Search by Drug Name or NDC
NDC 54879-0036-64 Carmustine Details
Carmustine
Carmustine is a KIT in the HUMAN PRESCRIPTION DRUG category. It is labeled and distributed by STI Pharma LLC. The primary component is .
MedlinePlus Drug Summary
Carmustine injection is used to treat certain types of brain tumors. Carmustine injection is also used along with prednisone to treat multiple myeloma (a type of cancer of the bone marrow). It is also used with other medications to treat Hodgkin's lymphoma (Hodgkin's disease) and non-Hodgkin's lymphoma (cancer that begins in the cells of the immune system) that has not improved or that has worsened after treatment with other medications. Carmustine is in a class of medications called alkylating agents. It works by slowing or stopping the growth of cancer cells in your body.
Related Packages: 54879-0036-64Last Updated: 11/30/2022
MedLinePlus Full Drug Details: Carmustine
Product Information
| NDC | 54879-0036 |
|---|---|
| Product ID | 54879-036_edaba575-f99e-f561-e053-2995a90a41e7 |
| Associated GPIs | 21102010002105 |
| GCN Sequence Number | 008782 |
| GCN Sequence Number Description | carmustine VIAL 100 MG INTRAVEN |
| HIC3 | V1A |
| HIC3 Description | ANTINEOPLASTIC - ALKYLATING AGENTS |
| GCN | 38440 |
| HICL Sequence Number | 003901 |
| HICL Sequence Number Description | CARMUSTINE |
| Brand/Generic | Generic |
| Proprietary Name | Carmustine |
| Proprietary Name Suffix | n/a |
| Non-Proprietary Name | Carmustine |
| Product Type | HUMAN PRESCRIPTION DRUG |
| Dosage Form | KIT |
| Route | n/a |
| Active Ingredient Strength | n/a |
| Active Ingredient Units | n/a |
| Substance Name | n/a |
| Labeler Name | STI Pharma LLC |
| Pharmaceutical Class | n/a |
| DEA Schedule | n/a |
| Marketing Category | ANDA |
| Application Number | ANDA209278 |
| Listing Certified Through | 2023-12-31 |
Package
Package Images



NDC 54879-0036-64 (54879003664)
| NDC Package Code | 54879-036-64 |
|---|---|
| Billing NDC | 54879003664 |
| Package | 1 KIT in 1 CARTON (54879-036-64) * 30 mL in 1 VIAL, SINGLE-DOSE (54879-023-51) * 3 mL in 1 VIAL (54879-024-13) |
| Marketing Start Date | 2019-05-16 |
| NDC Exclude Flag | N |
| Pricing Information | N/A |
Standard Product Labeling (SPL)/Prescribing Information SPL db5d5fec-1ebc-492a-8987-a5f449fd08a1 Details
HIGHLIGHTS OF PRESCRIBING INFORMATION
CARMUSTINE for injection, for intravenous use
Initial U.S. Approval: 1977
WARNING: MYELOSUPPRESSION and PULMONARY TOXICITY
See full prescribing information for complete boxed warning
• Suppression of marrow function, notably thrombocytopenia and leukopenia, is the most common and severe of the toxic effects of Carmustine for Injection. Monitor blood counts. (5, 6).
• Pulmonary toxicity from Carmustine for Injection appears to be dose related. Patients receiving greater than 1400 mg/m 2 cumulative dose are at significantly higher risk than those receiving less (5, 6).
INDICATIONS AND USAGE
Carmustine for Injection is a nitrosourea indicated as palliative therapy as a single agent or in established combination therapy with other approved chemotherapeutic agents in the following: (1)
- Brain tumors glioblastoma, brainstem glioma, medulloblastoma, astrocytoma, ependymoma, and metastatic brain tumors (1)
- Multiple myeloma-in combination with prednisone (1)
- Relapsed or refractory Hodgkin's lymphoma in combination with other approved drugs (1)
- Relapsed or refractory Non-Hodgkin's lymphomas in combination with other approved drugs (1)
DOSAGE AND ADMINISTRATION
Recommended Dosage: As a single agent, 150 to 200 mg/m 2 Carmustine for Injection intravenously every 6 weeks as a single dose or divided into daily injections such as 75 to 100 mg/m 2on 2 successive days. Adjust dose for combination therapy or in patients with reduced bone marrow reserve (2.1) Administer reconstituted solution only as a slow intravenous infusion over at least 2 hours. (2.2) (2)
DOSAGE FORMS AND STRENGTHS
For injection: 100 mg of carmustine lyophilized powder in a single-dose vial for reconstitution and a vial containing 3mL sterile diluent (Dehydrated Alcohol Injection, USP) (3)
CONTRAINDICATIONS
- Hypersensitivity (4)
WARNINGS AND PRECAUTIONS
- Administration Reactions: Extravasation may occur; monitor infusion site closely during administration (5.3)
- Carcinogenicity: Potentially carcinogenic to humans. Monitor patient periodically for such signs and apprise the patient of the symptoms for which they need to seek medical help. (5.4)
- Ocular Toxicity: Has occurred when administered via unapproved intraarterial intracarotid route. (5.5)
- Embryo-Fetal toxicity: Can cause fetal harm. Advise females of reproductive potential of the potential risk to a fetus and to avoid pregnancy. (5.6)
ADVERSE REACTIONS
Most common adverse reactions (>1%) are nausea, vomiting, renal toxicity, pneumonitis, pulmonary toxicity, myelosuppression ( 6)
To report SUSPECTED ADVERSE REACTIONS, contact STI Pharma LLC at 1-888-301-9680 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS
- Cimetidine: Increased myelosuppression with concomitant use. (7.1)
- Phenobarbital: Induces carmustine metabolism, reducing exposure. May lead to reduced efficacy. (7.1)
- Phenytoin: Carmustine for Injection may reduce the efficacy of phenytoin. (7.2)
USE IN SPECIFIC POPULATIONS
- Lactation: Advise lactating females not to breastfeed (8.2)
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 5/2019
Table Of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
WARNING: MYELOSUPPRESSION and PULMONARY TOXICITY
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Dosage
2.2 Preparation and Administration of Intravenous Solution
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Myelosuppression
5.2 Pulmonary Toxicity
5.3 Administration Reactions
5.4 Carcinogenicity
5.5 Ocular Toxicity
5.6 Embryo-Fetal Toxicity
6 ADVERSE REACTIONS
7 DRUG INTERACTIONS
7.1 Effects of Other Drugs on Carmustine for Injection
7.2 Effects of Carmustine for Injection on Other Drugs
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.3 Females and Males of Reproductive Potential
8.4 Pediatric Use
8.5 Geriatric Use
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
15 REFERENCES
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
16.2 Storage and Handling
17 PATIENT COUNSELING INFORMATION
- *
- Sections or subsections omitted from the full prescribing information are not listed.
WARNING
Carmustine for Injection causes suppression of marrow function (including thrombocytopenia and leukopenia), which may contribute to bleeding and overwhelming infections. [see Warnings and Precautions (5.1) and Adverse Reactions (6)] . Monitor blood counts weekly for at least 6 weeks after each dose. Adjust dosage based on nadir blood counts from the prior dose [see Dosage and Administration (2.1)] . Do not administer a repeat course of Carmustine for Injection until blood counts recover.
Pulmonary Toxicity
Carmustine for Injection causes dose-related pulmonary toxicity. Patients receiving greater than 1400 mg/m 2cumulative dose are at significantly higher risk than those receiving less. Delayed pulmonary toxicity can occur years after treatment, and can result in death, particularly in patients treated in childhood [see Adverse Reactions (6) and Use in Specific Populations (8.4)] .
1 INDICATIONS AND USAGE
Carmustine for injection is indicated as palliative therapy as a single agent or in established combination therapy in the following:
- Brain tumors glioblastoma, brainstem glioma, medulloblastoma, astrocytoma, ependymoma, and metastatic brain tumors.
- Multiple myeloma in combination with prednisone.
- Relapsed or refractory Hodgkin's lymphoma in combination with other approved drugs.
- Relapsed or refractory Non-Hodgkin's lymphomas in combination with other approved drugs.
2 DOSAGE AND ADMINISTRATION
2.1 Dosage
The recommended dose of Carmustine for Injection as a single agent in previously untreated patients is 150 to 200 mg/m 2 intravenously every 6 weeks. Administer as a single dose or divided into daily injections such as 75 to 100 mg/m 2 on two successive days. Lower the dose when Carmustine for Injection is used with other myelosuppressive drugs or in patients in whom bone marrow reserve is depleted. Administer Carmustine for Injection for the duration according to the established regimen. Premedicate each dose with anti-emetics.
Adjust doses subsequent to the initial dose according to the hematologic response of the patient to the preceding dose. The following schedule is suggested as a guide to dosage adjustment:
| Nadir After Prior Dose | Percentage of Prior Dose to
be Given |
|
| Leukocytes/mm 3 | Platelets/mm 3 | |
| >4000 | >100,000 | 100% |
| 3000-3999 | 75,000-99,999 | 100% |
| 2000-2999 | 25,000-74,999 | 70% |
| <2000 | <25,000 | 50% |
The hematologic toxicity can be delayed and cumulative. Monitor blood counts weekly. Do not administer a repeat course of Carmustine for Injection until circulating blood elements have returned to acceptable levels (platelets above 100 Gi/L, leukocytes above 4 Gi/L and absolute neutrophil count above 1 Gi/L). The usual interval between courses is 6 weeks.
Evaluate renal function prior to administration and periodically during treatment. For patients with compromised renal function, monitor for toxicity more frequently. Discontinue Carmustine for Injection if the creatinine clearance is less than 10 mL/min. Do not administer Carmustine for Injection to patients with compromised renal function. Monitor transaminases and bilirubin periodically during treatment. [see Adverse Reactions (6)].
2.2 Preparation and Administration of Intravenous Solution
- Dissolve carmustine for injection with 3 mL of the supplied sterile diluent (Dehydrated Alcohol Injection, USP).
- Aseptically add 27 mL Sterile Water for Injection, USP.
- Each mL of resulting solution contains 3.3 mg of carmustine in 10% ethanol. Such solutions should be protected from light.
- The reconstituted solution is a clear, colorless to yellowish solution.
- Once reconstituted, the solution must be further diluted with Sodium Chloride Injection, USP or 5% Dextrose Injection, USP.
- Examine reconstituted vials for crystal formation prior to use. If crystals are observed, they may be re-dissolved by warming the vial to room temperature with agitation.
- Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit.
- After reconstitution as recommended, carmustine for injection is stable for 24 hours under refrigeration (2°-8°C, 36°-46°F) in glass container. Examine reconstituted vials for crystal formation prior to use. If crystals are observed, they may be redissolved by warming the vial to room temperature with agitation.
- Vials reconstituted as directed and further diluted with 500 mL Sodium Chloride Injection, USP or 5% Dextrose Injection, USP, in glass or polypropylene containers to a concentration of 0.2 mg/mL, should be stored at room temperature, protected from light and utilized within 8 hours. These solutions are also stable 24 hours under refrigeration (2°-8°C, 36°-46°F) and an additional 6 hours at room temperature protected from light.
- Administer reconstituted solution by slow intravenous infusion over at least two hours. Administration of carmustine for injection over a period of less than two hours can lead to pain and burning at the site of injection. Monitor the injected area during the administration. The rate of administration of the intravenous infusion should not be more than 1.66 mg/m 2/min.
- See Section 16.2 for important instructions on the storage and handling of the injection. carmustine for injection is a cytotoxic drug. Follow applicable special handling and disposal procedures. 1
- The lyophilized dosage formulation contains no preservatives and is not intended for use as a multiple dose via.
Accidental contact of reconstituted carmustine for injection with the skin has caused transient hyperpigmentation of the affected areas. Wear impervious gloves to minimize the risk of dermal exposure impervious gloves when handling vials containing carmustine for injection. Immediately wash the skin or mucosa thoroughly with soap and water if carmustine for injection lyophilized material or solution contacts the skin or mucosa 1.
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Myelosuppression
Bone marrow toxicity is a dose-limiting, common and severe toxic effect of carmustine for injection occurring 4-6 weeks after drug administration (thrombocytopenia occurs at about 4 weeks post-administration persisting for 1 to 2 weeks; leukopenia occurs at 5 to 6 weeks after a dose of carmustine for injection persisting for 1 to 2 weeks; thrombocytopenia is generally more severe than leukopenia; anemia is less frequent and less severe compared to thrombocytopenia and/or leukopenia) Complete blood count should therefore be monitored weekly for at least six weeks after a dose. Repeat doses of carmustine for injection should not be given more frequently than every six weeks. The bone marrow toxicity of carmustine for injection is cumulative and therefore the dosage adjustment must be considered on the basis of nadir blood counts from prior dose [see Adverse Reactions ( 6)] . Greater myelotoxicity (e.g., leukopenia and neutropenia) has been reported when carmustine was combined with cimetidine [see Drug Interactions ( 7)] .
5.2 Pulmonary Toxicity
Cases of fatal pulmonary toxicity with carmustine for injection have been reported. Pulmonary toxicity characterized by pulmonary infiltrates and/or fibrosis has been reported to occur from 9 days to 43 months after treatment with carmustine for injection and related nitrosoureas. Pulmonary toxicity from carmustine for injection is dose-related. Patients receiving greater than 1400 mg/m 2 cumulative dose are at significantly higher risk than those receiving less. However, there have been reports of pulmonary fibrosis in patients receiving lower total doses. Interstitial fibrosis (with lower doses) occurred rarely. Additionally, delayed onset pulmonary fibrosis occurring up to 17 years after treatment has been reported in patients who received carmustine for injection (in cumulative doses ranging from 770 to 1800 mg/m 2 combined with cranial radiotherapy for intracranial tumors) in childhood and early adolescence. Other risk factors include past history of lung disease and duration of treatment. Baseline pulmonary function studies should be conducted along with frequent pulmonary function tests during treatment. Patients with a baseline below 70% of the predicted forced vital capacity (FVC) or carbon monoxide diffusing capacity (DLCO) are particularly at risk.
5.3 Administration Reactions
Injection site reactions may occur during the administration of carmustine for injection. Rapid intravenous infusion of carmustine for injection may produce intensive flushing of the skin and suffusion of the conjunctiva within 2 hours, lasting about 4 hours. It is also associated with burning at the site of injection although true thrombosis is rare. Given the possibility of extravasation, close monitoring of the infusion site for possible infiltration during drug administration is recommended. A specific treatment for extravasation reactions is unknown at this time.
5.4 Carcinogenicity
Long-term use of nitrosoureas, such as carmustine for injection, has been reported to be associated with the development of secondary malignancies. Carmustine was carcinogenic when administered to laboratory animals [see Nonclinical Toxicity (13.1)] . Nitrosourea therapy, such as carmustine for injection, has carcinogenic potential in humans. Patients treated with carmustine for injection should be monitored long-term for development of second malignancies.
5.5 Ocular Toxicity
Carmustine for injection has been administered through an intraarterial intracarotid route; this procedure is investigational and has been associated with ocular toxicity. Safety and effectiveness of the intra-arterial route have not been established.
5.6 Embryo-Fetal Toxicity
Carmustine was embryotoxic in rats and rabbits and teratogenic in rats when given in doses lower than the maximum cumulative human dose based on body surface area. There are no adequate and well- controlled studies in pregnant women. Advise pregnant women of the potential risk to the fetus [ see Use in Specific Populations ( 8.1, 8.3) ]. Advise females of reproductive potential to use highly effective contraception during and after treatment with carmustine for injection for at least 6 months after therapy. Advise males of reproductive potential to use effective contraception during and after treatment with carmustine for injection for at least 3 months after therapy [see Use in Specific Populations ( 8.1, 8.3)].
6 ADVERSE REACTIONS
The following serious adverse reactions are described elsewhere in the labeling:
- Myelosuppression [see Warnings and Precautions (5.1)]
- Pulmonary toxicity [see Warnings and Precautions (5.2)]
- Administration Reactions [see Warnings and Precautions (5.3)]
- Carcinogenicity [see Warnings and Precautions (5.4)]
- Ocular Toxicity [see Warnings and Precautions (5.5)]
The following adverse reactions associated with the use of carmustine for injection were identified in clinical studies or postmarketing reports. Because some of these reactions were reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Cardiac Disorders
Tachycardia and chest pain.
Eye Disorders
Conjunctival edema, conjunctival hemorrhage, blurred vision and loss of depth perception
Gastrointestinal Toxicity
Nausea, vomiting, anorexia, and diarrhea
Hepatotoxicity
Increased transaminase, increased alkaline phosphatase, increased bilirubin levels
Infections and Infestations
Opportunistic infection (including with fatal outcome).
Neoplasms Benign, Malignant and Unspecified (including cysts and polyps)
Acute leukemia, bone marrow dysplasias.
Nephrotoxicity
Progressive azotemia, decrease in kidney size, renal failure
Nervous System Disorders
Headaches, encephalopathy, and seizures
Pulmonary Toxicity
Pneumonitis, interstitial lung disease
Reproductive System and Breast Disorders
Gynecomastia
Skin and Subcutaneous Tissue Disorders
Burning sensation, hyperpigmentation, swelling, pain, erythema, skin necrosis, alopecia, allergic reaction
Vascular Disorders
Veno-occlusive disease.
7 DRUG INTERACTIONS
7.1 Effects of Other Drugs on Carmustine for Injection
Cimetidine: Greater myelosuppression (e.g., leukopenia and neutropenia) has been reported when oral cimetidine has been coadministered with carmustine. Consider alternative drugs to cimetidine.
Phenobarbital: Phenobarbital induces the metabolism of carmustine and may compromise antitumor activity of carmustine. Consider alternative drugs to phenobarbital.
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Carmustine for Injection can cause fetal harm when administered to a pregnant woman based on the mechanism of action [ see Clinical Pharmacology (12.1)] and findings in animals [see Data]. Limited available data with Carmustine for Injection use in pregnant women are insufficient to inform a drug-associated risk of major birth defects and miscarriage. Carmustine was embryotoxic in rats and rabbits and teratogenic in rats (thoracoabdominal closure, neural tube, and eye defects and malformations of the skeletal system of the fetus) when given in doses lower than the maximum cumulative human dose based on body surface area. Consider the benefits and risks of Carmustine for Injection for the mother and possible risks to the fetus when prescribing Carmustine for Injection to a pregnant woman.
Adverse outcomes in pregnancy occur regardless of the health of the mother or the use of medications. The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2-4% and 15-20%, respectively.
Data
Animal Data
Intraperitoneal (IP) administration of carmustine to pregnant rats 14 days prior to mating and during the period of organogenesis at cumulative doses ≥ 26 mg/kg (158 mg/ m 2), approximately 0.1 times the maximum cumulative human dose of 1400 mg/m 2, resulted in pre-implantation loss, increased resorptions (including completely resorbed litters), and reduced the number of live births in the presence of maternal toxicity.
Carmustine administered IP to pregnant rats during the period of organogenesis at cumulative doses ≥ 4 mg/kg (24 mg/m 2), approximately 0.02 times the maximum cumulative human dose based on a mg/m 2 basis, resulted in reduced fetal weight and various malformations, which included thoracoabdominal closure defects, neural tube defects, and eye defects, including microphthalmia/anophthalmia, and skeletal anomalies in the skull, sternebra, vertebrae and ribs, and reduced skeletal ossification) in the presence of maternal toxicity. Embryo-fetal death was observed at cumulative doses ≥ 8 mg/kg (48 mg/m 2), approximately 0.03 times the maximum cumulative human dose on a mg/ m 2 basis. Intravenous (IV) administration of carmustine to rats at a cumulative dose of 50 mg/kg (300 mg/ m 2), approximately 0.2 times the maximum cumulative human dose on a mg/m 2 basis, during the last quarter of pregnancy resulted in the death of offspring within 4 months. Carmustine administered IV to rabbits during the period of organogenesis resulted in spontaneous abortions in mothers and growth defects in the fetus, mainly at cumulative doses ≥ 13 mg/kg (156 mg/ m 2), approximately 0.1 times the maximum cumulative human dose on a mg/ m 2 basis.
8.2 Lactation
There is no information regarding the presence of carmustine in human milk, the effects on the breastfed infant, or the effects on milk production. Because many drugs are excreted in human milk and because of the potential for serious adverse events (e.g., carcinogenicity and myelosuppression) in nursing infants, nursing should be discontinued while taking Carmustine for Injection.
8.3 Females and Males of Reproductive Potential
Advise female patients to avoid pregnancy during treatment with carmustine for injection because of the risk of fetal harm [see Use in Specific Populations (8.1)] .
Advise female patients of reproductive potential to use highly effective contraception during and for up to six months after completion of treatment.
Advise males with female sexual partners of reproductive potential to use effective contraception during carmustine for injection treatment and for at least three months after the final dose of carmustine for injection [ see Nonclinical Toxicology (13.1)].
Infertility
Based on nonclinical findings, male fertility may be compromised by treatment with carmustine for injection [see Nonclinical Toxicology (13.1)].
8.4 Pediatric Use
Safety and effectiveness in children have not been established. Delayed onset pulmonary fibrosis occurring up to 17 years after treatment has been reported in a long-term study of patients who received carmustine for injection in childhood and early adolescence (1-16 years). Eight out of the 17 patients (47%) who survived childhood brain tumors, including all the 5 patients initially treated at less than 5 years of age, died of pulmonary fibrosis. [see Adverse Reactions (6.1)].
8.5 Geriatric Use
Clinical studies of carmustine for injection did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dose range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy.
Carmustine for injection and its metabolites are known to be substantially excreted by the kidney, and the risk of toxic reactions to this drug may be greater in patients with impaired renal function. Because elderly patients are more likely to have decreased renal function, care should be taken in dose selection, and renal function should be monitored.
10 OVERDOSAGE
11 DESCRIPTION
The active ingredient in Carmustine for injection, USP is a nitrosourea with the chemical name 1,3-bis(2-chloroethyl)-1-nitrosourea and a molecular weight of 214.06. The drug product is supplied as sterile lyophilized pale yellow flakes or a congealed mass, and it is highly soluble in alcohol and lipids, and poorly soluble in water. Carmustine for Injection, USP is administered by intravenous infusion after reconstitution, as recommended.
The structural formula of carmustine is:
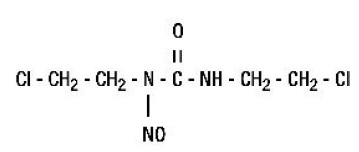
Carmustine for Injection, USP is available in 100-mg single dose vials of lyophilized material. Sterile diluent for constitution of Carmustine for Injection, USP is co-packaged with the active drug product for use in constitution of the lyophile. The diluent is supplied in a vial containing 3 mL of Dehydrated Alcohol Injection, USP.
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
The mechanism of action of carmustine is not fully understood. While carmustine alkylates DNA and RNA, it is not cross-resistant with other alkylators. As with other nitrosoureas, it may also inhibit several key enzymatic processes by carbamoylation of amino acids in proteins. The metabolites may contribute to antitumor activity and toxicities of carmustine.
12.3 Pharmacokinetics
Carmustine crosses the blood-brain barrier. Levels of radioactivity in the CSF are greater than or equal to 50% of those measured concurrently in plasma.
Elimination
Following a short intravenous infusion, the reported elimination half-life ranges from 15 minutes to 75 minutes.
Metabolism
Carmustine may be inactivated through denitrosation reactions catalyzed by both cytosolic and microsomal enzymes, including NADPH and glutathione-S-transferase.
Excretion
Approximately 60% to 70% of a total dose is excreted in the urine within 96 hours. Approximately 10% is eliminated as respiratory CO 2.
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carmustine is carcinogenic in rats and mice, producing a marked increase in tumor incidence in doses approximating those employed clinically. Nitrosourea therapy does have carcinogenic potential in humans [see Adverse Reactions (6.1)] .
Carmustine was mutagenic and clastogenic in multiple in vitro and in vivo genetic toxicology studies.
Male rats treated with carmustine at cumulative doses ≥ 36 mg/kg (216 mg/ m 2), approximately 0.15 times the maximum cumulative human dose on a mg/ m 2 basis, showed decreases in reproductive potential when mated with untreated female rats (e.g., decreased implantations, increased resorption rate, and a decrease in viable fetuses).
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
Carmustine for injection, USP. Each package includes a vial containing 100 mg carmustine and a vial containing 3 mL sterile diluent.
NDC 54879-036-64
16.2 Storage and Handling
Store product and diluent in a refrigerator (2°-8°C, 36°-46°F).
Stability
Store the unopened vial of the dry drug in a refrigerator (2°-8°C, 36°-46°F). Store the diluent vials in a refrigerator (2°-8°C, 36°-46°F). The recommended storage of unopened Carmustine for Injection, USP vials provides a stable product for up to 2 years.
Compatibility/ Incompatibility with Containers
The intravenous solution is unstable in polyvinyl chloride container. DO NOT USE PVC Containers.
Administer Carmustine for Injection, USP solution from the glass bottles or polypropylene container only. Ensure the polypropylene containers used are PVC free and DEHP free.
Important Note
Carmustine for Injection, USP has a low melting point (30.5°-32.0°C or 86.9°-89.6°F). Exposure of the drug to this temperature or above will cause the drug to liquefy and appear as an oil film on the vials. This is a sign of decomposition and vials should be discarded. If there is a question of adequate refrigeration upon receipt of this product, immediately inspect the vial in each individual carton. Hold the vial to a bright light for inspection. The Carmustine for Injection, USP will appear as a very small amount of dry flakes or dry congealed mass. If this is evident, the Carmustine for Injection, USP is suitable for use and should be refrigerated immediately.
17 PATIENT COUNSELING INFORMATION
Myelosuppression [see Warnings and Precautions (5.1)].
A serious and frequent toxicity of Carmustine for Injection is delayed myelosuppression and usually occurs 4 to 6 weeks after drug administration. Hence, patients should be advised to get blood counts monitored weekly for at least 6 weeks. The bone marrow toxicity of Carmustine for Injection is cumulative.
Pulmonary Toxicity [see Warnings and Precautions (5.2)].
Advise patients to contact a health care professional immediately for any of the following: shortness of breath, particularly during exercise, dry, hacking cough, fast, shallow breathing, gradual unintended weight loss, tiredness, aching joints and muscles, clubbing (widening and rounding) of the tips of the fingers or toes.
Seizures [see Adverse Reactions (6)]
Inform the patient that they may suffer from fits and advise them to get medical attention immediately in such cases.
Pregnancy [see Warnings and Precautions (5.6) and Use in Specific Populations ( 8.1 and 8.3)]
Advise pregnant women and females of reproductive potential that Carmustine for Injection exposure during pregnancy can result in fetal harm. Advise female patients to contact their healthcare provider with a known or suspected pregnancy. Advise women of reproductive potential to avoid becoming pregnant. Advise females of reproductive potential to use effective contraception during treatment.
Lactation [see Use in Specific Populations (8.2)]
Advise the female patient to discontinue nursing while taking Carmustine for Injection.
Manufactured in Germany For:
STI Pharma LLC
Newtown, PA 18940,
1.888.301.9680


PACKAGE LABEL.PRINCIPAL DISPLAY PANEL - diluent label
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL - vial label
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL - carton
INGREDIENTS AND APPEARANCE
| CARMUSTINE
carmustine kit |
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
| Labeler - STI Pharma LLC (832714070) |
| Registrant - STI Pharma LLC (832714070) |