Search by Drug Name or NDC
NDC 51672-1405-02 Oxymetazoline Hydrochloride 10 mg/g Details
Oxymetazoline Hydrochloride 10 mg/g
Oxymetazoline Hydrochloride is a TOPICAL CREAM in the HUMAN PRESCRIPTION DRUG category. It is labeled and distributed by Taro Pharmaceuticals U.S.A., Inc.. The primary component is OXYMETAZOLINE HYDROCHLORIDE.
MedlinePlus Drug Summary
Oxymetazoline is used to treat ongoing facial redness caused by rosacea (a skin disease that causes redness and pimples on the face). Oxymetazoline is in a class of medications called alpha1A adrenoceptor agonists. It works by narrowing the blood vessels in the skin.
Related Packages: 51672-1405-02Last Updated: 11/30/2022
MedLinePlus Full Drug Details: Oxymetazoline Topical
Product Information
| NDC | 51672-1405 |
|---|---|
| Product ID | 51672-1405_40827951-dbc4-4a28-a662-9e08d2cb0dcd |
| Associated GPIs | |
| GCN Sequence Number | n/a |
| GCN Sequence Number Description | n/a |
| HIC3 | n/a |
| HIC3 Description | n/a |
| GCN | n/a |
| HICL Sequence Number | n/a |
| HICL Sequence Number Description | n/a |
| Brand/Generic | n/a |
| Proprietary Name | Oxymetazoline Hydrochloride |
| Proprietary Name Suffix | n/a |
| Non-Proprietary Name | Oxymetazoline Hydrochloride |
| Product Type | HUMAN PRESCRIPTION DRUG |
| Dosage Form | CREAM |
| Route | TOPICAL |
| Active Ingredient Strength | 10 |
| Active Ingredient Units | mg/g |
| Substance Name | OXYMETAZOLINE HYDROCHLORIDE |
| Labeler Name | Taro Pharmaceuticals U.S.A., Inc. |
| Pharmaceutical Class | Imidazolines [CS], Increased Sympathetic Activity [PE], Vasoconstriction [PE], Vasoconstrictor [EPC] |
| DEA Schedule | n/a |
| Marketing Category | ANDA |
| Application Number | ANDA213584 |
| Listing Certified Through | 2024-12-31 |
Package
Package Images

NDC 51672-1405-02 (51672140502)
| NDC Package Code | 51672-1405-2 |
|---|---|
| Billing NDC | 51672140502 |
| Package | 1 TUBE in 1 CARTON (51672-1405-2) / 30 g in 1 TUBE |
| Marketing Start Date | 2021-10-04 |
| NDC Exclude Flag | N |
| Pricing Information | N/A |
Standard Product Labeling (SPL)/Prescribing Information SPL 8a7f2400-5e18-4215-8d2c-f55881706107 Details
HIGHLIGHTS OF PRESCRIBING INFORMATION
OXYMETAZOLINE HYDROCHLORIDE cream, for topical use
Initial U.S. Approval: 1964
INDICATIONS AND USAGE
Oxymetazoline hydrochloride cream is an alpha1A adrenoceptor agonist indicated for the topical treatment of persistent facial erythema associated with rosacea in adults. (1)
DOSAGE AND ADMINISTRATION
DOSAGE FORMS AND STRENGTHS
Cream, 1%. Each gram of cream contains 10 mg (1%) oxymetazoline hydrochloride, equivalent to 8.8 mg (0.88%) of oxymetazoline free base. (3)
CONTRAINDICATIONS
- None. (4)
WARNINGS AND PRECAUTIONS
- Alpha-adrenergic agonists as a class may impact blood pressure. Advise patients with cardiovascular disease, orthostatic hypotension, and/or uncontrolled hypertension or hypotension to seek medical care if their condition worsens. (5.1)
- Use with caution in patients with cerebral or coronary insufficiency, Raynaud's phenomenon, thromboangiitis obliterans, scleroderma, or Sjögren's syndrome and advise patients to seek medical care if signs and symptoms of potentiation of vascular insufficiency develop. (5.2)
- Advise patients to seek immediate medical care if signs and symptoms of acute narrow-angle glaucoma develop. (5.3)
ADVERSE REACTIONS
Most common adverse reactions (incidence ≥ 1%) are application site dermatitis, worsening inflammatory lesions of rosacea, application site pruritis, application site erythema, and application site pain. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Taro Pharmaceuticals U.S.A., Inc. at 1-866-923-4914 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 10/2021
Table Of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Potential Impacts on Cardiovascular Disease
5.2 Potentiation of Vascular Insufficiency
5.3 Risk of Angle Closure Glaucoma
6 ADVERSE REACTIONS
6.1 Clinical Studies Experience
7 DRUG INTERACTIONS
7.1 Anti-hypertensives/Cardiac Glycosides
7.2 Monoamine Oxidase Inhibitors
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- *
- Sections or subsections omitted from the full prescribing information are not listed.
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
For topical use only. Oxymetazoline hydrochloride is not for oral, ophthalmic, or intravaginal use.
Apply a pea-sized amount of oxymetazoline hydrochloride cream, once daily in a thin layer to cover the entire face (forehead, nose, each cheek, and chin) avoiding the eyes and lips. Wash hands immediately after applying oxymetazoline hydrochloride cream.
3 DOSAGE FORMS AND STRENGTHS
5 WARNINGS AND PRECAUTIONS
5.1 Potential Impacts on Cardiovascular Disease
Alpha-adrenergic agonists may impact blood pressure. Oxymetazoline hydrochloride should be used with caution in patients with severe or unstable or uncontrolled cardiovascular disease, orthostatic hypotension, and uncontrolled hypertension or hypotension. Advise patients with cardiovascular disease, orthostatic hypotension, and/or uncontrolled hypertension/hypotension to seek immediate medical care if their condition worsens.
5.2 Potentiation of Vascular Insufficiency
Oxymetazoline hydrochloride should be used with caution in patients with cerebral or coronary insufficiency, Raynaud's phenomenon, thromboangiitis obliterans, scleroderma, or Sjögren's syndrome. Advise patients to seek immediate medical care if signs and symptoms of potentiation of vascular insufficiency develop.
6 ADVERSE REACTIONS
6.1 Clinical Studies Experience
Because clinical trials are conducted under varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
A total of 489 subjects with persistent facial erythema associated with rosacea were treated with oxymetazoline hydrochloride once daily for 4 weeks in 3 controlled clinical trials. An additional 440 subjects with persistent facial erythema associated with rosacea were also treated with oxymetazoline hydrochloride once daily for up to one year in a long-term (open-label) clinical trial. Adverse reactions that occurred in at least 1% of subjects treated with oxymetazoline hydrochloride through 4 weeks of treatment are presented in Table 1 below.
| Adverse Reaction | Pooled Controlled Clinical Trials | |
|---|---|---|
| Oxymetazoline Hydrochloride Cream (N = 489) | Vehicle Cream (N = 483) |
|
| Application site dermatitis | 9 (2%) | 0 |
| Worsening inflammatory lesions of rosacea | 7 (1%) | 1 (<1%) |
| Application site pruritus | 5 (1%) | 4 (1%) |
| Application site erythema | 5 (1%) | 2 (<1%) |
| Application site pain | 4 (1%) | 1 (<1%) |
In the long-term (open-label) clinical trial, the rates of adverse reactions over a one-year treatment period were as follows: worsening inflammatory lesions of rosacea (3%), application site dermatitis (3%), application site pruritis (2%), application site pain (2%), and application site erythema (2%). Subjects with persistent erythema along with inflammatory lesions were allowed to use additional therapy for the inflammatory lesions of rosacea.
7 DRUG INTERACTIONS
7.1 Anti-hypertensives/Cardiac Glycosides
Alpha-adrenergic agonists, as a class, may impact blood pressure. Caution in using drugs such as beta-blockers, anti-hypertensives and/or cardiac glycosides is advised.
Caution should also be exercised in patients receiving alpha1 adrenergic receptor antagonists such as in the treatment of cardiovascular disease, benign prostatic hypertrophy, or Raynaud's disease.
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
There are no available data on oxymetazoline hydrochloride use in pregnant women to inform a drug-associated risk for major birth defects and miscarriage. A literature article describing intranasal decongestant use in pregnant women identified a potential association between second-trimester exposure to oxymetazoline (with no decongestant exposure in the first trimester) and renal collecting system anomalies [see Data]. In animal reproduction studies, there were no adverse developmental effects observed after oral administration of oxymetazoline hydrochloride in pregnant rats and rabbits at systemic exposures up to 3 times and 73 times, respectively, the exposure associated with the maximum recommended human dose (MRHD) [see Data]. The estimated background risks of major birth defects and miscarriage for the indicated population are unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2 to 4% and 15 to 20%, respectively.
Clinical Considerations
Fetal/Neonatal Adverse Reactions
Following repeated use of oxymetazoline hydrochloride solution nasal spray for the treatment of nasal congestion at a dose 5 times higher than recommended, one case of fetal distress was reported in a 41-week pregnant patient. The fetal distress resolved hours later, prior to the delivery of the healthy infant. The anticipated exposures for the case are 8- to 18-fold higher than plasma exposures after topical administration of oxymetazoline hydrochloride.
Data
Human Data
No adequate and well-controlled trials of oxymetazoline hydrochloride have been conducted in pregnant women. Across all clinical trials of oxymetazoline hydrochloride, two pregnancies were reported. One pregnancy resulted in the delivery of a healthy child. One pregnancy resulted in a spontaneous abortion, which was considered to be unrelated to the trial medication. A literature article summarizing the results of exploratory analyses of intranasal decongestant use during pregnancy identified a potential association between second-trimester exposure to oxymetazoline hydrochloride solution (with no decongestant exposure in the first trimester) and renal collecting system anomalies.
Animal Data
Effects on embryo-fetal development were evaluated in rats and rabbits following oral administration of oxymetazoline hydrochloride during the period of organogenesis. Oxymetazoline hydrochloride did not cause adverse effects to the fetus at oral doses up to 0.2 mg/kg/day in pregnant rats during the period of organogenesis (3 times the MRHD on an AUC comparison basis). Oxymetazoline hydrochloride did not cause adverse effects to the fetus at oral doses up to 1 mg/kg/day in pregnant rabbits during the period of organogenesis (73 times the MRHD on an AUC comparison basis). Maternal toxicity, such as decreased maternal body weight, was produced at the high dose of 1 mg/kg/day in pregnant rabbits and was associated with findings of delayed skeletal ossification.
In a rat perinatal and postnatal development study, oxymetazoline hydrochloride was orally administered to pregnant rats once daily from gestation day 6 through lactation day 20. Maternal toxicity was produced at the high dose of 0.2 mg/kg/day (3 times the MRHD on an AUC comparison basis) in pregnant rats and was associated with an increase in pup mortality and reduced pup body weights. Delayed sexual maturation was noted at 0.1 and 0.2 mg/kg/day (2 times the MRHD and 3 times the MRHD on an AUC comparison basis, respectively). Oxymetazoline hydrochloride did not have any adverse effects on fetal development at a dose of 0.05 mg/kg/day (one-half of the MRHD on an AUC comparison basis).
8.2 Lactation
No clinical data are available to assess the effects of oxymetazoline on the quantity or rate of breastmilk production, or to establish the level of oxymetazoline present in human breastmilk post-dose. Oxymetazoline was detected in the milk of lactating rats. The developmental and health benefits of breastfeeding should be considered along with the mother's clinical need for oxymetazoline hydrochloride and any potential adverse effects on the breastfed child from oxymetazoline hydrochloride or from the underlying maternal condition.
8.4 Pediatric Use
Safety and effectiveness of oxymetazoline hydrochloride have not been established in pediatric patients below the age of 18 years.
8.5 Geriatric Use
One hundred and ninety-three subjects aged 65 years and older received treatment with oxymetazoline hydrochloride (n = 135) or vehicle (n = 58) in clinical trials. No overall differences in safety or effectiveness were observed between subjects ≥ 65 years of age and younger subjects, based on available data. Clinical studies of oxymetazoline hydrochloride did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects.
10 OVERDOSAGE
Oxymetazoline hydrochloride is not for oral use. If oral ingestion occurs, seek medical advice. Monitor patient closely and administer appropriate supportive measures as necessary. Accidental ingestion of topical solutions (nasal sprays) containing imidazoline derivatives (e.g., oxymetazoline) in children has resulted in serious adverse events requiring hospitalization, including nausea, vomiting, lethargy, tachycardia, decreased respiration, bradycardia, hypotension, hypertension, sedation, somnolence, mydriasis, stupor, hypothermia, drooling, and coma. Keep oxymetazoline hydrochloride out of reach of children.
11 DESCRIPTION
Oxymetazoline hydrochloride cream, 1% contains oxymetazoline hydrochloride, an alpha1A adrenoceptor agonist. Oxymetazoline hydrochloride is a white to off-white cream. It has a chemical name of 3-[(4,5-Dihydro-1H-imidazol-2-yl)methyl]-6-(1,1-dimethylethyl)-2,4-dimethyl-phenol hydrochloride and a molecular weight of 296.8. It is freely soluble in water and ethanol and has a partition coefficient of 0.1 in 1-octanol/water. The molecular formula of oxymetazoline HCl is C16H25CIN2O and its structural formula is:

Each gram of oxymetazoline hydrochloride cream contains 10 mg (1%) oxymetazoline hydrochloride, equivalent to 8.8 mg (0.88%) of oxymetazoline free base. The cream contains the following inactive ingredients: butylated hydroxytoluene, ceteareth-6 (and) stearyl alcohol, ceteareth-25, cetostearyl alcohol, citric acid anhydrous, di-isopropyl adipate, edetate disodium, lanolin, medium chain triglycerides, methylparaben, oleyl alcohol super refined, phenoxyethanol, polyethylene glycol 300, polysorbate 60, propylparaben, purified water, sodium citrate dihydrate, and sorbitan monostearate.
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Oxymetazoline is an alpha1A adrenoceptor agonist. Oxymetazoline acts as a vasoconstrictor.
12.3 Pharmacokinetics
Absorption
The pharmacokinetics of oxymetazoline was evaluated following topical administration of oxymetazoline hydrochloride in a thin layer to cover the entire face in adult subjects with erythema associated with rosacea. The median weight of cream for each dose administration was 0.3 g. Plasma oxymetazoline concentrations were measurable in most of the subjects. Following the first dose application, the mean ± standard deviation (SD) peak concentrations (Cmax) and area under the concentration-time curves from time 0 to 24 hours (AUC0-24hr) were 60.5 ± 53.9 pg/mL and 895 ±798 pg*hr/mL, respectively. Following once daily applications for 28 days, the mean ± SD Cmax and AUC0-24hr were 66.4 ± 67.1 pg/mL and 1050 ± 992 pg*hr/mL, respectively. Following twice daily applications (twice the recommended frequency of application) for 28 days, the mean ± SD Cmax and AUC0-24hr were 68.8 ± 61.1 pg/mL and 1530 ± 922 pg*hr/mL, respectively.
Distribution
An in vitro study demonstrated that oxymetazoline is 56.7% to 57.5% bound to human plasma proteins.
Metabolism
In vitro studies using human liver microsomes showed that oxymetazoline was minimally metabolized, generating mono-oxygenated and dehydrogenated products of oxymetazoline. The percentage of parent drug oxymetazoline remaining was 95.9% after a 120-minute incubation with human liver microsomes.
Excretion
The excretion of oxymetazoline following administration of oxymetazoline hydrochloride has not been characterized in humans.
Drug Interaction
In vitro studies using human liver microsomes demonstrated that oxymetazoline up to the tested concentration of 100 nM had no inhibition on the activities of the cytochrome P450 (CYP) isoenzymes 1A2, 2B6, 2C8, 2C9, 2C19, 2D6, and 3A4/5. Treatment of cultured human hepatocytes with up to 100 nM oxymetazoline did not induce CYP1A2, CYP2B6, or CYP3A4.
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Oxymetazoline hydrochloride was not associated with an increased incidence of neoplastic or proliferative changes in transgenic mice given oral doses of 0.5, 1.0, or 2.5 mg/kg/day oxymetazoline hydrochloride for 6 months.
Oxymetazoline hydrochloride revealed no evidence of mutagenic or clastogenic potential based on the results of two in vitro genotoxicity tests (Ames assay and human lymphocyte chromosomal aberration assay) and one in vivo gentoxicity test (mouse micronucleus assay).
Effects on fertility and early embryonic development were evaluated in rats following oral administration of 0.05, 0.1, or 0.2 mg/kg/day oxymetazoline hydrochloride prior to and during mating and through early pregnancy. Decreased number of corpora lutea and increased post-implantation losses were noted at 0.2 mg/kg/day oxymetazoline hydrochloride (3 times the MRHD on an AUC comparison basis). However, no treatment related effects on fertility or mating parameters were noted at 0.2 mg/kg/day oxymetazoline hydrochloride (3 times the MRHD on an AUC comparison basis).
14 CLINICAL STUDIES
Oxymetazoline hydrochloride was evaluated for the treatment of persistent erythema associated with rosacea in two identical, randomized, double-blind, vehicle-controlled, parallel-group clinical trials. The trials enrolled 885 subjects aged 18 years and older. Overall, 90% of subjects were Caucasian and 79% were female. Subjects applied either oxymetazoline hydrochloride or vehicle once daily for 29 days.
Disease severity was graded by the clinician using a 5-point clinician erythema assessment (CEA) scale and by the subject on a similar 5-point subject self-assessment (SSA) scale, on which subjects scored either "moderate" or "severe" on both scales.
CEA and SSA were measured over a 12-hour period at equally-spaced timepoints (hours 3, 6, 9, and 12) post-dose on Days 1, 15, and 29. The primary efficacy endpoint was defined as the proportion of subjects with at least a 2-grade reduction in erythema (improvement) from baseline (pre-dose on Day 1) on both the CEA and SSA measured at hours 3, 6, 9, and 12 on Day 29. The results from both trials on the composite endpoint for Day 29 are presented in Table 2.
| Trial 1 | Trial 2 | |||
|---|---|---|---|---|
| Time-point (Hour) | Oxymetazoline Hydrochloride Cream (N=222) | Vehicle Cream (N=218) | Oxymetazoline Hydrochloride Cream (N=224) | Vehicle Cream (N=221) |
|
||||
| 3 | 12% | 6% | 14% | 7% |
| 6 | 16% | 8% | 13% | 5% |
| 9 | 18% | 6% | 16% | 9% |
| 12 | 15% | 6% | 12% | 6% |
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
Advise the patient and/or caregiver to read the FDA-approved patient labeling (Patient Information and Instructions for Use).
Important Administration Instructions
Advise patients of the following:
- Oxymetazoline hydrochloride cream is for topical use only.
- Do not to apply oxymetazoline hydrochloride cream to irritated skin or open wounds.
- Avoid contact with the eyes and lips.
- Wash hands immediately after application.
- Keep oxymetazoline hydrochloride cream out of reach of children.
SPL UNCLASSIFIED SECTION
SPL UNCLASSIFIED SECTION
| This Patient Information has been approved by the U.S. Food and Drug Administration. | ||||
| PATIENT INFORMATION Oxymetazoline Hydrochloride (ox" i me taz' oh leen hye" droe klor' ride) Cream |
||||
| Important: Oxymetazoline hydrochloride cream is for skin (topical) use on the face only. Do not use oxymetazoline hydrochloride cream in your eyes, mouth, or vagina. Keep oxymetazoline hydrochloride cream out of the reach of children. Get medical help right away if you, a child, or anyone else swallows oxymetazoline hydrochloride cream. |
||||
| What is oxymetazoline hydrochloride cream?
Oxymetazoline hydrochloride cream is a prescription medicine used on the skin (topical) to treat facial redness due to rosacea that does not go away (persistent) in adults. It is not known if oxymetazoline hydrochloride cream is safe and effective in children under 18 years of age. |
||||
Before you use oxymetazoline hydrochloride cream, tell your healthcare provider about all of your medical conditions, including if you:
|
||||
How should I use oxymetazoline hydrochloride cream?
|
||||
| What are the possible side effects of oxymetazoline hydrochloride cream?
The most common side effects of oxymetazoline hydrochloride cream include application site reactions of: |
||||
|
|
|
||
| These are not all the possible side effects of oxymetazoline hydrochloride cream. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088. |
||||
How should I store oxymetazoline hydrochloride cream?
|
||||
| General information about the safe and effective use of oxymetazoline hydrochloride cream
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use oxymetazoline hydrochloride cream for a condition for which it was not prescribed. Do not give oxymetazoline hydrochloride cream to other people, even if they have the same symptoms that you have. It may harm them. You can ask your pharmacist or healthcare provider for information about oxymetazoline hydrochloride cream that is written for health professionals. |
||||
| What are the ingredients in oxymetazoline hydrochloride cream? Active ingredient: oxymetazoline hydrochloride Inactive ingredients: butylated hydroxytoluene, ceteareth-6 (and) stearyl alcohol, ceteareth-25, cetostearyl alcohol, citric acid anhydrous, di-isopropyl adipate, edetate disodium, lanolin, medium chain triglycerides, methylparaben, oleyl alcohol super refined, phenoxyethanol, polyethylene glycol 300, polysorbate 60, propylparaben, purified water, sodium citrate dihydrate, and sorbitan monostearate. Manufactured by: Taro Pharmaceuticals Inc., Brampton, Ontario, Canada L6T 1C1 Distributed by: Taro Pharmaceuticals U.S.A., Inc., Hawthorne, NY 10532 Revised: October 2021 5217318 1021 55 |
||||
INSTRUCTIONS FOR USE
Oxymetazoline Hydrochloride (ox" i me taz' oh leen hye" droe klor' ride) Cream
Tube
Important:
|
Read and follow the steps below so that you use your tube of oxymetazoline hydrochloride cream correctly:
| Step 1: Open the tube by gently squeezing and pressing down on the child-resistant cap and twisting it counterclockwise until the cap is removed. Do not squeeze the tube while opening or closing. |  |
| Note: When the cap is removed, the tube is not child-resistant. | 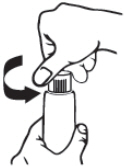 |
| Step 2: To apply oxymetazoline hydrochloride cream to your face, squeeze a pea-sized amount of oxymetazoline hydrochloride cream from the tube onto your fingertip. | 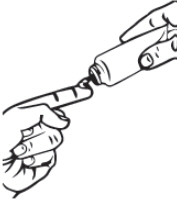 |
Step 3: Apply the pea-sized amount of oxymetazoline hydrochloride cream to cover your entire face (forehead, nose, each cheek, and chin) 1 time each day. Spread the cream smoothly and evenly in a thin layer over your face.
|
|
| Step 4: To close your oxymetazoline hydrochloride cream tube, place the cap back on the tube. Press down on the child-resistant cap and twist clockwise until it stops. The tube is child-resistant again. |  |
| Step 5: Wash your hands right away after applying oxymetazoline hydrochloride cream. | |
How do I store oxymetazoline hydrochloride cream?
|
|
This Instructions for Use has been approved by the U.S. Food and Drug Administration.
Manufactured by: Taro Pharmaceuticals Inc.
Brampton, Ontario, Canada L6T 1C1
Distributed by: Taro Pharmaceuticals U.S.A., Inc., Hawthorne, NY 10532
Revised: October 2021
5217318
1021
55
PRINCIPAL DISPLAY PANEL - 30 g Tube Carton
INGREDIENTS AND APPEARANCE
| OXYMETAZOLINE HYDROCHLORIDE
oxymetazoline hydrochloride cream |
||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||
| Labeler - Taro Pharmaceuticals U.S.A., Inc. (145186370) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Taro Pharmaceuticals Inc. | 206263295 | MANUFACTURE(51672-1405) | |