Search by Drug Name or NDC
NDC 55289-0124-12 Amitriptyline Hydrochloride 10 mg/1 Details
Amitriptyline Hydrochloride 10 mg/1
Amitriptyline Hydrochloride is a ORAL TABLET, FILM COATED in the HUMAN PRESCRIPTION DRUG category. It is labeled and distributed by PD-Rx Pharmaceuticals, Inc.. The primary component is AMITRIPTYLINE HYDROCHLORIDE.
MedlinePlus Drug Summary
Amitriptyline is used to treat symptoms of depression. Amitriptyline is in a class of medications called tricyclic antidepressants. It works by increasing the amounts of certain natural substances in the brain that are needed to maintain mental balance.
Related Packages: 55289-0124-12Last Updated: 11/30/2022
MedLinePlus Full Drug Details: Amitriptyline
Product Information
| NDC | 55289-0124 |
|---|---|
| Product ID | 55289-124_d2313aa0-7b6c-17cc-e053-2995a90a8cfc |
| Associated GPIs | 58200010100305 |
| GCN Sequence Number | 046043 |
| GCN Sequence Number Description | amitriptyline HCl TABLET 10 MG ORAL |
| HIC3 | H2U |
| HIC3 Description | TRICYCLIC ANTIDEPRESSANTS,REL.NON-SEL.REUPT-INHIB |
| GCN | 16512 |
| HICL Sequence Number | 001643 |
| HICL Sequence Number Description | AMITRIPTYLINE HCL |
| Brand/Generic | Generic |
| Proprietary Name | Amitriptyline Hydrochloride |
| Proprietary Name Suffix | n/a |
| Non-Proprietary Name | Amitriptyline Hydrochloride |
| Product Type | HUMAN PRESCRIPTION DRUG |
| Dosage Form | TABLET, FILM COATED |
| Route | ORAL |
| Active Ingredient Strength | 10 |
| Active Ingredient Units | mg/1 |
| Substance Name | AMITRIPTYLINE HYDROCHLORIDE |
| Labeler Name | PD-Rx Pharmaceuticals, Inc. |
| Pharmaceutical Class | Tricyclic Antidepressant [EPC] |
| DEA Schedule | n/a |
| Marketing Category | ANDA |
| Application Number | ANDA040218 |
| Listing Certified Through | 2022-12-31 |
Package
Package Images

NDC 55289-0124-12 (55289012412)
| NDC Package Code | 55289-124-12 |
|---|---|
| Billing NDC | 55289012412 |
| Package | 12 TABLET, FILM COATED in 1 BOTTLE, PLASTIC (55289-124-12) |
| Marketing Start Date | 2010-04-13 |
| NDC Exclude Flag | N |
| Pricing Information | N/A |
Standard Product Labeling (SPL)/Prescribing Information SPL b6815ba3-df6e-4208-a14b-74c3aea54a4c Details
WARNING
Antidepressants increased the risk compared to placebo of suicidal thinking and behavior (suicidality) in children, adolescents, and young adults in short-term studies of major depressive disorder (MDD) and other psychiatric disorders. Anyone considering the use of amitriptyline hydrochloride tablets or any other antidepressant in a child, adolescent, or young adult must balance this risk with the clinical need. Short-term studies did not show an increase in the risk of suicidality with antidepressants compared to placebo in adults beyond age 24; there was a reduction in risk with antidepressants compared to placebo in adults aged 65 and older. Depression and certain other psychiatric disorders are themselves associated with increases in the risk of suicide. Patients of all ages who are started on antidepressant therapy should be monitored appropriately and observed closely for clinical worsening, suicidality, or unusual changes in behavior. Families and caregivers should be advised of the need for close observation and communication with the prescriber. Amitriptyline hydrochloride tablets are not approved for use in pediatric patients (see Warnings: Clinical Worsening and Suicide Risk, Precautions: Information for Patients, and Precautions: Pediatric Use.)
DESCRIPTION
Amitriptyline HCl is 3-(10,11-dihydro-5H-dibenzo [a, d] cycloheptene-5-ylidene)-N,N-dimethyl-1-propanamine hydrochloride. Its empirical formula is C 20H 23N•HCl, and its structural formula is:
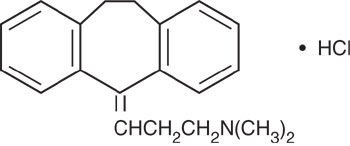
Amitriptyline HCl, a dibenzocycloheptadiene derivative, has a molecular weight of 313.87. It is a white, odorless, crystalline compound which is freely soluble in water.
Amitriptyline HCl is supplied as 10 mg, 25 mg, 50 mg, 75 mg, 100 mg or 150 mg tablets. Each tablet contains the following inactive ingredients: colloidal silicon dioxide, hypromellose, lactose monohydrate, magnesium stearate, microcrystalline cellulose, polyethylene glycol, polysorbate, sodium starch glycolate and titanium dioxide. The 10 mg tablets also contain FD&C blue #1 lake. The 25 mg tablets also contain D&C yellow #10 lake and FD&C blue #2 lake. The 50 mg tablets also contain synthetic black iron oxide, synthetic red iron oxide and synthetic yellow iron oxide. The 75 mg tablets also contain FD&C yellow #6 lake. The 100 mg tablets also contain D&C red #33 lake and FD&C red #40 lake. The 150 mg tablets also contain FD&C blue #2 lake and FD&C yellow #6 lake.
CLINICAL PHARMACOLOGY
Amitriptyline hydrochloride is an antidepressant with sedative effects. Its mechanism of action in man is not known. It is not a monoamine oxidase inhibitor, and it does not act primarily by stimulation of the central nervous system.
Amitriptyline inhibits the membrane pump mechanism responsible for uptake of norepinephrine and serotonin in adrenergic and serotonergic neurons. Pharmacologically this action may potentiate or prolong neuronal activity since reuptake of these biogenic amines is important physiologically in terminating transmitting activity. This interference with the reuptake of norepinephrine and/or serotonin is believed by some to underlie the antidepressant activity of amitriptyline.
INDICATIONS AND USAGE
CONTRAINDICATIONS
Amitriptyline hydrochloride is contraindicated in patients who have shown prior hypersensitivity to it.
It should not be given concomitantly with monoamine oxidase inhibitors. Hyperpyretic crises, severe convulsions, and deaths have occurred in patients receiving tricyclic antidepressant and monoamine oxidase inhibiting drugs simultaneously. When it is desired to replace a monoamine oxidase inhibitor with amitriptyline hydrochloride, a minimum of 14 days should be allowed to elapse after the former is discontinued. Amitriptyline hydrochloride should then be initiated cautiously with a gradual increase in dosage until optimum response is achieved.
Amitriptyline hydrochloride should not be given with Cisapride due to the potential for increased QT interval and increased risk for arrhythmia.
This drug is not recommended for use during the acute recovery phase following myocardial infarction.
WARNINGS
Clinical Worsening and Suicide Risk:
Patients with major depressive disorder (MDD), both adult and pediatric, may experience worsening of their depression and/or the emergence of suicidal ideation and behavior (suicidality) or unusual changes in behavior, whether or not they are taking antidepressant medications, and this risk may persist until significant remission occurs. Suicide is a known risk of depression and certain other psychiatric disorders, and these disorders themselves are the strongest predictors of suicide. There has been a long-standing concern, however, that antidepressants may have a role in inducing worsening of depression and the emergence of suicidality in certain patients during the early phases of treatment. Pooled analyses of short-term placebo-controlled trials of antidepressant drugs (SSRIs and others) showed that these drugs increase the risk of suicidal thinking and behavior (suicidality) in children, adolescents, and young adults (ages 18–24) with major depressive disorder (MDD) and other psychiatric disorders. Short-term studies did not show an increase in the risk of suicidality with antidepressants compared to placebo in adults beyond age 24; there was a reduction with antidepressants compared to placebo in adults aged 65 and older.
The pooled analyses of placebo-controlled trials in children and adolescents with MDD, obsessive compulsive disorder (OCD), or other psychiatric disorders included a total of 24 short-term trials of 9 antidepressant drugs in over 4400 patients. The pooled analyses of placebo-controlled trials in adults with MDD or other psychiatric disorders included a total of 295 short-term trials (median duration of 2 months) of 11 antidepressant drugs in over 77,000 patients. There was considerable variation in risk of suicidality among drugs, but a tendency toward an increase in the younger patients for almost all drugs studied. There were differences in absolute risk of suicidality across the different indications, with the highest incidence in MDD. The risk differences (drug vs placebo), however, were relatively stable within age strata and across indications. These risk differences (drug-placebo difference in the number of cases of suicidality per 1000 patients treated) are provided in Table 1.
| Table 1 | |
| Age Range | Drug-Placebo Difference in
Number of Cases of Suicidality per 1000 Patients Treated |
| Increases Compared to Placebo | |
| <18 | 14 additional cases |
| 18–24 | 5 additional cases |
| Decreases Compared to Placebo | |
| 25–64 | 1 fewer case |
| ≥65 | 6 fewer cases |
No suicides occurred in any of the pediatric trials. There were suicides in the adult trials, but the number was not sufficient to reach any conclusion about drug effect on suicide.
It is unknown whether the suicidality risk extends to longer-term use, i.e., beyond several months. However, there is substantial evidence from placebo-controlled maintenance trials in adults with depression that the use of antidepressants can delay the recurrence of depression.
All patients being treated with antidepressants for any indication should be monitored appropriately and observed closely for clinical worsening, suicidality, and unusual changes in behavior, especially during the initial few months of a course of drug therapy, or at times of dose changes, either increases or decreases.
The following symptoms, anxiety, agitation, panic attacks, insomnia, irritability, hostility, aggressiveness, impulsivity, akathisia (psychomotor restlessness), hypomania, and mania, have been reported in adult and pediatric patients being treated with antidepressants for major depressive disorder as well as for other indications, both psychiatric and nonpsychiatric. Although a causal link between the emergence of such symptoms and either the worsening of depression and/or the emergence of suicidal impulses has not been established, there is concern that such symptoms may represent precursors to emerging suicidality.
Consideration should be given to changing the therapeutic regimen, including possibly discontinuing the medication, in patients whose depression is persistently worse, or who are experiencing emergent suicidality or symptoms that might be precursors to worsening depression or suicidality, especially if these symptoms are severe, abrupt in onset, or were not part of the patient's presenting symptoms.
Families and caregivers of patients being treated with antidepressants for major depressive disorder or other indications, both psychiatric and nonpsychiatric, should be alerted about the need to monitor patients for the emergence of agitation, irritability, unusual changes in behavior, and the other symptoms described above, as well as the emergence of suicidality, and to report such symptoms immediately to health care providers. Such monitoring should include daily observation by families and caregivers. Prescriptions for amitriptyline hydrochloride tablets should be written for the smallest quantity of tablets consistent with good patient management, in order to reduce the risk of overdose.
Screening Patients for Bipolar Disorder:
A major depressive episode may be the initial presentation of bipolar disorder. It is generally believed (though not established in controlled trials) that treating such an episode with an antidepressant alone may increase the likelihood of precipitation of a mixed/manic episode in patients at risk for bipolar disorder. Whether any of the symptoms described above represent such a conversion is unknown. However, prior to initiating treatment with an antidepressant, patients with depressive symptoms should be adequately screened to determine if they are at risk for bipolar disorder; such screening should include a detailed psychiatric history, including a family history of suicide, bipolar disorder, and depression. It should be noted that amitriptyline hydrochloride tablets are not approved for use in treating bipolar depression.
Amitriptyline hydrochloride may block the antihypertensive action of guanethidine or similarly acting compounds.
It should be used with caution in patients with a history of seizures and, because of its atropine-like action, in patients with a history of urinary retention or angle-closure glaucoma. In patients with angle-closure glaucoma, even average doses may precipitate an attack.
Patients with cardiovascular disorders should be watched closely. Tricyclic antidepressant drugs, including amitriptyline hydrochloride, particularly when given in high doses, have been reported to produce arrhythmias, sinus tachycardia, and prolongation of the conduction time. Myocardial infarction and stroke have been reported with drugs of this class.
Close supervision is required when amitriptyline hydrochloride is given to hyperthyroid patients or those receiving thyroid medication.
Amitriptyline hydrochloride may enhance the response to alcohol and the effects of barbiturates and other CNS depressants. In patients who may use alcohol excessively, it should be borne in mind that the potentiation may increase the danger inherent in any suicide attempt or overdosage. Delirium has been reported with concurrent administration of amitriptyline and disulfiram.
Angle-Closure Glaucoma: The pupillary dilation that occurs following use of many antidepressant drugs, including amitriptyline hydrochloride tablets, may trigger an angle closure attack in a patient with anatomically narrow angles who does not have a patent iridectomy.
Usage in Pregnancy:
Pregnancy Category C –
Teratogenic effects were not observed in mice, rats, or rabbits when amitriptyline was given orally at doses of 2 to 40 mg/kg/day (up to 13 times the maximum recommended human dose*). Studies in literature have shown amitriptyline to be teratogenic in mice and hamsters when given by various routes of administration at doses of 28 to 100 mg/kg/day (9 to 33 times the maximum recommended human dose), producing multiple malformations. Another study in the rat reported that an oral dose of 25 mg/kg/day (8 times the maximum recommended human dose) produced delays in ossification of fetal vertebral bodies without other signs of embryotoxicity. In rabbits, an oral dose of 60 mg/kg/day (20 times the maximum recommended human dose) was reported to cause incomplete ossification of the cranial bones.
Amitriptyline has been shown to cross the placenta. Although a causal relationship has not been established, there have been a few reports of adverse events, including CNS effects, limb deformities, or developmental delay, in infants whose mothers had taken amitriptyline during pregnancy. There are no adequate and well-controlled studies in pregnant women. Amitriptyline hydrochloride should be used during pregnancy only if the potential benefit to the mother justifies the potential risk to the fetus.
Nursing Mothers:
Amitriptyline is excreted into breast milk. In one report in which a patient received amitriptyline 100 mg/day while nursing her infant, levels of 83 to 141 ng/mL were detected in the mother's serum. Levels of 135 to 151 ng/mL were found in the breast milk, but no trace of the drug could be detected in the infant's serum.
Because of the potential for serious adverse reactions in nursing infants from amitriptyline, a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother.
PRECAUTIONS
Schizophrenic patients may develop increased symptoms of psychosis; patients with paranoid symptomatology may have an exaggeration of such symptoms. Depressed patients, particularly those with known manic-depressive illness, may experience a shift to mania or hypomania. In these circumstances the dose of amitriptyline may be reduced or a major tranquilizer such as perphenazine may be administered concurrently.
The possibility of suicide in depressed patients remains until significant remission occurs. Potentially suicidal patients should not have access to large quantities of this drug. Prescriptions should be written for the smallest amount feasible.
Concurrent administration of amitriptyline hydrochloride and electroshock therapy may increase the hazards associated with such therapy. Such treatment should be limited to patients for whom it is essential.
When possible, the drug should be discontinued several days before elective surgery.
Both elevation and lowering of blood sugar levels have been reported.
Amitriptyline hydrochloride should be used with caution in patients with impaired liver function.
Information for Patients:
Prescribers or other health professionals should inform patients, their families, and their caregivers about the benefits and risks associated with treatment with amitriptyline hydrochloride tablets and should counsel them in its appropriate use. A patient Medication Guide about "Antidepressant Medicines, Depression and other Serious Mental Illness, and Suicidal Thoughts or Actions" is available for amitriptyline hydrochloride tablets. The prescriber or health professional should instruct patients, their families, and their caregivers to read the Medication Guide and should assist them in understanding its contents. Patients should be given the opportunity to discuss the contents of the Medication Guide and to obtain answers to any questions they may have. The complete text of the Medication Guide is reprinted at the end of this document.
Patients should be advised of the following issues and asked to alert their prescriber if these occur while taking amitriptyline hydrochloride tablets.
While on therapy with amitriptyline hydrochloride, patients should be advised as to the possible impairment of mental and/or physical abilities required for performance of hazardous tasks, such as operating machinery or driving a motor vehicle.
Patients should be advised that taking amitriptyline hydrochloride tablets can cause mild pupillary dilation, which in susceptible individuals, can lead to an episode of angle-closure glaucoma. Pre-existing glaucoma is almost always open-angle glaucoma because angle-closure glaucoma, when diagnosed, can be treated definitively with iridectomy. Open-angle glaucoma is not a risk factor for angle-closure glaucoma. Patients may wish to be examined to determine whether they are susceptible to angle closure, and have a prophylactic procedure (e.g., iridectomy), if they are susceptible.
Clinical Worsening and Suicide Risk:
Patients, their families, and their caregivers should be encouraged to be alert to the emergence of anxiety, agitation, panic attacks, insomnia, irritability, hostility, aggressiveness, impulsivity, akathisia (psychomotor restlessness), hypomania, mania, other unusual changes in behavior, worsening of depression, and suicidal ideation, especially early during antidepressant treatment and when the dose is adjusted up or down. Families and caregivers of patients should be advised to look for the emergence of such symptoms on a day-to-day basis, since changes may be abrupt. Such symptoms should be reported to the patient's prescriber or health professional, especially if they are severe, abrupt in onset, or were not part of the patient's presenting symptoms. Symptoms such as these may be associated with an increased risk for suicidal thinking and behavior and indicate a need for very close monitoring and possibly changes in the medication.
Drug Interactions:
Topiramate –
Some patients may experience a large increase in amitriptyline concentration in the presence of topiramate and any adjustments in amitriptyline dose should be made according to the patient's clinical response and not on the basis of plasma levels.
Drugs Metabolized by P450 2D6 –
The biochemical activity of the drug metabolizing isozyme cytochrome P450 2D6 (debrisoquin hydroxylase) is reduced in a subset of the Caucasian population (about 7 to 10% of Caucasians are so called "poor metabolizers"); reliable estimates of the prevalence of reduced P450 2D6 isozyme activity among Asian, African and other populations are not yet available. Poor metabolizers have higher than expected plasma concentrations of tricyclic antidepressants (TCAs) when given usual doses. Depending on the fraction of drug metabolized by P450 2D6, the increase in plasma concentration may be small, or quite large (8-fold increase in plasma AUC of the TCA).
In addition, certain drugs inhibit the activity of this isozyme and make normal metabolizers resemble poor metabolizers. An individual who is stable on a given dose of TCA may become abruptly toxic when given one of these inhibiting drugs as concomitant therapy. The drugs that inhibit cytochrome P450 2D6 include some that are not metabolized by the enzyme (quinidine; cimetidine) and many that are substrates for P450 2D6 (many other antidepressants, phenothiazines, and the Type 1C antiarrhythmics propafenone and flecainide). While all the selective serotonin reuptake inhibitors (SSRIs), e.g., fluoxetine, sertraline, and paroxetine, inhibit P450 2D6, they may vary in the extent of inhibition. The extent to which SSRI-TCA interactions may pose clinical problems will depend on the degree of inhibition and the pharmacokinetics of the SSRI involved. Nevertheless, caution is indicated in the coadministration of TCAs with any of SSRIs and also in switching from one class to the other. Of particular importance, sufficient time must elapse before initiating TCA treatment in a patient being withdrawn from fluoxetine, given the long half-life of the parent and active metabolite (at least 5 weeks may be necessary).
Concomitant use of tricyclic antidepressants with drugs that can inhibit cytochrome P450 2D6 may require lower doses than usually prescribed for either the tricyclic antidepressant or the other drug. Furthermore, whenever one of these other drugs is withdrawn from co-therapy, an increased dose of tricyclic antidepressant may be required. It is desirable to monitor TCA plasma levels whenever a TCA is going to be coadministered with another drug known to be an inhibitor of P450 2D6.
Monoamine oxidase inhibitors –
see CONTRAINDICATIONS section. Guanethidine or similarly acting compounds; thyroid medication; alcohol, barbiturates and other CNS depressants; and disulfiram – see WARNINGS section.
When amitriptyline hydrochloride is given with anticholinergic agents or sympathomimetic drugs, including epinephrine combined with local anesthetics, close supervision and careful adjustment of dosages are required.
Hyperpyrexia has been reported when amitriptyline hydrochloride is administered with anticholinergic agents or with neuroleptic drugs, particularly during hot weather.
Paralytic ileus may occur in patients taking tricyclic antidepressants in combination with anticholinergic-type drugs.
Cimetidine is reported to reduce hepatic metabolism of certain tricyclic antidepressants, thereby delaying elimination and increasing steady-state concentrations of these drugs. Clinically significant effects have been reported with the tricyclic antidepressants when used concomitantly with cimetidine. Increases in plasma levels of tricyclic antidepressants, and in the frequency and severity of side effects, particularly anticholinergic, have been reported when cimetidine was added to the drug regimen. Discontinuation of cimetidine in well-controlled patients receiving tricyclic antidepressants and cimetidine may decrease the plasma levels and efficacy of the antidepressants.
Caution is advised if patients receive large doses of ethchlorvynol concurrently. Transient delirium has been reported in patients who were treated with one gram of ethchlorvynol and 75 to 150 mg of amitriptyline hydrochloride.
Pediatric Use:
Safety and effectiveness in the pediatric population have not been established (see BOX WARNING and WARNINGS-Clinical Worsening and Suicide Risk). Anyone considering the use of amitriptyline hydrochloride tablets in a child or adolescent must balance the potential risks with the clinical need.
Geriatric Use:
Clinical experience has not identified differences in responses between elderly and younger patients. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic function, concomitant disease and other drug therapy in elderly patients.
Geriatric patients are particularly sensitive to the anticholinergic side effects of tricyclic antidepressants including amitriptyline hydrochloride. Peripheral anticholinergic effects include tachycardia, urinary retention, constipation, dry mouth, blurred vision, and exacerbation of narrow-angle glaucoma. Central nervous system anticholinergic effects include cognitive impairment, psychomotor slowing, confusion, sedation, and delirium. Elderly patients taking amitriptyline hydrochloride may be at increased risk for falls. Elderly patients should be started on low doses of amitriptyline hydrochloride and observed closely (see DOSAGE AND ADMINISTRATION).
ADVERSE REACTIONS
Within each category the following adverse reactions are listed in order of decreasing severity. Included in the listing are a few adverse reactions which have not been reported with this specific drug. However, pharmacological similarities among the tricyclic antidepressant drugs require that each of the reactions be considered when amitriptyline is administered.
Cardiovascular: Myocardial infarction; stroke; nonspecific ECG changes and changes in AV conduction; heart block; arrhythmias; hypotension, particularly orthostatic hypotension; syncope; hypertension; tachycardia; palpitation.
CNS and Neuromuscular: Coma; seizures; hallucinations; delusion; confusional states; disorientation; incoordination; ataxia; tremors; peripheral neuropathy; numbness, tingling and paresthesias of the extremities; extrapyramidal symptoms including abnormal involuntary movements and tardive dyskinesia; dysarthria; disturbed concentration; excitement; anxiety; insomnia; restlessness; nightmares; drowsiness; dizziness; weakness; fatigue; headache; syndrome of inappropriate ADH (antidiuretic hormone) secretion; tinnitus; alteration in EEG patterns.
Anticholinergic: Paralytic ileus; hyperpyrexia; urinary retention; dilatation of the urinary tract; constipation; blurred vision, disturbance of accommodation, increased ocular pressure, mydriasis; dry mouth.
Allergic: Skin rash; urticaria; photosensitization; edema of face and tongue.
Hematologic: Bone marrow depression including agranulocytosis, leukopenia, thrombocytopenia; purpura; eosinophilia.
Gastrointestinal: Rarely hepatitis (including altered liver function and jaundice); nausea; epigastric distress; vomiting; anorexia; stomatitis; peculiar taste; diarrhea; parotid swelling; black tongue.
Endocrine: Testicular swelling and gynecomastia in the male; breast enlargement and galactorrhea in the female; increased or decreased libido; impotence; elevation and lowering of blood sugar levels.
Other: Alopecia; edema; weight gain or loss; urinary frequency; increased perspiration.
Withdrawal Symptoms: After prolonged administration, abrupt cessation of treatment may produce nausea, headache, and malaise. Gradual dosage reductions has been reported to produce, within two weeks, transient symptoms including irritability, restlessness, and dream and sleep disturbance.
These symptoms are not indicative of addiction. Rare instances have been reported of mania or hypomania occurring within 2 to 7 days following cessation of chronic therapy with tricyclic antidepressants.
Causal Relationship Unknown: Other reactions, reported under circumstances where a causal relationship could not be established, are listed to serve as alerting information to physicians.
Body as a Whole: Lupus-like syndrome (migratory arthritis, positive ANA and rheumatoid factor).
Digestive: Hepatic failure, ageusia.
Postmarketing Adverse Events: A syndrome resembling neuroleptic malignant syndrome (NMS) has been very rarely reported after starting or increasing the dose of amitriptyline hydrochloride, with and without concomitant medications known to cause NMS. Symptoms have included muscle rigidity, fever, mental status changes, diaphoresis, tachycardia, and tremor.
Very rare cases of serotonin syndrome (SS) have been reported with amitriptyline hydrochloride in combination with other drugs that have a recognized association with SS.
Very rare cases of cardiomyopathy have been reported with amitriptyline.
OVERDOSAGE
Deaths may occur from overdosage with this class of drugs. Multiple drug ingestion (including alcohol) is common in deliberate tricyclic antidepressant overdose. As the management is complex and changing, it is recommended that the physician contact a poison control center for current information on treatment. Signs and symptoms of toxicity develop rapidly after tricyclic antidepressant overdose; therefore, hospital monitoring is required as soon as possible.
Manifestations:
Critical manifestations of overdose include: cardiac dysrhythmias, severe hypotension, convulsions, and CNS depression, including coma. Changes in the electrocardiogram, particularly in QRS axis or width, are clinically significant indicators of tricyclic antidepressant toxicity. In addition, a rightward axis shift in the terminal QRS complex together with a prolonged QT interval and sinus tachycardia are specific and sensitive indicators of first generation tricyclic overdose. The absence of these findings is not exclusionary. Prolonged PR interval, ST-T wave changes, ventricular tachycardia and fibrillation may also occur.
Other signs of overdose may include: impaired myocardial contractility, confusion, disturbed concentration, transient visual hallucinations, dilated pupils, disorders of ocular motility, agitation, hyperactive reflexes, polyradiculoneuropathy, stupor, drowsiness, muscle rigidity, vomiting, hypothermia, hyperpyrexia, or any of the symptoms listed under ADVERSE REACTIONS.
Management:
General –
Obtain an ECG and immediately initiate cardiac monitoring. Protect the patient's airway, establish an intravenous line and initiate gastric decontamination. A minimum of six hours of observation with cardiac monitoring and observation for signs of CNS or respiratory depression, hypotension, cardiac dysrhythmias and/or conduction blocks, and seizures is necessary. If signs of toxicity occur at any time during the period, extended monitoring is required. There are case reports of patients succumbing to fatal dysrhythmias late after overdose; these patients had clinical evidence of significant poisoning prior to death and most received inadequate gastrointestinal decontamination. Monitoring of plasma drug levels should not guide management of the patient.
Gastrointestinal Decontamination:
All patients suspected of tricyclic antidepressant overdose should receive gastrointestinal decontamination. This should include large volume gastric lavage followed by activated charcoal. If consciousness is impaired, the airway should be secured prior to lavage. EMESIS IS CONTRAINDICATED.
Cardiovascular:
A maximal limb-lead QRS duration of ≥0.10 seconds may be the best indication of the severity of the overdose. Intravenous sodium bicarbonate should be used to maintain the serum pH in the range of 7.45 to 7.55. If the pH response is inadequate, hyperventilation may also be used. Concomitant use of hyperventilation and sodium bicarbonate should be done with extreme caution, with frequent pH monitoring. A pH > 7.60 or a pCO 2 < 20 mm Hg is undesirable. Dysrhythmias unresponsive to sodium bicarbonate therapy/hyperventilation may respond to lidocaine, bretylium or phenytoin. Type 1A and 1C antiarrhythmics are generally contraindicated (e.g., quinidine, disopyramide, and procainamide).
In rare instances, hemoperfusion may be beneficial in acute refractory cardiovascular instability in patients with the acute toxicity. However, hemodialysis, peritoneal dialysis, exchange transfusions, and forced diuresis generally have been reported as ineffective in tricyclic antidepressant poisoning.
CNS:
In patients with CNS depression early intubation is advised because of the potential for abrupt deterioration. Seizures should be controlled with benzodiazepines, or if these are ineffective, other anticonvulsants (e.g., phenobarbital, phenytoin).
Physostigmine is not recommended except to treat life-threatening symptoms that have been unresponsive to other therapies, and then only in consultation with a poison control center.
DOSAGE AND ADMINISTRATION
Dosage should be initiated at a low level and increased gradually, noting carefully the clinical response and any evidence of intolerance.
Initial Dosage for Adults:
For outpatients 75 mg of amitriptyline HCl a day in divided doses is usually satisfactory. If necessary, this may be increased to a total of 150 mg per day. Increases are made preferably in the late afternoon and/or bedtime doses. A sedative effect may be apparent before the antidepressant effect is noted, but an adequate therapeutic effect may take as long as 30 days to develop.
An alternate method of initiating therapy in outpatients is to begin with 50 to 100 mg amitriptyline HCl at bedtime. This may be increased by 25 or 50 mg as necessary in the bedtime dose to a total of 150 mg per day.
Hospitalized patients may require 100 mg a day initially. This can be increased gradually to 200 mg a day if necessary. A small number of hospitalized patients may need as much as 300 mg a day.
Adolescent and Elderly Patients:
In general, lower dosages are recommended for these patients. Ten mg 3 times a day with 20 mg at bedtime may be satisfactory in adolescent and elderly patients who do not tolerate higher dosages.
Maintenance:
The usual maintenance dosage of amitriptyline HCl is 50 to 100 mg per day. In some patients 40 mg per day is sufficient. For maintenance therapy the total daily dosage may be given in a single dose preferably at bedtime. When satisfactory improvement has been reached, dosage should be reduced to the lowest amount that will maintain relief of symptoms. It is appropriate to continue maintenance therapy 3 months or longer to lessen the possibility of relapse.
Usage in Pediatric Patients
In view of the lack of experience with the use of this drug in pediatric patients, it is not recommended at the present time for patients under 12 years of age.
Plasma Levels
Because of the wide variation in the absorption and distribution of tricyclic antidepressants in body fluids, it is difficult to directly correlate plasma levels and therapeutic effect. However, determination of plasma levels may be useful in identifying patients who appear to have toxic effects and may have excessively high levels, or those in whom lack of absorption or noncompliance is suspected. Because of increased intestinal transit time and decreased hepatic metabolism in elderly patients, plasma levels are generally higher for a given oral dose of amitriptyline hydrochloride than in younger patients. Elderly patients should be monitored carefully and quantitative serum levels obtained as clinically appropriate. Adjustment in dosage should be made according to the patient's clinical response and not on the basis of plasma levels.**
HOW SUPPLIED
SPL UNCLASSIFIED SECTION
METABOLISM
Studies in man following oral administration of 14C-labeled drug indicated that amitriptyline is rapidly absorbed and metabolized. Radioactivity of the plasma was practically negligible, although significant amounts of radioactivity appeared in the urine by 4 to 6 hours and one-half to one-third of the drug was excreted within 24 hours.
Amitriptyline is metabolized by N-demethylation and bridge hydroxylation in man, rabbit and rat. Virtually the entire dose is excreted as glucuronide or sulfate conjugate of metabolites, with little unchanged drug appearing in the urine. Other metabolic pathways may be involved.
REFERENCES
Ayd FJ Jr: Amitriptyline therapy for depressive reactions. Psychosomatics 1960;1:320–325.
Diamond S: Human metabolizer of amitriptyline tagged with carbon 14. Curr Ther Res, Mar 1965, pp 170–175.
Dorfman W: Clinical experiences with amitriptyline: A preliminary report. Psychosomatics 1960;1:153–155.
Fallette JM, Stasney CR, Mintz AA: Amitriptyline poisoning treated with physostigmine. South Med J 1970;63:1492–1493.
Hollister LE, Overall JE, Johnson M, et al: Controlled comparison of amitriptyline, imipramine and placebo in hospitalized depressed patients. J Nerv Ment Dis 1964;139:370–375.
Hordern A, Burt CG, Holt NF: Depressive states: A pharmacotherapeutic study, Springfield study. Springfield, Ill, Charles C. Thomas, 1965.
Jenike MA: Treatment of Affective Illness in the Elderly with Drugs and Electroconvulsive Therapy. J Geriatr Psychiatry 1989; 22(1):77–112.
Klerman GL, Cole JO: Clinical pharmacology of imipramine and related antidepressant compounds. Int J Psychiatry 1976;3:267–304.
Liu B, Anderson G, Mittman N, et al: Use of selective serotonin-reuptake inhibitors or tricyclic antidepressants and risk of hip fractures in elderly people. Lancet 1998; 351(9112):1303–1307.
McConaghy N, Joffe AD, Kingston WA, et al: Correlation of clinical features of depressed out-patients with response to amitriptyline and protriptyline. Br J Psychiatry 1968;114:103–106.
McDonald IM, Perkins M, Marjerrison G, et al: A controlled comparison of amitriptyline and electroconvulsive therapy in the treatment of depression. Am J Psychiatry 1966;122:1427–1431.
Slovis T, Ott J, Teitelbaum D, et al: Physostigmine therapy in acute tricyclic antidepressant poisoning. Clin Toxicol 1971;4:451–459.
Symposium on depression with special studies of a new antidepressant, amitriptyline. Dis Nerv Syst, (Sect 2) May 1961, pp 5–56.
*Based on a maximum recommended amitriptyline dose of 150 mg/day or 3 mg/kg/day for a 50 kg patient.
**Hollister LE: Monitoring Tricyclic Antidepressant Plasma Concentrations. JAMA 1979; 241(23):2530–2533.
SPL UNCLASSIFIED SECTION
Medication Guide Antidepressant Medicines, Depression and Other Serious Mental Illnesses, and Suicidal Thoughts or Actions
Read the Medication Guide that comes with you or your family member's antidepressant medicine. This Medication Guide is only about the risk of suicidal thoughts and actions with antidepressant medicines. Talk to your, or your family member's, healthcare provider about:
- all risks and benefits of treatment with antidepressant medicines
- all treatment choices for depression or other serious mental illness
What is the most important information I should know about antidepressant medicines, depression and other serious mental illnesses, and suicidal thoughts or actions?
- Antidepressant medicines may increase suicidal thoughts or actions in some children, teenagers, and young adults within the first few months of treatment.
- Depression and other serious mental illnesses are the most important causes of suicidal thoughts and actions. Some people may have a particularly high risk of having suicidal thoughts or actions. These include people who have (or have a family history of) bipolar illness (also called manic-depressive illness) or suicidal thoughts or actions.
-
How can I watch for and try to prevent suicidal thoughts and actions in myself or a family member?
- Pay close attention to any changes, especially sudden changes, in mood, behaviors, thoughts, or feelings. This is very important when an antidepressant medicine is started or when the dose is changed.
- Call the healthcare provider right away to report new or sudden changes in mood, behavior, thoughts, or feelings.
- Keep all follow-up visits with the healthcare provider as scheduled. Call the healthcare provider between visits as needed, especially if you have concerns about symptoms.
Call a healthcare provider right away if you or your family member has any of the following symptoms, especially if they are new, worse, or worry you:
- thoughts about suicide or dying
- attempts to commit suicide
- new or worse depression
- new or worse anxiety
- feeling very agitated or restless
- panic attacks
- trouble sleeping (insomnia)
- new or worse irritability
- acting aggressive, being angry, or violent
- acting on dangerous impulses
- an extreme increase in activity and talking (mania)
- other unusual changes in behavior or mood
- Visual problems: eye pain, changes in vision, swelling or redness in or around the eye
What else do I need to know about antidepressant medicines?
- Never stop an antidepressant medicine without first talking to a healthcare provider. Stopping an antidepressant medicine suddenly can cause other symptoms.
- Visual problems. Only some people are at risk for these problems. You may want to undergo an eye examination to see if you are at risk and receive preventative treatment if you are.
- Antidepressants are medicines used to treat depression and other illnesses. It is important to discuss all the risks of treating depression and also the risks of not treating it. Patients and their families or other caregivers should discuss all treatment choices with the healthcare provider, not just the use of antidepressants.
- Antidepressant medicines have other side effects. Talk to the healthcare provider about the side effects of the medicine prescribed for you or your family member.
- Antidepressant medicines can interact with other medicines. Know all of the medicines that you or your family member takes. Keep a list of all medicines to show the healthcare provider. Do not start new medicines without first checking with your healthcare provider.
- Not all antidepressant medicines prescribed for children are FDA approved for use in children. Talk to your child's healthcare provider for more information.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
This Medication Guide has been approved by the U.S. Food and Drug Administration for all antidepressants.
Distributed by:
Par Pharmaceutical
Chestnut Ridge, NY 10977
8181699
Revised: 08/17
R7
INGREDIENTS AND APPEARANCE
| AMITRIPTYLINE HYDROCHLORIDE
amitriptyline hydrochloride tablet, film coated |
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
| Labeler - PD-Rx Pharmaceuticals, Inc. (156893695) |
| Registrant - PD-Rx Pharmaceuticals, Inc. (156893695) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| PD-Rx Pharmaceuticals, Inc. | 156893695 | repack(55289-124) | |