Search by Drug Name or NDC
NDC 51672-1402-04 Calcipotriene and Betamethasone Dipropionate 50; 50 mg/g; ug/g Details
Calcipotriene and Betamethasone Dipropionate 50; 50 mg/g; ug/g
Calcipotriene and Betamethasone Dipropionate is a TOPICAL SUSPENSION in the HUMAN PRESCRIPTION DRUG category. It is labeled and distributed by Taro Pharmaceuticals U.S.A., Inc.. The primary component is BETAMETHASONE DIPROPIONATE; CALCIPOTRIENE.
MedlinePlus Drug Summary
Betamethasone topical is used to treat the itching, redness, dryness, crusting, scaling, inflammation, and discomfort of various skin conditions, including psoriasis (a skin disease in which red, scaly patches form on some areas of the body) and eczema (a skin disease that causes the skin to be dry and itchy and to sometimes develop red, scaly rashes). Betamethasone is in a class of medications called corticosteroids. It works by activating natural substances in the skin to reduce swelling, redness, and itching.
Related Packages: 51672-1402-04Last Updated: 11/30/2022
MedLinePlus Full Drug Details: Betamethasone Topical
Calcipotriene is used to treat psoriasis (a skin disease in which red, scaly patches form due to increased production of skin cells on some areas of the body). Calcipotriene is in a class of medications called synthetic vitamin D3 derivatives. It works by slowing the excessive production of skin cells.
Related Packages: 51672-1402-04Last Updated: 11/30/2022
MedLinePlus Full Drug Details: Calcipotriene Topical
Product Information
| NDC | 51672-1402 |
|---|---|
| Product ID | 51672-1402_68ae0c48-7f7c-43c6-a767-676e32be34dc |
| Associated GPIs | 90559902321825 |
| GCN Sequence Number | 063988 |
| GCN Sequence Number Description | calcipotriene/betamethasone SUSPENSION 0.005-.064 TOPICAL |
| HIC3 | T0A |
| HIC3 Description | TOPICAL VIT D ANALOG/ANTI-INFLAMMATORY STEROID |
| GCN | 99699 |
| HICL Sequence Number | 022851 |
| HICL Sequence Number Description | CALCIPOTRIENE/BETAMETHASONE DIPROPIONATE |
| Brand/Generic | Generic |
| Proprietary Name | Calcipotriene and Betamethasone Dipropionate |
| Proprietary Name Suffix | n/a |
| Non-Proprietary Name | Calcipotriene and Betamethasone Dipropionate |
| Product Type | HUMAN PRESCRIPTION DRUG |
| Dosage Form | SUSPENSION |
| Route | TOPICAL |
| Active Ingredient Strength | 50; 50 |
| Active Ingredient Units | mg/g; ug/g |
| Substance Name | BETAMETHASONE DIPROPIONATE; CALCIPOTRIENE |
| Labeler Name | Taro Pharmaceuticals U.S.A., Inc. |
| Pharmaceutical Class | Corticosteroid Hormone Receptor Agonists [MoA], Corticosteroid [EPC], Vitamin D Analog [EPC], Vitamin D [CS] |
| DEA Schedule | n/a |
| Marketing Category | ANDA |
| Application Number | ANDA213269 |
| Listing Certified Through | 2024-12-31 |
Package
Package Images

NDC 51672-1402-04 (51672140204)
| NDC Package Code | 51672-1402-4 |
|---|---|
| Billing NDC | 51672140204 |
| Package | 1 BOTTLE in 1 CARTON (51672-1402-4) / 60 g in 1 BOTTLE |
| Marketing Start Date | 2020-09-02 |
| NDC Exclude Flag | N |
| Pricing Information | |
| Price Per Unit | 3.10467 |
| Pricing Unit | GM |
| Effective Date | 2023-01-18 |
| NDC Description | CALCIPOTRIENE-BETAMETHASONE DP 0.005%-0.064% SUSPENSION |
| Pharmacy Type Indicator | C/I |
| OTC | N |
| Explanation Code | 4, 5 |
| Classification for Rate Setting | G |
| As of Date | 2024-01-10 |
Standard Product Labeling (SPL)/Prescribing Information SPL 1d0ab2e6-1ab1-4d3e-ad55-7fa26446f191 Details
HIGHLIGHTS OF PRESCRIBING INFORMATION
CALCIPOTRIENE and BETAMETHASONE DIPROPIONATE, for topical suspension
Initial U.S. Approval: 2006
RECENT MAJOR CHANGES
| Warnings and Precautions, Ophthalmic Adverse Reactions (5.5) | 7/2019 |
INDICATIONS AND USAGE
Calcipotriene and Betamethasone Dipropionate Topical Suspension is a combination of calcipotriene, a vitamin D analog, and betamethasone dipropionate, a corticosteroid, indicated for the topical treatment of plaque psoriasis of the scalp in patients 12 years and older and plaque psoriasis of the scalp and body in patients 18 years and older. (1)
DOSAGE AND ADMINISTRATION
- Shake bottle before use. (2)
- Apply Calcipotriene and Betamethasone Dipropionate Topical Suspension to affected areas on the scalp and body once daily for up to 8 weeks. Discontinue therapy when control is achieved. (2)
- Patients age 12 to 17 years should not use more than 60 grams per week. (2)
- Adult patients should not use more than 100 grams per week. (2)
- Do not use with occlusive dressings unless directed by a healthcare provider. (2)
- Avoid use on the face, groin, or axillae, or if skin atrophy is present at the treatment site. (2)
- Not for oral, ophthalmic, or intravaginal use. (2)
DOSAGE FORMS AND STRENGTHS
Topical Suspension: 0.005%/0.064% - each gram of Calcipotriene and Betamethasone Dipropionate Topical Suspension contains 50 mcg of calcipotriene and 0.643 mg of betamethasone dipropionate. (3)
CONTRAINDICATIONS
None (4)
WARNINGS AND PRECAUTIONS
- Hypercalcemia and Hypercalciuria: Hypercalcemia and hypercalciuria have been reported. If either occurs, discontinue until parameters of calcium metabolism normalize. (5.1)
- Effects on Endocrine System: Can cause reversible hypothalamic-pituitary-adrenal (HPA) axis suppression with the potential for glucocorticosteroid insufficiency during and after withdrawal of treatment. Risk factors include the use of high-potency topical corticosteroid, use over a large surface area or to areas under occlusion, prolonged use, altered skin barrier, liver failure, and use in pediatric patients. Modify use should HPA axis suppression develop. (5.2, 8.4)
- Ophthalmic Adverse Reactions: May increase the risk of cataracts and glaucoma. If visual symptoms occur, consider referral to an ophthalmologist. (5.5)
ADVERSE REACTIONS
The most common adverse reactions (≥1%) are folliculitis and burning sensation of skin (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Taro Pharmaceuticals U.S.A., Inc. at 1-866-923-4914 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Additional pediatric use information is approved for LEO Pharma A/S's Taclonex® (calcipotriene and betamethasone dipropionate) Topical Suspension. However, due to LEO Pharma A/S's marketing exclusivity rights, this drug product is not labeled with that information.
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 3/2020
Table Of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Hypercalcemia and Hypercalciuria
5.2 Effects on Endocrine System
5.3 Allergic Contact Dermatitis with Topical Corticosteroids
5.4 Allergic Contact Dermatitis with Topical Calcipotriene
5.5 Ophthalmic Adverse Reactions
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Postmarketing Experience
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
17 PATIENT COUNSELING INFORMATION
- *
- Sections or subsections omitted from the full prescribing information are not listed.
1 INDICATIONS AND USAGE
Calcipotriene and Betamethasone Dipropionate Topical Suspension is indicated for the topical treatment of plaque psoriasis of the scalp in patients 12 years and older and plaque psoriasis of the scalp and body in patients 18 years and older.
Additional pediatric use information is approved for LEO Pharma A/S's Taclonex® (calcipotriene and betamethasone dipropionate) Topical Suspension. However, due to LEO Pharma A/S's marketing exclusivity rights, this drug product is not labeled with that information.
2 DOSAGE AND ADMINISTRATION
Instruct patients to shake bottle prior to using calcipotriene and betamethasone dipropionate topical suspension. Apply calcipotriene and betamethasone dipropionate topical suspension to affected areas on the scalp and body once daily for up to 8 weeks. Calcipotriene and betamethasone dipropionate topical suspension should be discontinued when control is achieved. Instruct patients to wash their hands after applying the product. Inform patients that they should not take a bath or shower or wash their hair right after application of calcipotriene and betamethasone dipropionate topical suspension.
Patients 12 to 17 years should not use more than 60 grams per week and patients 18 years and older should not use more than 100 grams per week.
Calcipotriene and Betamethasone Dipropionate Topical Suspension should not be:
- Used with occlusive dressings unless directed by a healthcare provider.
- Used on the face, groin, or axillae, or if skin atrophy is present at the treatment site.
- Applied to the scalp in the 12 hours before or after any chemical treatments to the hair.
Calcipotriene and Betamethasone Dipropionate Topical Suspension is not for oral, ophthalmic, or intravaginal use.
3 DOSAGE FORMS AND STRENGTHS
5 WARNINGS AND PRECAUTIONS
5.1 Hypercalcemia and Hypercalciuria
Hypercalcemia and hypercalciuria have been observed with use of calcipotriene and betamethasone dipropionate topical suspension. If hypercalcemia or hypercalciuria develop, discontinue treatment until parameters of calcium metabolism have normalized. The incidence of hypercalcemia and hypercalciuria following calcipotriene and betamethasone dipropionate topical suspension treatment of more than 8 weeks has not been evaluated [see Clinical Pharmacology (12.2)].
5.2 Effects on Endocrine System
Hypothalamic-Pituitary-Adrenal Axis Suppression
Calcipotriene and betamethasone dipropionate topical suspension can cause reversible hypothalamic-pituitary-adrenal (HPA) axis suppression with the potential for clinical glucocorticosteroid insufficiency. This may occur during treatment or upon withdrawal of treatment. Factors that predispose a patient to HPA axis suppression include the use of high-potency steroids, large treatment surface areas, prolonged use, use of occlusive dressings, altered skin barrier, liver failure, and young age.
Evaluation for HPA axis suppression may be done by using the adrenocorticotropic hormone (ACTH) stimulation test. If HPA axis suppression is documented, gradually withdraw calcipotriene and betamethasone dipropionate topical suspension, reduce the frequency of application, or substitute with a less potent corticosteroid.
The following trials evaluated the effects of calcipotriene and betamethasone dipropionate topical suspension on HPA axis suppression:
- In a trial evaluating the effects of calcipotriene and betamethasone dipropionate topical suspension and calcipotriene and betamethasone dipropionate ointment on the HPA axis, 32 adult subjects applied both calcipotriene and betamethasone dipropionate topical suspension on the scalp and calcipotriene and betamethasone dipropionate ointment on the body. Adrenal suppression was identified in 5 of 32 subjects (16%) after 4 weeks of treatment and in 2 of 11 subjects (18%) who continued treatment for 8 weeks. In another trial, 36 adult subjects applied calcipotriene and betamethasone dipropionate topical suspension on the body and scalp and 7 subjects applied calcipotriene and betamethasone dipropionate topical suspension on the body. Adrenal suppression occurred in 3 out of 43 subjects (7%) after 4 weeks of treatment and in none of the 36 subjects who continued treatment for 8 weeks [see Clinical Pharmacology (12.2)].
- In two trials, the effects of calcipotriene and betamethasone dipropionate topical suspension on the HPA axis were evaluated in 31 and 30 pediatric subjects aged 12 to 17 years old who applied calcipotriene and betamethasone dipropionate topical suspension on the scalp and the scalp/body, respectively. Adrenal suppression occurred in 1 of 30 evaluable subjects (3%) after 4 weeks of treatment (scalp) and 5 of 31 evaluable subjects (16%) after up to 8 weeks of treatment (scalp and body) [see Use in Specific Populations (8.4) and Clinical Pharmacology (12.2)].
Cushing's Syndrome and Hyperglycemia
Cushing's syndrome and hyperglycemia may occur due to the systemic effects of the topical corticosteroid. These complications are rare and generally occur after prolonged exposure to excessively large doses, especially of high-potency topical corticosteroids.
Additional Considerations for Endocrine Adverse Reactions
Pediatric patients may be more susceptible to systemic toxicity due to their larger skin surface to body mass ratios [see Use in Specific Populations (8.4) and Clinical Pharmacology (12.2)].
Use of more than one corticosteroid-containing product at the same time may increase the total systemic corticosteroid exposure.
5.3 Allergic Contact Dermatitis with Topical Corticosteroids
Allergic contact dermatitis to a topical corticosteroid is usually diagnosed by observing a failure to heal rather than a clinical exacerbation. Such an observation should be corroborated with appropriate diagnostic patch testing.
5.4 Allergic Contact Dermatitis with Topical Calcipotriene
Allergic contact dermatitis has been observed with use of topical calcipotriene. Such an observation should be corroborated with appropriate diagnostic patch testing.
5.5 Ophthalmic Adverse Reactions
Use of topical corticosteroids, including calcipotriene and betamethasone dipropionate topical suspension, may increase the risk of posterior subcapsular cataracts and glaucoma. Cataracts and glaucoma have been reported with the postmarketing use of topical corticosteroid products [see Adverse Reactions (6.2)]. Avoid contact of calcipotriene and betamethasone dipropionate topical suspension with eyes. Calcipotriene and betamethasone dipropionate topical suspension may cause eye irritation. Advise patients to report any visual symptoms and consider referral to an ophthalmologist for evaluation.
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Clinical Trials Conducted in Subjects 18 years and older with Psoriasis of the Scalp
The rates of adverse reactions described below were from randomized, multicenter, vehicle- and/or active controlled clinical trials in adult subjects with psoriasis of the scalp [see Clinical Studies (14)]. Subjects applied study product once daily for 8 weeks, and the median weekly dose was 12.6 grams.
Adverse reactions that occurred in ≥1% of subjects treated with calcipotriene and betamethasone dipropionate topical suspension and at a rate higher than in subjects treated with vehicle are presented in Table 1.
| Calcipotriene and Betamethasone Dipropionate Topical Suspension | Betamethasone dipropionate in vehicle | Calcipotriene in vehicle | Vehicle | |
|---|---|---|---|---|
| N=1,953 | N=1,214 | N=979 | N=173 | |
| Event | # of subjects (%) | |||
| Folliculitis | 16 (1%) | 12 (1%) | 5 (1%) | 0 (0%) |
| Burning sensation of skin | 13 (1%) | 10 (1%) | 29 (3%) | 0 (0%) |
Other less common adverse reactions (<1% but >0.1%) were, in decreasing order of incidence: acne, exacerbation of psoriasis, eye irritation, and pustular rash.
In a 52-week trial, adverse reactions that were reported by >1% of subjects treated with calcipotriene and betamethasone dipropionate topical suspension were pruritus (3.6%), psoriasis (2.4%), erythema (2.1%), skin irritation (1.4%), and folliculitis (1.2%).
Clinical Trials Conducted in Subjects18 years and older with Psoriasis of the Body
In randomized, multicenter, vehicle- and/or active controlled clinical trials in adult subjects with plaque psoriasis on non-scalp areas, 824 subjects applied calcipotriene and betamethasone dipropionate topical suspension once daily for 8 weeks [see Clinical Studies (14)].The median weekly dose was 22.6grams.
There were no adverse reactions that occurred in ≥1%of subjects treated with calcipotriene and betamethasone dipropionate topical suspension and at a rate higher than in subjects treated with vehicle. Other less common adverse reactions (<1%but >0.1%) were, in decreasing order of incidence: rash and folliculitis.
Additional pediatric use information is approved for LEO Pharma A/S's Taclonex® (calcipotriene and betamethasone dipropionate) Topical Suspension. However, due to LEO Pharma A/S's marketing exclusivity rights, this drug product is not labeled with that information.
Clinical Trials Conducted in Subjects 12 to 17 years with Psoriasis of the Scalp
In two uncontrolled clinical trials, 109 subjects aged 12 to 17 years with plaque psoriasis of the scalp applied calcipotriene and betamethasone dipropionate topical suspension once daily for up to 8 weeks. The median weekly dose was 40 grams. Adverse reactions included acne, acneiform dermatitis and application site pruritus (0.9% each) [see Use in Specific Populations (8.4) and Clinical Pharmacology (12.2)].
6.2 Postmarketing Experience
Because adverse reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Postmarketing reports for local adverse reactions to topical corticosteroids included atrophy, striae, telangiectasias, itching, dryness, hypopigmentation, perioral dermatitis, secondary infection, and miliaria.
Ophthalmic adverse reactions of cataracts, glaucoma, increased intraocular pressure, and central serous chorioretinopathy have been reported during use of topical corticosteroids, including topical betamethasone products.
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
Available data with calcipotriene and betamethasone dipropionate topical suspension are not sufficient to evaluate a drug-associated risk for major birth defects, miscarriages, or adverse maternal or fetal outcomes. Although there are no available data on use of the calcipotriene component in pregnant women, systemic exposure to calcipotriene after topical administration of calcipotriene and betamethasone dipropionate topical suspension is likely to be low [see Clinical Pharmacology (12.3)].
Observational studies suggest an increased risk of having low birth weight infants with the maternal use of potent or super potent topical corticosteroids (see Data). Advise pregnant women that calcipotriene and betamethasone dipropionate topical suspension may increase the potential risk of having a low birth weight infant and to use calcipotriene and betamethasone dipropionate topical suspension on the smallest area of skin and for the shortest duration possible.
In animal reproduction studies, oral administration of calcipotriene to pregnant rats during the period of organogenesis resulted in an increased incidence of minor skeletal abnormalities, including enlarged fontanelles and extra ribs (see Data). Oral administration of calcipotriene to pregnant rabbits during the period of organogenesis had no apparent effects on embryo-fetal development. Subcutaneous administration of betamethasone dipropionate to pregnant rats and rabbits during the period of organogenesis resulted in fetal toxicity, including fetal deaths, reduced fetal weight, and fetal malformations (cleft palate and crooked or short tail) (see Data).
The available data do not allow the calculation of relevant comparisons between the systemic exposures of calcipotriene and betamethasone dipropionate observed in animal studies to the systemic exposures that would be expected in humans after topical use of calcipotriene and betamethasone dipropionate topical suspension.
The estimated background risk of major birth defects and miscarriage of the indicated population is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively.
Data
Human Data
Available observational studies in pregnant women did not identify a drug-associated risk of major birth defects, preterm delivery, or fetal mortality with the use of topical corticosteroids of any potency. However, when the dispensed amount of potent or super potent topical corticosteroids exceeded 300 grams during the entire pregnancy, maternal use was associated with an increased risk of low birth weight in infants.
Animal Data
Embryo-fetal development studies with calcipotriene were performed by the oral route in rats and rabbits. Pregnant rats received dosages of 0, 6, 18, or 54 mcg/kg/day (0, 36, 108, and 324 mcg/m2/day, respectively) on days 6 to 15 of gestation (the period of organogenesis). There were no apparent effects on maternal survival, behavior, or body weight gain, no effects on litter parameters, and no effects on the incidence of major malformations in fetuses. Fetuses from dams dosed at 54 mcg/kg/day exhibited a significantly increased incidence of minor skeletal abnormalities, including enlarged fontanelles and extra ribs.
Pregnant rabbits were dosed daily with calcipotriene at exposures of 0, 4, 12, or 36 mcg/kg/day (0, 48, 144, and 432 mcg/m2/day, respectively) on days 6 to 18 of gestation (the period of organogenesis). Mean maternal body weight gain was reduced in animals dosed at 12 or 36 mcg/kg/day. The incidence of fetal deaths was increased in the group dosed at 36 mcg/kg/day; reduced fetal weight was also observed in this group. The incidence of major malformations among fetuses was not affected. An increase in the incidence of minor skeletal abnormalities, including incomplete ossification of sternebrae, pubic bones, and forelimb phalanges, was observed in the group dosed at 36 mcg/kg/day.
Embryo-fetal development studies with betamethasone dipropionate were performed via subcutaneous injection in mice and rabbits. Pregnant mice were administered doses of 0, 156, 625, or 2500 mcg/kg/day (0, 468, 1875, and 7500 mcg/m2/day, respectively) on days 7 through 13 of gestation (the period of organogenesis). Betamethasone dipropionate induced fetal toxicity, including fetal deaths, reduced fetal weight, malformations (increased incidence of the cleft palate and crooked or short tail), and minor skeletal abnormalities (delayed ossification of vertebra and sternebrae). Fetal toxicity was observed at the lowest exposure that was evaluated (156 mcg/kg/day).
Pregnant rabbits were injected subcutaneously at dosages of 0, 0.625, 2.5, and 10 mcg/kg/day (0, 7.5, 30, and 120 mcg/m2/day, respectively) on days 6 through 18 of gestation (the period of organogenesis). Betamethasone dipropionate induced fetal toxicity, including fetal deaths, reduced fetal weight, external malformations (including malformed ears, cleft palate, umbilical hernia, kinked tail, club foot, and club hand), and skeletal malformations (including absence of phalanges of the first digit and cranial dysplasia) at dosages of 2.5 mcg/kg/day and above.
Calcipotriene was evaluated for effects on peri- and post-natal development when orally administered to pregnant rats at dosages of 0, 6, 18 or 54 mcg/kg/day (0, 36, 108, and 324 mcg/m2/day, respectively) from gestation day 15 through day 20 postpartum. No remarkable effects were observed on any parameter, including survival, behavior, body weight, litter parameters, or the ability to nurse or rear pups.
Betamethasone dipropionate was evaluated for effects on peri- and post-natal development when orally administered to pregnant rats at dosages of 0, 100, 300, and 1000 mcg/kg/day (0, 600, 1800, and 6000 mcg/m2/day, respectively) from gestation day 6 through day 20 postpartum. Mean maternal body weight was significantly reduced on gestation day 20 in animals dosed at 300 and 1000 mcg/kg/day. The mean duration of gestation was slightly, but statistically significantly, increased at 100, 300, and 1000 mcg/kg/day. The mean percentage of pups that survived to day 4 was reduced in relation to dosage. On lactation day 5, the percentage of pups with a reflex to right themselves when placed on their back was significantly reduced at 1000 mcg/kg/day. No effects on the ability of pups to learn were observed, and the ability of the offspring of treated rats to reproduce was not affected.
8.2 Lactation
Risk Summary
There is no information regarding the presence of topically administered calcipotriene and betamethasone dipropionate in human milk, the effects on the breastfed infant, or the effects on milk production. Concentrations of calcipotriene in plasma are low after topical administration, and therefore, concentrations in human milk are likely to be low [see Clinical Pharmacology (12.3)]. It is not known whether topical administration of large amounts of betamethasone dipropionate could result in sufficient systemic absorption to produce detectable quantities in human milk (see Clinical Considerations). The developmental and health benefits of breastfeeding should be considered along with the mother's clinical need for calcipotriene and betamethasone dipropionate topical suspension and any potential adverse effects on the breastfed child from calcipotriene and betamethasone dipropionate topical suspension or from the underlying maternal condition.
Clinical Considerations
To minimize potential exposure to the breastfed infant via breast milk, use calcipotriene and betamethasone dipropionate topical suspension on the smallest area of skin and for the shortest duration possible while breastfeeding. Advise breastfeeding women not to apply calcipotriene and betamethasone dipropionate topical suspension directly to the nipple and areola to avoid direct infant exposure [see Use in Specific Populations (8.4)].
8.4 Pediatric Use
The safety and effectiveness of calcipotriene and betamethasone dipropionate topical suspension for the treatment of plaque psoriasis of the scalp have been established in pediatric patients age 12 to 17 years. The use of calcipotriene and betamethasone dipropionate topical suspension for this indication is supported by evidence from adequate and well-controlled trials in adults and from uncontrolled trials in pediatric subjects that enrolled 109 adolescents with moderate psoriasis of the scalp. After 4 weeks of once daily treatment with calcipotriene and betamethasone dipropionate topical suspension, HPA axis suppression was observed in 3% of adolescents with psoriasis of the scalp and 16% of adolescents with psoriasis of the scalp and body [see Warnings and Precautions (5.2), Adverse Reactions (6.1), and Clinical Pharmacology (12.2)].
Because of a higher ratio of skin surface area to body mass, pediatric patients are at a greater risk than adults of systemic toxicity when treated with topical corticosteroids. Pediatric patients are, therefore, also at greater risk of HPA axis suppression and adrenal insufficiency with the use of topical corticosteroids including calcipotriene and betamethasone dipropionate topical suspension [see Clinical Pharmacology (12.2)].
Rare systemic toxicities such as Cushing's syndrome, linear growth retardation, delayed weight gain, and intracranial hypertension have been reported in pediatric patients, especially those with prolonged exposure to large doses of high potency topical corticosteroids. Local adverse reactions including striae have also been reported with use of topical corticosteroids in pediatric patients.
The safety and effectiveness of calcipotriene and betamethasone dipropionate topical suspension in pediatric patients less than 12 years of age have not been established.
Additional pediatric use information is approved for LEO Pharma A/S's Taclonex® (calcipotriene and betamethasone dipropionate) Topical Suspension. However, due to LEO Pharma A/S's marketing exclusivity rights, this drug product is not labeled with that information.
8.5 Geriatric Use
Clinical studies of calcipotriene and betamethasone dipropionate topical suspension in plaque psoriasis on non-scalp areas included 124 subjects who were 65 years of age or older, and 36 were 75 years of age or older. Clinical studies of calcipotriene and betamethasone dipropionate topical suspension in subjects with psoriasis of the scalp included 334 subjects who were 65 years or older and 84 subjects who were 75 years or older.
No overall differences in safety or effectiveness of calcipotriene and betamethasone dipropionate topical suspension were observed between these subjects and younger subjects, and other reported clinical experience has not identified any differences in responses between the elderly and younger subjects, but greater sensitivity of some older individuals cannot be ruled out.
11 DESCRIPTION
Calcipotriene and betamethasone dipropionate topical suspension contains calcipotriene hydrate and betamethasone dipropionate. It is for topical use only. Calcipotriene hydrate is a synthetic vitamin D3 analog.
Calcipotriene Hydrate
Calcipotriene hydrate is a vitamin D analog and has the chemical name 9,10-secochola-5,7,10(19),22-tetraene-1,3,24-triol,24-cyclopropyl-,monohydrate, (1α,3β,5Z,7E,22E,24S) with the empirical formula C27H40O3∙H2O, a molecular weight of 430.6, and the following structural formula (calcipotriene hydrate is a white to almost white, crystalline compound):

Betamethasone Dipropionate
Betamethasone dipropionate is a synthetic corticosteroid and has the chemical name pregna-1,4-diene-3,20-dione-9-fluoro-11hydroxy-16-methyl-17,21-bis(1-oxypropoxy)-(11β,16β), with the empirical formula C28H37FO7, a molecular weight of 504.6, and the following structural formula (betamethasone dipropionate is a white to almost white, crystalline powder):

Calcipotriene and Betamethasone Dipropionate Topical Suspension
Each gram of calcipotriene and betamethasone dipropionate topical suspension contains 50 mcg of calcipotriene (equivalent to 52.18 mcg of calcipotriene hydrate) and 0.643 mg of betamethasone dipropionate (equivalent to 0.5 mg of betamethasone) in a base of all-rac-alpha-tocopherol, butylhydroxytoluene, hydrogenated castor oil, mineral oil, and polyoxypropylene stearyl ether. Calcipotriene and betamethasone dipropionate topical suspension is an odorless, clear to slightly off-white suspension.
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Calcipotriene and betamethasone dipropionate topical suspension combines the pharmacological effects of calcipotriene hydrate as a synthetic vitamin D3 analog and betamethasone dipropionate as a synthetic corticosteroid. However, while their pharmacologic and clinical effects are known, the exact mechanisms of their actions in the treatment of plaque psoriasis of the scalp and body are unknown.
12.2 Pharmacodynamics
Hypothalamic-Pituitary-Adrenal (HPA) Axis Suppression:
HPA axis suppression was evaluated in four trials (Trial A, B, C, and D) following the application of calcipotriene and betamethasone dipropionate topical suspension. In all these trials, adrenal suppression was defined by a 30-minute post-stimulation cortisol level ≤18 mcg/dL.
- In Trial A, HPA axis suppression was evaluated in adult subjects (N=32) with extensive psoriasis involving at least 30% of the scalp and, in total, 15 to 30% of the body surface area. Treatment consisted of once daily application of calcipotriene and betamethasone dipropionate topical suspension on the scalp in combination with calcipotriene and betamethasone dipropionate ointment on the body for 4 to 8 weeks. Adrenal suppression was observed in 5 of 32 subjects (16%) after 4 weeks of treatment and in 2 of 11 subjects (18%) who continued treatment for 8 weeks.
- In Trial B, HPA axis suppression was evaluated in adult subjects (N=43) with extensive psoriasis involving 15 to 30% of the body surface area (including the scalp). Treatment consisted of once daily application of calcipotriene and betamethasone dipropionate topical suspension to the body (including the scalp in 36 out of 43 subjects) for 4 to 8 weeks. Adrenal suppression was observed in 3 out of 43 subjects (7%) after 4 weeks of treatment and in none of the 36 subjects (0%) who continued treatment for 8 weeks.
- In Trial C, HPA axis suppression was evaluated in pediatric subjects 12 to 17 years (N=30) with plaque psoriasis of the scalp involving at least 20% of the scalp area. Treatment consisted of once daily application of calcipotriene and betamethasone dipropionate topical suspension to the affected area on the scalp for up to 8 weeks. Adrenal suppression was observed in 1 of 30 evaluable subjects (3%) after 4 weeks of treatment and in no subjects (0%) who continued treatment for 8 weeks [see Use in Specific Populations (8.4)].
- In Trial D, HPA axis suppression was evaluated in a subset of pediatric subjects aged 12 to 17 years (N=31) with plaque psoriasis of the scalp and body involving 10% to 29% of the body surface area. Adrenal suppression was observed in 5 of 31 subjects (16%): 3 subjects after 4 weeks of treatment, 1 subject after 8 weeks of treatment, and 1 subject after both 4 and 8 weeks of treatment [see Use in Specific Populations (8.4)].
Effects on Calcium Metabolism
The effect on calcium metabolism was evaluated following the application of calcipotriene and betamethasone dipropionate topical suspension (these trials are described above).
- In Trial A, elevated urinary calcium levels outside the normal range were observed in two subjects (one at 4 weeks and one at 8 weeks).
- In Trial B, there was no change in mean serum or urinary calcium levels. Elevated urinary calcium levels outside the normal range were observed in two subjects (one at 4 weeks and one at 8 weeks).
- In Trial C (N=109), including 31 subjects with at least 20% scalp involvement and 78 subjects with at least 10% scalp involvement, no cases of hypercalcemia and no clinically relevant changes in urinary calcium were reported.
12.3 Pharmacokinetics
Absorption
The systemic effect of calcipotriene and betamethasone dipropionate topical suspension in psoriasis was investigated in Trials A and B [see Clinical Pharmacology (12.2)].
In Trial A, the serum levels of calcipotriene and betamethasone dipropionate and their major metabolites were measured after 4 and 8 weeks of once daily application of calcipotriene and betamethasone dipropionate topical suspension on the scalp in combination with calcipotriene and betamethasone dipropionate ointment on the body. Calcipotriene and betamethasone dipropionate were below the lower limit of quantification in all serum samples of the 34 subjects evaluated. However, one major metabolite of calcipotriene (MC1080) was quantifiable in 10 of 34 (29%) subjects at week 4 and in 5 of 12 (42%) subjects at week 8. The major metabolite of betamethasone dipropionate, betamethasone 17propionate (B17P) was also quantifiable in 19 of 34 (56%) subjects at week 4 and 7 of 12 (58%) subjects at week 8. The serum concentrations for MC1080 ranged from 20-75 pg/mL. The clinical significance of this finding is unknown.
In Trial B, the plasma levels of calcipotriene and betamethasone dipropionate and their major metabolites were measured after 4 weeks of once daily application of calcipotriene and betamethasone dipropionate topical suspension. Calcipotriene and its metabolite MC1080 were below the lower limit of quantification in all plasma samples. Betamethasone dipropionate was quantifiable in 4 of 43 (9) subjects. The metabolite of betamethasone dipropionate (B17P) was quantifiable in 16 of 43 (37%) subjects. The plasma concentrations of betamethasone dipropionate ranged from 30.9-63.5 pg/mL and that of its metabolite betamethasone 17-propionate ranged from 30.5 to 257 pg/mL. The clinical significance of this finding is unknown.
Elimination
Metabolism
Calcipotriene: Calcipotriene metabolism following systemic uptake is rapid and occurs in the liver. The primary metabolites of calcipotriene are less potent than the parent compound.
Calcipotriene is metabolized to MC1046 (the α,β-unsaturated ketone analog of calcipotriene), which is metabolized further to MC1080 (a saturated ketone analog). MC1080 is the major metabolite in plasma. MC1080 is slowly metabolized to calcitroic acid.
Betamethasone dipropionate: Betamethasone dipropionate is metabolized to betamethasone 17-propionate and betamethasone, including the 6β-hydroxy derivatives of those compounds by hydrolysis. Betamethasone 17-propionate (B17P) is the primary metabolite.
Additional pediatric use information is approved for LEO Pharma A/S's Taclonex® (calcipotriene and betamethasone dipropionate) Topical Suspension. However, due to LEO Pharma A/S's marketing exclusivity rights, this drug product is not labeled with that information.
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
When calcipotriene was applied topically to mice for up to 24 months at dosages of 3, 10, and 30 mcg/kg/day (9, 30, and 90 mcg/m2/day, respectively), no significant changes in tumor incidence were observed when compared to control.
A 104-week oral carcinogenicity study was conducted with calcipotriene in male and female rats at doses of 1, 5 and 15 mcg/kg/day (6, 30, and 90 mcg/m2/day, respectively). Beginning week 71, the dosage for high-dose animals of both genders was reduced to 10 mcg/kg/day (60 mcg/m2/day). A treatment-related increase in benign C-cell adenomas was observed in the thyroid of females that received 15 mcg/kg/day. A treatment-related increase in benign pheochromocytomas was observed in the adrenal glands of males that received 15 mcg/kg/day. No other statistically significant differences in tumor incidence were observed when compared to control. The relevance of these findings to patients is unknown.
When betamethasone dipropionate was applied topically to CD-1 mice for up to 24 months at dosages approximating 1.3, 4.2, and 8.5 mcg/kg/day in females, and 1.3, 4.2, and 12.9 mcg/kg/day in males (up to 26 mcg/m2/day and 39 mcg/m2/day, in females and males, respectively), no significant changes in tumor incidence were observed when compared to control.
When betamethasone dipropionate was administered via oral gavage to male and female Sprague Dawley rats for up to 24 months at dosages of 20, 60, and 200 mcg/kg/day (120, 360, and 1200 mcg/m2/day, respectively), no significant changes in tumor incidence were observed when compared to control.
Calcipotriene did not elicit any genotoxic effects in the Ames mutagenicity assay, the mouse lymphoma TK locus assay, the human lymphocyte chromosome aberration test, or the mouse micronucleus test. Betamethasone dipropionate did not elicit any genotoxic effects in the Ames mutagenicity assay, the mouse lymphoma TK locus assay, or in the rat micronucleus test.
Studies in rats with oral doses of up to 54 mcg/kg/day (324 mcg/m2/day) of calcipotriene indicated no impairment of fertility or general reproductive performance. Studies in male rats at oral doses of up to 200 mcg/kg/day (1200 mcg/m2/day), and in female rats at oral doses of up to 1000 mcg/kg/day (6000 mcg/m2/day), of betamethasone dipropionate indicated no impairment of fertility.
14 CLINICAL STUDIES
Clinical Trials Conducted in Subjects 18 Years and Older with Psoriasis of the Scalp
Two multicenter, randomized, double-blind trials were conducted in adult subjects with moderate to very severe psoriasis of the scalp.
- In Trial One, 1,407 subjects were randomized to 1 of 4 treatment groups: calcipotriene and betamethasone dipropionate topical suspension, betamethasone dipropionate in the same vehicle, calcipotriene in the same vehicle, or the vehicle alone.
- In Trial Two, 1,280 subjects were randomized to 1 of 3 treatment groups: calcipotriene and betamethasone dipropionate topical suspension, betamethasone dipropionate in the same vehicle, or calcipotriene in the same vehicle.
Both trials enrolled subjects with moderate to very severe psoriasis of the scalp. The majority of subjects had disease of moderate severity at baseline. Subjects were treated once daily for 8 weeks. Efficacy was assessed as the proportion of subjects at Week 8 with absent or very mild disease according to the Investigator's Global Assessment of Disease Severity. "Clear" was defined as no evidence of redness, thickness or scaling. "Almost clear" was defined as an overall clinical picture of lesions with the presence of minimal erythema. Table 2 contains the response rates in each of these 2 trials.
| Calcipotriene and Betamethasone Dipropionate Topical Suspension | Betamethasone Dipropionate in vehicle | Calcipotriene in vehicle | Vehicle | |
|---|---|---|---|---|
| Trial One | (N=494) | (N=531) | (N=256) | (N=126) |
| Week 2 | 55.5% | 46.1% | 18.4% | 9.5% |
| Week 8 | 70.0% | 63.1% | 36.7% | 19.8% |
| Trial Two | (N=512) | (N=517) | (N=251) | - |
| Week 2 | 47.1% | 36.4% | 12.7% | - |
| Week 8 | 67.2% | 59.6% | 41.0% | - |
Clinical Trials Conducted in Subjects 18 Years and Older with Psoriasis of the Body
One multicenter, randomized, double-blind trial was conducted in subjects with mild to moderate plaque psoriasis on non-scalp areas, excluding face, axillae, and groin. In this trial, 1152 subjects were randomized to 1 of 4 treatment groups: calcipotriene and betamethasone dipropionate topical suspension, betamethasone dipropionate in the same vehicle, calcipotriene in the same vehicle, or the vehicle alone. Seventy eight percent (78%) of subjects had disease of moderate severity at baseline. Subjects were treated once daily for 8 weeks. Efficacy was assessed at Week 4 and Week 8 as the proportion of subjects who were "Clear" or "Almost clear" according to the Investigator's Global Assessment of Disease Severity. Subjects with mild disease at baseline were required to be "Clear" to be considered a success. Table 3 contains the response rates in this trial.
| Calcipotriene and Betamethasone Dipropionate Topical Suspension | Betamethasone Dipropionate in vehicle | Calcipotriene in vehicle | Vehicle | |
|---|---|---|---|---|
| (N=482) | (N=479) | (N=96) | (N=95) | |
|
||||
| Week 4 | 13.3% | 12.5% | 5.2% | 2.1% |
| Week 8 | 29.0% | 21.5% | 14.6% | 6.3% |
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
See FDA-approved patient labeling (Patient Information and Instructions for Use).
Administration Instructions
- Instruct pediatric patients (12 to 17 years) not to use more than 60 grams per week.
- Instruct adult patients (18 years and older) not to use more than 100 grams per week.
- Instruct patients to discontinue therapy when control is achieved unless directed otherwise by the healthcare provider.
- Advise patients not to apply calcipotriene and betamethasone dipropionate topical suspension to the scalp in the 12 hours before or after any chemical treatments to the hair since hair treatments may involve strong chemicals. Talk with the healthcare provider first.
- Inform patients that they should not take a bath or shower or wash their hair right after application of calcipotriene and betamethasone dipropionate topical suspension.
- Advise patients to avoid use of calcipotriene and betamethasone dipropionate topical suspension, on the face, underarms, groin or eyes. If this medicine gets on face or in eyes, wash area right away.
- Advise patients not to occlude the treatment area with a bandage or other covering unless directed by the healthcare provider.
- Instruct patients to shake bottle prior to using calcipotriene and betamethasone dipropionate topical suspension and to wash hands after application.
Local Reactions and Skin Atrophy
Advise patients that local reactions and skin atrophy are more likely to occur with occlusive use, prolonged use or use of higher potency corticosteroids.
Hypercalcemia and Hypercalciuria
Advise patients that hypercalcemia and hypercalciuria have been observed with the use of calcipotriene and betamethasone dipropionate topical suspension [see Warnings and Precautions (5.1)].
HPA Axis Suppression, Cushing's Syndrome, and Hyperglycemia
Advise patients that calcipotriene and betamethasone dipropionate topical suspension can cause HPA access suppression, Cushing's syndrome, and/or hyperglycemia [see Warnings and Precautions (5.2)].
Ophthalmic Adverse Reactions
Advise patients to avoid contact of calcipotriene and betamethasone dipropionate topical suspension with eyes and to report any visual symptoms [see Warnings and Precautions (5.5)].
Possible Avoidance of Other Products Containing Calcipotriene or a Corticosteroid
Instruct patients not to use other products containing calcipotriene or a corticosteroid with calcipotriene and betamethasone dipropionate topical suspension without first talking to the healthcare provider.
Pregnancy and Lactation
- Advise pregnant women that calcipotriene and betamethasone dipropionate topical suspension may increase the potential risk of having a low birth weight infant and to use calcipotriene and betamethasone dipropionate topical suspension on the smallest area of skin and for the shortest duration possible [see Use in Specific Populations (8.1)].
- Advise breastfeeding women not to apply calcipotriene and betamethasone dipropionate topical suspension directly to the nipple and areola to avoid direct infant exposure [see Use in Specific Populations (8.2)].
SPL UNCLASSIFIED SECTION
SPL UNCLASSIFIED SECTION
| PATIENT INFORMATION Calcipotriene and Betamethasone Dipropionate (kal si poe trye´ een and bay" ta meth´ a sone dye proe´ pee oh nate) Topical Suspension |
||
|---|---|---|
| This Patient Information has been approved by the U.S. Food and Drug Administration. Issued: March, 2020 PK-9018-0 0320-0 39 |
||
| Important: Calcipotriene and betamethasone dipropionate topical suspension is for use on skin only (topical). Do not get calcipotriene and betamethasone dipropionate topical suspension near or in your mouth, eyes, or vagina. | ||
| There are other medicines that contain the same medicine that is in calcipotriene and betamethasone dipropionate topical suspension and are used to treat plaque psoriasis. Do not use other products containing calcipotriene or a corticosteroid medicine with calcipotriene and betamethasone dipropionate topical suspension without talking to your healthcare provider first. | ||
| What is Calcipotriene and Betamethasone Dipropionate Topical Suspension? | ||
| Calcipotriene and betamethasone dipropionate topical suspension is a prescription medicine used on the skin (topical) to treat plaque psoriasis of the scalp in people 12 years and older and plaque psoriasis of the scalp and body in people 18 years and older. | ||
| It is not known if calcipotriene and betamethasone dipropionate topical suspension is safe and effective in children under 12 years of age. | ||
| Before you use Calcipotriene and Betamethasone Dipropionate Topical Suspension, tell your healthcare provider about all of your medical conditions, including if you: | ||
|
||
| Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. | ||
| How should I use Calcipotriene and Betamethasone Dipropionate Topical Suspension? | ||
| See the "Instructions for Use" for detailed information about the right way to use Calcipotriene and Betamethasone Dipropionate Topical Suspension. | ||
|
||
| What are the possible side effects of Calcipotriene and Betamethasone Dipropionate Topical Suspension? | ||
| Calcipotriene and Betamethasone Dipropionate Topical Suspension may cause serious side effects, including: | ||
|
||
|
|
|
|
||
| The most common side effects of Calcipotriene and Betamethasone Dipropionate Topical Suspension include inflamed hair pores (folliculitis) and skin burning. | ||
| Call your doctor for medical advice about side effects. You may report side effects to FDA at 1800-FDA-1088. | ||
| How should I store Calcipotriene and Betamethasone Dipropionate Topical Suspension? | ||
|
||
| Keep Calcipotriene and Betamethasone Dipropionate Topical Suspension and all medicines out of reach of children. | ||
| General information about Calcipotriene and Betamethasone Dipropionate Topical Suspension. | ||
| Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use calcipotriene and betamethasone dipropionate topical suspension for a condition for which it was not prescribed. Do not give calcipotriene and betamethasone dipropionate topical suspension to other people, even if they have the same symptoms you have. It may harm them. You can ask your pharmacist or healthcare provider for information about calcipotriene and betamethasone dipropionate topical suspension that is written for health professionals. | ||
| What are the ingredients in calcipotriene and betamethasone dipropionate topical suspension? | ||
| Active ingredients: calcipotriene hydrate and betamethasone dipropionate. | ||
| Inactive ingredients: all-rac-alpha-tocopherol, butylhydroxytoluene, hydrogenated castor oil, mineral oil, and polyoxypropylene stearyl ether | ||
| Additional pediatric use information is approved for LEO Pharma A/S's Taclonex® (calcipotriene and betamethasone dipropionate) Topical Suspension. However, due to LEO Pharma A/S's marketing exclusivity rights, this drug product is not labeled with that information. | ||
| Mfd. by: Taro Pharmaceuticals Inc., Brampton, Ontario, Canada L6T 1C1 | ||
| Dist. by: Taro Pharmaceuticals U.S.A., Inc., Hawthorne, NY 10532 | ||
| For more information, go to www.taro.com or call 1-866-923-4914 | ||
INSTRUCTIONS FOR USE
| Keep calcipotriene and betamethasone dipropionate topical suspension and all medicines out of the reach of children.
This Instructions for Use has been approved by the U.S. Food and Drug Administration. Mfd. by: Taro Pharmaceuticals Inc., Brampton, Ontario, Canada L6T 1C1 Dist. by: Taro Pharmaceuticals U.S.A., Inc., Hawthorne, NY 10532 Issued: March, 2020 PK-9018-0 0320-0 39 |
||||
| Instructions for Use Calcipotriene and Betamethasone Dipropionate (kal si poe trye´ een and bay" ta meth´ a sone dye proe´ pee oh nate) Topical Suspension |
||||
| Important: Calcipotriene and betamethasone dipropionate topical suspension is for use on skin only (topical). Do not get calcipotriene and betamethasone dipropionate topical suspension near or in your mouth, eyes, or vagina. | ||||
| Read this Instructions for Use before you start using calcipotriene and betamethasone dipropionate topical suspension and each time you get a refill. There may be new information. This information does not take the place of talking with your healthcare provider about your medical condition or treatment. | ||||
| How to apply calcipotriene and betamethasone dipropionate topical suspension to your body: | ||||
| Follow your healthcare provider's instructions of how much calcipotriene and betamethasone dipropionate topical suspension to use and where to use it. Apply calcipotriene and betamethasone dipropionate topical suspension directly to areas affected by plaque psoriasis and gently rub in. Wash your hands after applying calcipotriene and betamethasone dipropionate topical suspension, unless you are treating areas on your hands. | ||||
| How to apply calcipotriene and betamethasone dipropionate topical suspension to your scalp: | ||||
| You do not need to wash your hair before you apply calcipotriene and betamethasone dipropionate topical suspension. | ||||
 | Step 1: Shake the bottle before use. Remove the cap from the bottle. (See Figure A). | |||
 | Step 2: Locate the area to treat using your fingers and part your hair. (See Figure B). | |||
 | Step 3: Squeeze a drop of calcipotriene and betamethasone dipropionate topical suspension to your fingertip. (See Figure C). | |||
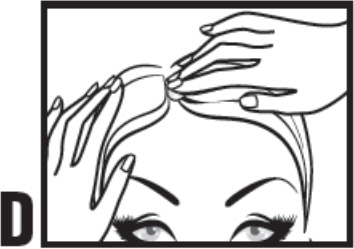 | Step 4: Use your fingers to apply the drop of calcipotriene and betamethasone dipropionate topical suspension directly to scalp affected by plaque psoriasis. Gently rub in. (See Figure D). | |||
| Step 5: After applying calcipotriene and betamethasone dipropionate topical suspension, put the cap back on the bottle. | ||||
| Step 6: Wash your hands after applying calcipotriene and betamethasone dipropionate topical suspension. Do not wash your hair right after you apply calcipotriene and betamethasone dipropionate topical suspension to your scalp. | ||||
| How should I store calcipotriene and betamethasone dipropionate topical suspension? | ||||
|
||||
PRINCIPAL DISPLAY PANEL - 60 g Bottle Carton
INGREDIENTS AND APPEARANCE
| CALCIPOTRIENE AND BETAMETHASONE DIPROPIONATE
calcipotriene and betamethasone dipropionate suspension |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Labeler - Taro Pharmaceuticals U.S.A., Inc. (145186370) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Taro Pharmaceuticals Inc. | 206263295 | MANUFACTURE(51672-1402) | |