAZOLE.
MedlinePlus Drug Summary
Topical clotrimazole is used to treat tinea corporis (ringworm; fungal skin infection that causes a red scaly rash on different parts of the body), tinea cruris (jock itch; fungal infection of the skin in the groin or buttocks), and tinea pedis (athlete's foot; fungal infection of the skin on the feet and between the toes). Clotrimazole is in a class of antifungal medications called imidazoles. It works by stopping the growth of fungi that cause infection.
Related Packages: 51672-1275-07Last Updated: 11/30/2022
MedLinePlus Full Drug Details: Clotrimazole Topical
Product Information
| NDC | 51672-1275 |
|---|---|
| Product ID | 51672-1275_e3356d10-c970-4e82-be44-6e36d4414f5a |
| Associated GPIs | 90154020003705 |
| GCN Sequence Number | 007361 |
| GCN Sequence Number Description | clotrimazole CREAM (G) 1 % TOPICAL |
| HIC3 | Q5F |
| HIC3 Description | TOPICAL ANTIFUNGALS |
| GCN | 30370 |
| HICL Sequence Number | 004137 |
| HICL Sequence Number Description | CLOTRIMAZOLE |
| Brand/Generic | Generic |
| Proprietary Name | Clotrimazole |
| Proprietary Name Suffix | n/a |
| Non-Proprietary Name | Clotrimazole |
| Product Type | HUMAN PRESCRIPTION DRUG |
| Dosage Form | CREAM |
| Route | TOPICAL |
| Active Ingredient Strength | 10 |
| Active Ingredient Units | mg/g |
| Substance Name | CLOTRIMAZOLE |
| Labeler Name | Taro Pharmaceuticals U.S.A., Inc. |
| Pharmaceutical Class | Azole Antifungal [EPC], Azoles [CS] |
| DEA Schedule | n/a |
| Marketing Category | ANDA |
| Application Number | ANDA072640 |
| Listing Certified Through | 2024-12-31 |
Package
Package Images
NDC 51672-1275-07 (51672127507)
| NDC Package Code | 51672-1275-7 |
|---|---|
| Billing NDC | 51672127507 |
| Package | 2 TUBE in 1 CARTON (51672-1275-7) / 45 g in 1 TUBE |
| Marketing Start Date | 1993-08-31 |
| NDC Exclude Flag | N |
| Pricing Information | N/A |
Standard Product Labeling (SPL)/Prescribing Information SPL 77883cfd-f13c-40cb-88b2-8f777e1cdce9 Details
DESCRIPTION
Clotrimazole Cream USP, 1% contains clotrimazole, a synthetic antifungal agent having the chemical name {1-(o-Chloro-α, α-diphenylbenzyl)imidazole}; the molecular formula C22H17ClN2; a molecular weight of 344.84; and the structural formula:

Clotrimazole is an odorless, white crystalline substance. It is practically insoluble in water, sparingly soluble in ether and very soluble in polyethylene glycol 400, ethanol and chloroform.
Each gram of clotrimazole cream USP contains 10 mg clotrimazole, dispersed in a vanishing cream base of cetostearyl alcohol, cetyl esters wax, 2-octyldodecanol, polysorbate 60, purified water, sorbitan monostearate, and benzyl alcohol (1%) as preservative.
CLINICAL PHARMACOLOGY
Clotrimazole is a broad-spectrum antifungal agent that is used for the treatment of dermal infections caused by various species of pathogenic dermatophytes, yeasts, and Malassezia furfur. The primary action of clotrimazole is against dividing and growing organisms.
In vitro, clotrimazole exhibits fungistatic and fungicidal activity against isolates of Trichophyton rubrum, Trichophyton mentagrophytes, Epidermophyton floccosum, Microsporum canis and Candida species including Candida albicans. In general, the in vitro activity of clotrimazole corresponds to that of tolnaftate and griseofulvin against the mycelia of dermatophytes (Trichophyton, Microsporum, and Epidermophyton), and to that of the polyenes (amphotericin B and nystatin) against budding fungi (Candida). Using an in vivo (mouse) and an in vitro (mouse kidney homogenate) testing system, clotrimazole and miconazole were equally effective in preventing the growth of the pseudomycelia and mycelia of Candida albicans.
Strains of fungi having a natural resistance to clotrimazole are rare. Only a single isolate of Candida guilliermondi has been reported to have primary resistance to clotrimazole.
No single-step or multiple-step resistance to clotrimazole has developed during successive passages of Candida albicans and Trichophyton mentagrophytes. No appreciable change in sensitivity was detected after successive passage of isolates of C. albicans, C. krusei, or C. pseudotropicalis in liquid or solid media containing clotrimazole. Also, resistance could not be developed in chemically induced mutant strains of polyene-resistant strains of polyene-resistant isolates of C. albicans. Slight, reversible resistance was noted in three isolates of C. albicans tested by one investigator. There is a single report that records the clinical emergence of C. albicans strain with considerable resistance to flucytosine and miconazole, and with cross-resistance to clotrimazole, the strain remained sensitive to nystatin and amphotericin B.
In studies of the mechanism of action, the minimum fungicidal concentration of clotrimazole caused leakage of intracellular phosphorus compounds into the ambient medium with concomitant breakdown of cellular nucleic acids and accelerated potassium efflux. Both these events began rapidly and extensively after addition of the drug.
Clotrimazole appears to be well absorbed in humans following oral administration and is eliminated mainly as inactive metabolites. Following topical and vaginal administration, however, clotrimazole appears to be minimally absorbed.
Six hours after the application of radioactive clotrimazole 1% cream and 1% solution onto intact and acutely inflamed skin, the concentration of clotrimazole varied from 100 mcg/cm3 in the stratum corneum to 0.5 to 1 mcg/cm3 in the stratum reticulare, and 0.1 mcg/cm3 in the subcutis. No measurable amount of radioactivity (≤0.001 mcg/mL) was found in the serum within 48 hours after application under occlusive dressing of 0.5 mL of the solution or 0.8 g of the cream. Only 0.5% or less of the applied radioactivity was excreted in the urine.
Following intravaginal administration of 100 mg 14C-clotrimazole vaginal tablets to nine adult females, an average peak serum level, corresponding to only 0.03 μg equivalents/mL of clotrimazole, was reached one to two days after application. After intravaginal administration of 5 g of 1% 14C-clotrimazole vaginal cream containing 50 mg active drug, to five subjects (one with candidal colpitis), serum levels corresponding to approximately 0.01 μg equivalents/mL were reached between 8 and 24 hours after application.
INDICATIONS AND USAGE
Clotrimazole cream USP is indicated for the topical treatment of candidiasis due to Candida albicans and tinea versicolor due to Malassezia furfur.
Clotrimazole is also available as a nonprescription item which is indicated for the topical treatment of the following dermal infections: tinea pedis, tinea cruris, and tinea corporis due to Trichophyton rubrum, Trichophyton mentagrophytes, Epidermophyton floccosum, and Microsporum canis.
CONTRAINDICATIONS
PRECAUTIONS
General
If irritation or sensitivity develops with the use of clotrimazole cream, treatment should be discontinued and appropriate therapy instituted.
Information for Patients
The patient should be advised to:
- Use the medication for the full treatment time even though the symptoms may have improved.
Notify the physician if there is no improvement after four weeks of treatment. - Inform the physician if the area of application shows signs of increased irritation (redness, itching, burning, blistering, swelling, oozing) indicative of possible sensitization.
- Avoid the use of occlusive wrappings or dressings.
- Avoid sources of infection or reinfection.
Laboratory Tests
If there is a lack of response to clotrimazole cream, appropriate microbiological studies should be repeated to confirm the diagnosis and rule out other pathogens before instituting another course of antimycotic therapy.
Drug Interactions
Synergism or antagonism between clotrimazole and nystatin, or amphotericin B, or flucytosine against strains of C. albicans has not been reported.
Carcinogenesis, Mutagenesis, Impairment of Fertility
An 18-month oral dosing study with clotrimazole in rats has not revealed any carcinogenic effect.
In tests for mutagenesis, chromosomes of the spermatophores of Chinese hamsters which had been exposed to clotrimazole were examined for structural changes during the metaphase. Prior to testing, the hamsters had received five oral clotrimazole doses of 100 mg/kg body weight. The results of this study showed that clotrimazole had not mutagenic effect.
Usage in Pregnancy
Pregnancy Category B
The disposition of 14C-clotrimazole has been studied in humans and animals. Clotrimazole is very poorly absorbed following dermal application or intravaginal administration to humans. (See CLINICAL PHARMACOLOGY.)
In clinical trials, use of vaginally applied clotrimazole in pregnant women in their second and third trimesters has not been associated with ill effects. There are, however, no adequate and well-controlled studies in pregnant women during their first trimester of pregnancy.
Studies in pregnant rats with intravaginal doses up to 100 mg/kg have revealed no evidence of harm to the fetus due to clotrimazole.
High oral doses of clotrimazole in rats and mice ranging from 50 to 120 mg/kg resulted in embryotoxicity (possibly secondary to maternal toxicity), impairment of mating, decreased litter size and number of viable young and decreased pup survival to weaning. However, clotrimazole was not teratogenic in mice, rabbits and rats at oral doses up to 200, 180 and 100 mg/kg respectively. Oral absorption in the rats amounts to approximately 90% of the administered dose.
Because animal reproductive studies are not always predictive of human response, this drug should be used only if clearly indicated during the first trimester of pregnancy.
ADVERSE REACTIONS
OVERDOSAGE
DOSAGE AND ADMINISTRATION
Gently massage sufficient Clotrimazole Cream USP, 1% into the affected and surrounding skin areas twice a day, in the morning and evening.
Clinical improvement, with relief of pruritus, usually occurs within the first week of treatment with clotrimazole cream. If the patient shows no clinical improvement after four weeks of treatment with clotrimazole cream, the diagnosis should be reviewed.
HOW SUPPLIED
SPL UNCLASSIFIED SECTION
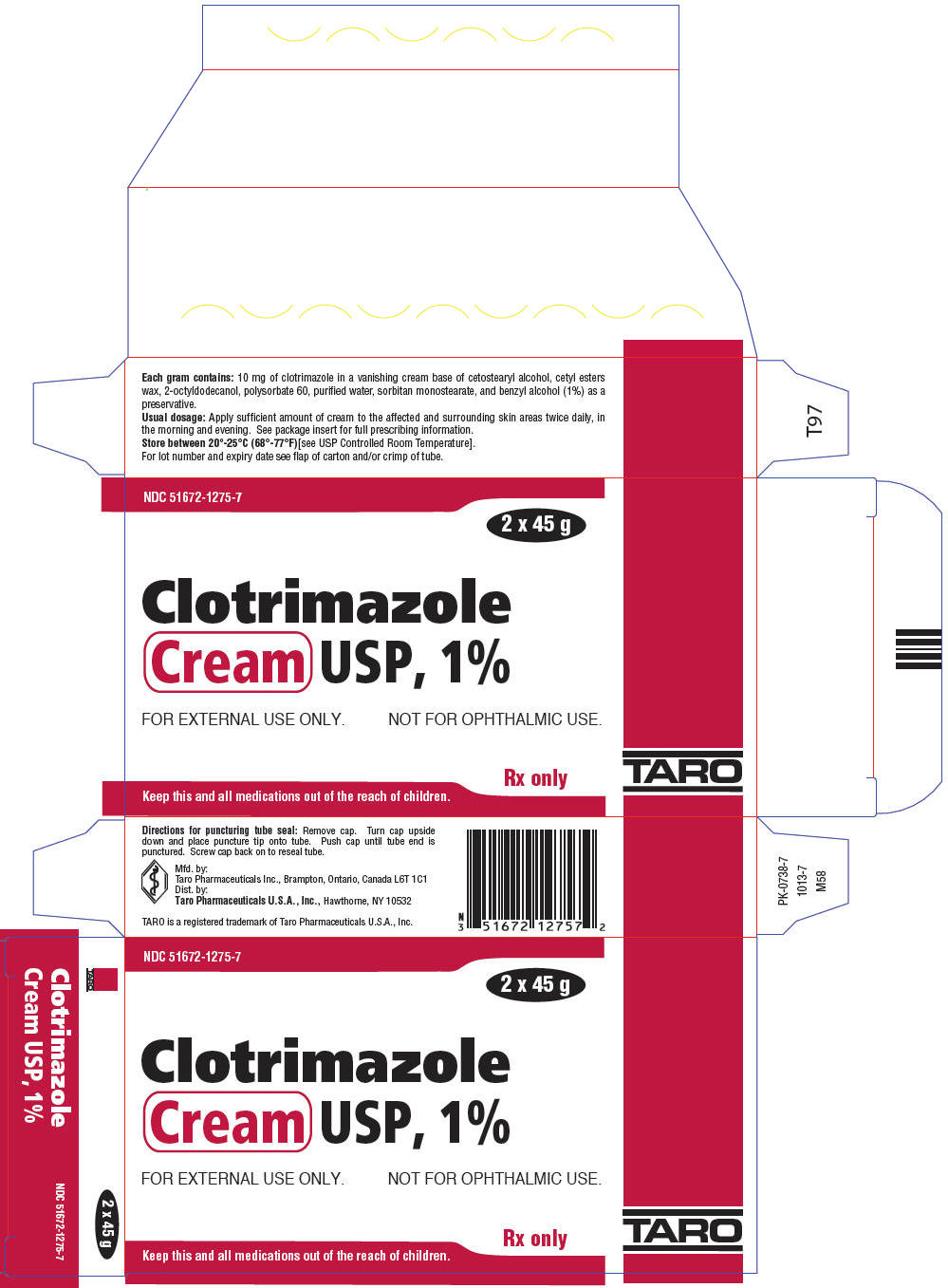
PRINCIPAL DISPLAY PANEL - 2 x 45 g Tube Carton
INGREDIENTS AND APPEARANCE
| CLOTRIMAZOLE
clotrimazole cream |
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
| Labeler - Taro Pharmaceuticals U.S.A., Inc. (145186370) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Taro Pharmaceuticals Inc. | 206263295 | MANUFACTURE(51672-1275) | |