Search by Drug Name or NDC
NDC 57237-0061-90 Finasteride 1 mg/1 Details
Finasteride 1 mg/1
Finasteride is a ORAL TABLET, FILM COATED in the HUMAN PRESCRIPTION DRUG category. It is labeled and distributed by Rising Health, LLC. The primary component is FINASTERIDE.
MedlinePlus Drug Summary
Finasteride (Proscar) is used alone or in combination with another medication (doxazosin [Cardura]) to treat benign prostatic hypertrophy (BPH, enlargement of the prostate gland). Finasteride is used to treat symptoms of BPH such as frequent and difficult urination and may reduce the chance of acute urinary retention (sudden inability to urinate). It also may decrease the chance that prostate surgery will be needed. Finasteride (Propecia) is also used to treat male pattern hair loss (gradual thinning of the hair on the scalp, leading to a receding hairline or balding on the top of the head in men.) Finasteride (Propecia) has not been shown to treat thinning hair at the temples and is not used to treat hair loss in women or children. Finasteride is in a class of medications called 5-alpha reductase inhibitors. Finasteride treats BPH by blocking the body's production of a male hormone that causes the prostate to enlarge. Finasteride treats male pattern hair loss by blocking the body's production of a male hormone in the scalp that stops hair growth.
Related Packages: 57237-0061-90Last Updated: 11/30/2022
MedLinePlus Full Drug Details: Finasteride
Product Information
| NDC | 57237-0061 |
|---|---|
| Product ID | 57237-061_62d045bb-28d3-4812-a54a-4f2fed4317a6 |
| Associated GPIs | 90736030000310 |
| GCN Sequence Number | 037050 |
| GCN Sequence Number Description | finasteride TABLET 1 MG ORAL |
| HIC3 | L1C |
| HIC3 Description | HYPERTRICHOTIC AGENTS, SYSTEMIC/INCL. COMBINATIONS |
| GCN | 29248 |
| HICL Sequence Number | 006421 |
| HICL Sequence Number Description | FINASTERIDE |
| Brand/Generic | Generic |
| Proprietary Name | Finasteride |
| Proprietary Name Suffix | n/a |
| Non-Proprietary Name | Finasteride |
| Product Type | HUMAN PRESCRIPTION DRUG |
| Dosage Form | TABLET, FILM COATED |
| Route | ORAL |
| Active Ingredient Strength | 1 |
| Active Ingredient Units | mg/1 |
| Substance Name | FINASTERIDE |
| Labeler Name | Rising Health, LLC |
| Pharmaceutical Class | 5-alpha Reductase Inhibitor [EPC], 5-alpha Reductase Inhibitors [MoA] |
| DEA Schedule | n/a |
| Marketing Category | ANDA |
| Application Number | ANDA203687 |
| Listing Certified Through | 2024-12-31 |
Package
Package Images

NDC 57237-0061-90 (57237006190)
| NDC Package Code | 57237-061-90 |
|---|---|
| Billing NDC | 57237006190 |
| Package | 90 TABLET, FILM COATED in 1 BOTTLE (57237-061-90) |
| Marketing Start Date | 2013-11-05 |
| NDC Exclude Flag | N |
| Pricing Information | |
| Price Per Unit | 0.04543 |
| Pricing Unit | EA |
| Effective Date | 2024-02-21 |
| NDC Description | FINASTERIDE 1 MG TABLET |
| Pharmacy Type Indicator | C/I |
| OTC | N |
| Explanation Code | 1 |
| Classification for Rate Setting | G |
| As of Date | 2024-02-21 |
Standard Product Labeling (SPL)/Prescribing Information SPL 01b88593-99d3-4dd6-a7b6-5438c15bd7b7 Details
HIGHLIGHTS OF PRESCRIBING INFORMATION
FINASTERIDE tablets, for oral use
Initial U.S. Approval: 1992
INDICATIONS AND USAGE
DOSAGE AND ADMINISTRATION
DOSAGE FORMS AND STRENGTHS
1 mg tablets (3).
CONTRAINDICATIONS
WARNINGS AND PRECAUTIONS
- Finasteride is not indicated for use in women or pediatric patients (5.1, 5.4).
- Women should not handle crushed or broken finasteride tablets when they are pregnant or may potentially be pregnant due to potential risk to a male fetus (5.1, 8.1, 16).
- Finasteride causes a decrease in serum PSA levels. Any confirmed increase in PSA while on finasteride may signal the presence of prostate cancer and should be evaluated, even if those values are still within the normal range for men not taking a 5α-reductase inhibitor (5.2).
- 5α-reductase inhibitors may increase the risk of high-grade prostate cancer (5.3, 6.1).
ADVERSE REACTIONS
The most common adverse reactions, reported in ≥1% of patients treated with finasteride and greater than in patients treated with placebo are: decreased libido, erectile dysfunction and ejaculation disorder (6.1).
To report SUSPECTED ADVERSE REACTIONS, contact Rising Health, LLC at 1-833-395-6928 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 8/2022
Table Of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Exposure of Women - Risk to Male Fetus
5.2 Effects on Prostate Specific Antigen (PSA)
5.3 Increased Risk of High-Grade Prostate Cancer with 5α-Reductase Inhibitors
5.4 Pediatric Patients
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Postmarketing Experience
7 DRUG INTERACTIONS
7.1 Cytochrome P450-Linked Drug Metabolizing Enzyme System
7.2 Other Concomitant Therapy
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.3 Nursing Mothers
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Hepatic Impairment
8.7 Renal Impairment
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
14.1 Studies in Men
14.2 Study in Women
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- *
- Sections or subsections omitted from the full prescribing information are not listed.
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
Finasteride tablets may be administered with or without meals.
The recommended dose of finasteride is one tablet (1 mg) taken once daily.
In general, daily use for three months or more is necessary before benefit is observed. Continued use is recommended to sustain benefit, which should be re-evaluated periodically. Withdrawal of treatment leads to reversal of effect within 12 months.
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
Finasteride tablets are contraindicated in the following:
- Pregnancy. Finasteride use is contraindicated in women when they are or may potentially be pregnant. Because of the ability of Type II 5α-reductase inhibitors to inhibit the conversion of testosterone to 5α-dihydrotestosterone (DHT), finasteride may cause abnormalities of the external genitalia of a male fetus of a pregnant woman who receives finasteride. If this drug is used during pregnancy, or if pregnancy occurs while taking this drug, the pregnant woman should be apprised of the potential hazard to the male fetus. [See Warnings and Precautions (5.1), Use in Specific Populations (8.1), How Supplied/Storage and Handling (16) and Patient Counseling Information (17).] In female rats, low doses of finasteride administered during pregnancy have produced abnormalities of the external genitalia in male offspring.
- Hypersensitivity to any component of this medication.
5 WARNINGS AND PRECAUTIONS
5.1 Exposure of Women - Risk to Male Fetus
Finasteride is not indicated for use in women. Women should not handle crushed or broken finasteride tablets when they are pregnant or may potentially be pregnant because of the possibility of absorption of finasteride and the subsequent potential risk to a male fetus. Finasteride tablets are coated and will prevent contact with the active ingredient during normal handling, provided that the tablets have not been broken or crushed. [See Indications and Usage (1), Contraindications (4), Use in Specific Populations (8.1), How Supplied/Storage and Handling (16) and Patient Counseling Information (17).]
5.2 Effects on Prostate Specific Antigen (PSA)
In clinical studies with finasteride, 1 mg in men 18 to 41 years of age, the mean value of serum prostate specific antigen (PSA) decreased from 0.7 ng/mL at baseline to 0.5 ng/mL at Month 12. Further, in clinical studies with finasteride, 5 mg when used in older men who have benign prostatic hyperplasia (BPH), PSA levels are decreased by approximately 50%. Other studies with finasteride 5 mg showed it may also cause decreases in serum PSA in the presence of prostate cancer. These findings should be taken into account for proper interpretation of serum PSA when evaluating men treated with finasteride. Any confirmed increase from the lowest PSA value while on finasteride may signal the presence of prostate cancer and should be evaluated, even if PSA levels are still within the normal range for men not taking a 5α-reductase inhibitor. Non-compliance to therapy with finasteride may also affect PSA test results.
5.3 Increased Risk of High-Grade Prostate Cancer with 5α-Reductase Inhibitors
Men aged 55 and over with a normal digital rectal examination and PSA ≤3 ng/mL at baseline taking finasteride 5 mg/day (5 times the dose of finasteride) in the 7-year Prostate Cancer Prevention Trial (PCPT) had an increased risk of Gleason score 8 to 10 prostate cancer (finasteride 1.8% vs. placebo 1.1%). [See Adverse Reactions (6.1).] Similar results were observed in a 4-year placebo-controlled clinical trial with another 5α-reductase inhibitor (dutasteride, AVODART) (1% dutasteride vs. 0.5% placebo). 5α-reductase inhibitors may increase the risk of development of high-grade prostate cancer. Whether the effect of 5α-reductase inhibitors to reduce prostate volume, or study-related factors, impacted the results of these studies has not been established.
5.4 Pediatric Patients
Finasteride is not indicated for use in pediatric patients [see Use in Specific Populations (8.4)].
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in clinical practice.
Clinical Studies for Finasteride 1 mg in the Treatment of Male Pattern Hair Loss
In three controlled clinical trials for finasteride of 12-month duration, 1.4% of patients taking finasteride (n=945) were discontinued due to adverse experiences that were considered to be possibly, probably or definitely drug-related (1.6% for placebo; n=934).
Clinical adverse experiences that were reported as possibly, probably or definitely drug-related in ≥1% of patients treated with finasteride or placebo are presented in Table 1.
TABLE 1: Drug-Related Adverse Experiences for Finasteride 1 mg in Year 1 (%) MALE PATTERN HAIR LOSS
| | Finasteride
N=945 | Placebo
N=934 |
| Decreased Libido | 1.8 | 1.3 |
| Erectile Dysfunction | 1.3 | 0.7 |
| Ejaculation Disorder (Decreased Volume of Ejaculate) | 1.2 (0.8) | 0.7 (0.4) |
| Discontinuation due to drug-related sexual adverse experiences | 1.2 | 0.9 |
Integrated analysis of clinical adverse experiences showed that during treatment with finasteride, 36 (3.8%) of 945 men had reported one or more of these adverse experiences as compared to 20 (2.1%) of 934 men treated with placebo (p=0.04). Resolution occurred in men who discontinued therapy with finasteride due to these side effects and in most of those who continued therapy. The incidence of each of the above adverse experiences decreased to ≤0.3% by the fifth year of treatment with finasteride.
In a study of finasteride 1 mg daily in healthy men, a median decrease in ejaculate volume of 0.3 mL (-11%) compared with 0.2 mL (-8%) for placebo was observed after 48 weeks of treatment. Two other studies showed that finasteride at 5 times the dosage of finasteride (5 mg daily) produced significant median decreases of approximately 0.5 mL (-25%) compared to placebo in ejaculate volume, but this was reversible after discontinuation of treatment.
In the clinical studies with finasteride, the incidences for breast tenderness and enlargement, hypersensitivity reactions, and testicular pain in finasteride-treated patients were not different from those in patients treated with placebo.
Controlled Clinical Trials and Long-Term Open Extension Studies for Finasteride 5 mg and AVODART (dutasteride) in the Treatment of Benign Prostatic Hyperplasia
In the finasteride 5 mg long-term efficacy and safety study, a 4-year controlled clinical study, 3040 patients between the ages of 45 and 78 with symptomatic BPH and an enlarged prostate were evaluated for safety over a period of 4 years (1524 on finasteride 5 mg/day and 1516 on placebo). 3.7% (57 patients) treated with finasteride 5 mg and 2.1% (32 patients) treated with placebo discontinued therapy as a result of adverse reactions related to sexual function, which are the most frequently reported adverse reactions.
Table 2 presents the only clinical adverse reactions considered possibly, probably or definitely drug related by the investigator, for which the incidence on finasteride 5 mg was ≥1% and greater than placebo over the 4 years of the study. In years 2 to 4 of the study, there was no significant difference between treatment groups in the incidences of impotence, decreased libido and ejaculation disorder.
TABLE 2: Drug-Related Adverse Experiences for Finasteride 5 mg BENIGN PROSTATIC HYPERPLASIA
| | Year 1
(%) | Years 2, 3 and 4*
(%) |
||
| Finasteride
5 mg | Placebo
| Finasteride
5 mg | Placebo
|
|
| Impotence | 8.1 | 3.7 | 5.1 | 5.1 |
| Decreased Libido | 6.4 | 3.4 | 2.6 | 2.6 |
| Decreased Volume of Ejaculate | 3.7 | 0.8 | 1.5 | 0.5 |
| Ejaculation Disorder | 0.8 | 0.1 | 0.2 | 0.1 |
| Breast Enlargement | 0.5 | 0.1 | 1.8 | 1.1 |
| Breast Tenderness | 0.4 | 0.1 | 0.7 | 0.3 |
| Rash | 0.5 | 0.2 | 0.5 | 0.1 |
| *Combined Years 2 to 4
N = 1524 and 1516, finasteride vs. placebo, respectively |
||||
The adverse experience profiles in the 1-year, placebo-controlled, Phase III BPH studies and the 5-year open extensions with finasteride 5 mg and long-term efficacy and safety study were similar.
There is no evidence of increased sexual adverse experiences with increased duration of treatment with finasteride 5 mg. New reports of drug-related sexual adverse experiences decreased with duration of therapy.
During the 4- to 6-year placebo- and comparator-controlled Medical Therapy of Prostatic Symptoms (MTOPS) study that enrolled 3047 men, there were 4 cases of breast cancer in men treated with finasteride 5 mg but no cases in men not treated with finasteride 5 mg. During the 4-year placebo-controlled long-term efficacy and safety study that enrolled 3040 men, there were 2 cases of breast cancer in placebo-treated men, but no cases were reported in men treated with finasteride 5 mg.
During the 7-year placebo-controlled Prostate Cancer Prevention Trial (PCPT) that enrolled 18,882 men, there was 1 case of breast cancer in men treated with finasteride 5 mg, and 1 case of breast cancer in men treated with placebo. The relationship between long-term use of finasteride and male breast neoplasia is currently unknown.
The PCPT trial was a 7-year randomized, double-blind, placebo-controlled trial that enrolled 18,882 healthy men ≥55 years of age with a normal digital rectal examination and a PSA ≤3 ng/mL. Men received either finasteride 5 mg or placebo daily. Patients were evaluated annually with PSA and digital rectal exams. Biopsies were performed for elevated PSA, an abnormal digital rectal exam, or the end of study. The incidence of Gleason score 8 to 10 prostate cancer was higher in men treated with finasteride (1.8%) than in those treated with placebo (1.1%). In a 4-year placebo-controlled clinical trial with another 5α-reductase inhibitor [AVODART (dutasteride)], similar results for Gleason score 8 to 10 prostate cancer were observed (1% dutasteride vs. 0.5% placebo). The clinical significance of these findings with respect to use of finasteride by men is unknown.
No clinical benefit has been demonstrated in patients with prostate cancer treated with finasteride 5 mg. Finasteride 5 mg is not approved to reduce the risk of developing prostate cancer.
Sexual Function Questionnaire
A sexual function questionnaire was self-administered by patients participating in the two vertex baldness trials to detect more subtle changes in sexual function. At Month 12, statistically significant differences in favor of placebo were found in 3 of 4 domains (sexual interest, erections, and perception of sexual problems). However, no significant difference was seen in the question on overall satisfaction with sex life.
In one of the two vertex baldness studies, patients were questioned on non-scalp body hair growth. Finasteride did not appear to affect non-scalp body hair.
6.2 Postmarketing Experience
The following adverse reactions have been identified during post approval use of finasteride. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure:
Hypersensitivity Reaction: hypersensitivity reactions such as rash, pruritus, urticaria, and angioedema (including swelling of the lips, tongue, throat, and face);
Reproductive System: sexual dysfunction that continued after discontinuation of treatment, including erectile dysfunction, libido disorders, ejaculation disorders, and orgasm disorders; male infertility and/or poor seminal quality (normalization or improvement of seminal quality has been reported after discontinuation of finasteride); testicular pain; hematospermia.
Neoplasms: male breast cancer;
Breast Disorders: breast tenderness and enlargement;
Nervous System/Psychiatric: depression, suicidal ideation and behavior.
7 DRUG INTERACTIONS
7.1 Cytochrome P450-Linked Drug Metabolizing Enzyme System
No drug interactions of clinical importance have been identified. Finasteride does not appear to affect the cytochrome P450-linked drug-metabolizing enzyme system. Compounds that have been tested in man include antipyrine, digoxin, propranolol, theophylline, and warfarin and no clinically meaningful interactions were found.
7.2 Other Concomitant Therapy
Although specific interaction studies were not performed, finasteride doses of 1 mg or more were concomitantly used in clinical studies with acetaminophen, acetylsalicylic acid, α-blockers, analgesics, angiotensin-converting enzyme (ACE) inhibitors, anticonvulsants, benzodiazepines, beta blockers, calcium-channel blockers, cardiac nitrates, diuretics, H2 antagonists, HMG-CoA reductase inhibitors, prostaglandin synthetase inhibitors (also referred to as NSAIDs), and quinolone anti-infectives without evidence of clinically significant adverse interactions.
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Pregnancy Category X [see Contraindications (4)]. Finasteride is contraindicated for use in women who are or may become pregnant. Finasteride is a Type II 5α-reductase inhibitor that prevents conversion of testosterone to 5α-dihydrotestosterone (DHT), a hormone necessary for normal development of male genitalia. In animal studies, finasteride caused abnormal development of external genitalia in male fetuses. If this drug is used during pregnancy, or if the patient becomes pregnant while taking this drug, the patient should be apprised of the potential hazard to the male fetus.
Abnormal male genital development is an expected consequence when conversion of testosterone to 5α-dihydrotestosterone (DHT) is inhibited by 5α-reductase inhibitors. These outcomes are similar to those reported in male infants with genetic 5α-reductase deficiency. Women could be exposed to finasteride through contact with crushed or broken finasteride tablets or semen from a male partner taking finasteride. With regard to finasteride exposure through the skin, finasteride tablets are coated and will prevent skin contact with finasteride during normal handling if the tablets have not been crushed or broken. Women who are pregnant or may become pregnant should not handle crushed or broken finasteride tablets because of possible exposure of a male fetus. If a pregnant woman comes in contact with crushed or broken finasteride tablets, the contact area should be washed immediately with soap and water. With regard to potential finasteride exposure through semen, a study has been conducted in men receiving finasteride 1 mg/day that measured finasteride concentrations in semen [see Clinical Pharmacology (12.3)].
In an embryo-fetal development study, pregnant rats received finasteride during the period of major organogenesis (gestation days 6 to 17). At maternal doses of oral finasteride approximately 1 to 684 times the recommended human dose (RHD) of 1 mg/day (based on AUC at animal doses of 0.1 to 100 mg/kg/day) there was a dose-dependent increase in hypospadias that occurred in 3.6 to 100% of male offspring. Exposure multiples were estimated using data from nonpregnant rats. Days 16 to 17 of gestation is a critical period in male fetal rats for differentiation of the external genitalia. At oral maternal doses approximately 0.2 times the RHD (based on AUC at animal dose of 0.03 mg/kg/day), male offspring had decreased prostatic and seminal vesicular weights, delayed preputial separation and transient nipple development. Decreased anogenital distance occurred in male offspring of pregnant rats that received approximately 0.02 times the RHD (based on AUC at animal dose of 0.003 mg/kg/day). No abnormalities were observed in female offspring exposed to any dose of finasteride in utero.
No developmental abnormalities were observed in the offspring of untreated females mated with finasteride-treated male rats that received approximately 488 times the RHD (based on AUC at animal dose of 80 mg/kg/day). Slightly decreased fertility was observed in male offspring after administration of about 20 times the RHD (based on AUC at animal dose of 3 mg/kg/day) to female rats during late gestation and lactation. No effects on fertility were seen in female offspring under these conditions.
No evidence of male external genital malformations or other abnormalities were observed in rabbit fetuses exposed to finasteride during the period of major organogenesis (gestation days 6 to 18) at maternal doses up to 100 mg/kg/day (finasteride exposure levels were not measured in rabbits). However, this study may not have included the critical period for finasteride effects on development of male external genitalia in the rabbit.
The fetal effects of maternal finasteride exposure during the period of embryonic and fetal development were evaluated in the rhesus monkey (gestation days 20 to 100), in a species and development period more predictive of specific effects in humans than the studies in rats and rabbits. Intravenous administration of finasteride to pregnant monkeys at doses as high as 800 ng/day (estimated maximal blood concentration of 1.86 ng/mL or about 930 times the highest estimated exposure of pregnant women to finasteride from semen of men taking 1 mg/day) resulted in no abnormalities in male fetuses. In confirmation of the relevance of the rhesus model for human fetal development, oral administration of a dose of finasteride (2 mg/kg/day or approximately 120,000 times the highest estimated blood levels of finasteride from semen of men taking 1 mg/day) to pregnant monkeys resulted in external genital abnormalities in male fetuses. No other abnormalities were observed in male fetuses and no finasteride-related abnormalities were observed in female fetuses at any dose.
8.3 Nursing Mothers
Finasteride is not indicated for use in women.
It is not known whether finasteride is excreted in human milk.
8.4 Pediatric Use
Finasteride is not indicated for use in pediatric patients.
Safety and effectiveness in pediatric patients have not been established.
8.5 Geriatric Use
Clinical efficacy studies with finasteride did not include subjects aged 65 and over. Based on the pharmacokinetics of finasteride 5 mg, no dosage adjustment is necessary in the elderly for finasteride [see Clinical Pharmacology (12.3)]. However the efficacy of finasteride in the elderly has not been established.
8.6 Hepatic Impairment
Caution should be exercised in the administration of finasteride in those patients with liver function abnormalities, as finasteride is metabolized extensively in the liver [see Clinical Pharmacology (12.3)].
10 OVERDOSAGE
In clinical studies, single doses of finasteride up to 400 mg and multiple doses of finasteride up to 80 mg/day for three months did not result in adverse reactions. Until further experience is obtained, no specific treatment for an overdose with finasteride can be recommended.
Significant lethality was observed in male and female mice at single oral doses of 1500 mg/m2(500 mg/kg) and in female and male rats at single oral doses of 2360 mg/m2 (400 mg/kg) and 5900 mg/m2(1000 mg/kg), respectively.
11 DESCRIPTION
Finasteride tablets USP contain finasteride USP as the active ingredient. Finasteride, a synthetic 4-azasteroid compound, is a specific inhibitor of steroid Type II 5α-reductase, an intracellular enzyme that converts the androgen testosterone into 5α-dihydrotestosterone (DHT).
The chemical name of finasteride is N-tert-Butyl-3-oxo-4-aza-5α-androst-1-ene-17β-carboxamide. The molecular formula of finasteride is C23H36N2O2 and its molecular weight is 372.55. Its structural formula is:
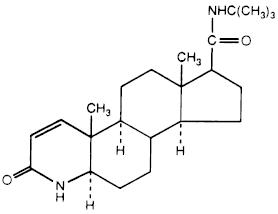
Finasteride USP is a white to off-white, crystalline solid with a melting point between 254°C and 262°C. It is freely soluble in chloroform and in lower alcohol solvents but is practically insoluble in water.
Finasteride tablets USP are film-coated tablets for oral administration. Each tablet contains 1 mg of finasteride USP and the following inactive ingredients: docusate sodium, hydroxypropyl cellulose, hypromellose, lactose monohydrate, magnesium stearate, microcrystalline cellulose, pregelatinised starch (maize), sodium starch glycolate, talc, and titanium dioxide.
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Finasteride is a competitive and specific inhibitor of Type II 5α-reductase, an intracellular enzyme that converts the androgen testosterone into DHT. Two distinct isozymes are found in mice, rats, monkeys, and humans: Type I and II. Each of these isozymes is differentially expressed in tissues and developmental stages. In humans, Type I 5α-reductase is predominant in the sebaceous glands of most regions of skin, including scalp, and liver. Type I 5α-reductase is responsible for approximately one-third of circulating DHT. The Type II 5α-reductase isozyme is primarily found in prostate, seminal vesicles, epididymides, and hair follicles as well as liver, and is responsible for two-thirds of circulating DHT.
In humans, the mechanism of action of finasteride is based on its preferential inhibition of the Type II isozyme. Using native tissues (scalp and prostate), in vitro binding studies examining the potential of finasteride to inhibit either isozyme revealed a 100-fold selectivity for the human Type II 5α-reductase over Type I isozyme (IC50=500 and 4.2 nM for Type I and II, respectively). For both isozymes, the inhibition by finasteride is accompanied by reduction of the inhibitor to dihydrofinasteride and adduct formation with NADP+. The turnover for the enzyme complex is slow (t1/2 approximately 30 days for the Type II enzyme complex and 14 days for the Type I complex). Inhibition of Type II 5α-reductase blocks the peripheral conversion of testosterone to DHT, resulting in significant decreases in serum and tissue DHT concentrations.
In men with male pattern hair loss (androgenetic alopecia), the balding scalp contains miniaturized hair follicles and increased amounts of DHT compared with hairy scalp. Administration of finasteride decreases scalp and serum DHT concentrations in these men. The relative contributions of these reductions to the treatment effect of finasteride have not been defined. By this mechanism, finasteride appears to interrupt a key factor in the development of androgenetic alopecia in those patients genetically predisposed.
12.2 Pharmacodynamics
Finasteride produces a rapid reduction in serum DHT concentration, reaching 65% suppression within 24 hours of oral dosing with a 1 mg tablet. Mean circulating levels of testosterone and estradiol were increased by approximately 15% as compared to baseline, but these remained within the physiologic range.
Finasteride has no affinity for the androgen receptor and has no androgenic, antiandrogenic, estrogenic, antiestrogenic, or progestational effects. The relationship between these pharmacodynamic activities and the mechanisms(s) by which finasteride exerts its clinical effect is unknown. In studies with finasteride, no clinically meaningful changes in luteinizing hormone (LH), follicle-stimulating hormone (FSH) or prolactin were detected. In healthy volunteers, treatment with finasteride did not alter the response of LH and FSH to gonadotropin-releasing hormone indicating that the hypothalamic-pituitary-testicular axis was not affected. Finasteride had no effect on circulating levels of cortisol, thyroid-stimulating hormone, or thyroxine, nor did it affect the plasma lipid profile (e.g., total cholesterol, low-density lipoproteins, high-density lipoproteins and triglycerides) or bone mineral density.
12.3 Pharmacokinetics
Absorption
In a study in 15 healthy young male subjects, the mean bioavailability of finasteride 1 mg tablets was 65% (range 26 to 170%), based on the ratio of area under the curve (AUC) relative to an intravenous (IV) reference dose. At steady state following dosing with 1 mg/day (n=12), maximum finasteride plasma concentration averaged 9.2 ng/mL (range, 4.9 to 13.7 ng/mL) and was reached 1 to 2 hours postdose; AUC(0-24 hr) was 53 ng•hr/mL (range, 20 to 154 ng•hr/mL). Bioavailability of finasteride was not affected by food.
Distribution
Mean steady-state volume of distribution was 76 liters (range, 44 to 96 liters; n=15). Approximately 90% of circulating finasteride is bound to plasma proteins. There is a slow accumulation phase for finasteride after multiple dosing.
Finasteride has been found to cross the blood-brain barrier.
Semen levels have been measured in 35 men taking finasteride 1 mg/day for 6 weeks. In 60% (21 of 35) of the samples, finasteride levels were undetectable (<0.2 ng/mL). The mean finasteride level was 0.26 ng/mL and the highest level measured was 1.52 ng/mL. Using the highest semen level measured and assuming 100% absorption from a 5 mL ejaculate per day, human exposure through vaginal absorption would be up to 7.6 ng per day, which is 650-fold less than the dose of finasteride (5 mcg) that had no effect on circulating DHT levels in men. [See Use in Specific Populations (8.1).]
Metabolism
Finasteride is extensively metabolized in the liver, primarily via the cytochrome P450 3A4 enzyme subfamily. Two metabolites, the t-butyl side chain monohydroxylated and monocarboxylic acid metabolites, have been identified that possess no more than 20% of the 5α-reductase inhibitory activity of finasteride.
Excretion
Following intravenous infusion in healthy young subjects (n=15), mean plasma clearance of finasteride was 165 mL/min (range, 70 to 279 mL/min). Mean terminal half-life in plasma was 4.5 hours (range, 3.3 to 13.4 hours; n=12). Following an oral dose of 14C-finasteride in man (n=6), a mean of 39% (range, 32 to 46%) of the dose was excreted in the urine in the form of metabolites; 57% (range, 51 to 64%) was excreted in the feces.
Mean terminal half-life is approximately 5 to 6 hours in men 18 to 60 years of age and 8 hours in men more than 70 years of age.
| Mean (± SD) n=15 |
|
|---|---|
| *Range |
|
| Bioavailability | 65% (26 to 170%)* |
| Clearance (mL/min) | 165 (55) |
| Volume of Distribution (L) | 76 (14) |
| Mean (± SD) (n=12) |
|
|---|---|
| *First-dose values; all other parameters are last-dose values |
|
| AUC (ng•hr/mL) | 53 (33.8) |
| Peak Concentration (ng/mL) | 9.2 (2.6) |
| Time to Peak (hours) | 1.3 (0.5) |
| Half-Life (hours)* | 4.5 (1.6) |
Renal Impairment
No dosage adjustment is necessary in patients with renal impairment. In patients with chronic renal impairment, with creatinine clearances ranging from 9 to 55 mL/min, AUC, maximum plasma concentration, half-life, and protein binding after a single dose of 14C-finasteride were similar to those obtained in healthy volunteers. Urinary excretion of metabolites was decreased in patients with renal impairment. This decrease was associated with an increase in fecal excretion of metabolites. Plasma concentrations of metabolites were significantly higher in patients with renal impairment (based on a 60% increase in total radioactivity AUC). However, finasteride has been tolerated in men with normal renal function receiving up to 80 mg/day for 12 weeks where exposure of these patients to metabolites would presumably be much greater.
Hepatic Impairment
The effect of hepatic impairment on finasteride pharmacokinetics has not been studied. Caution should be used in the administration of finasteride in patients with liver function abnormalities, as finasteride is metabolized extensively in the liver.
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
No evidence of a tumorigenic effect was observed in a 24-month study in Sprague-Dawley rats receiving doses of finasteride up to 160 mg/kg/day in males and 320 mg/kg/day in females. These doses produced respective systemic exposure in rats of 888 and 2192 times those observed in man receiving the recommended human dose of 1 mg/day. All exposure calculations were based on calculated AUC(0-24 hr) for animals and mean AUC(0-24 hr) for man (0.05 mcg•hr/mL).
In a 19-month carcinogenicity study in CD-1 mice, a statistically significant (p≤0.05) increase in the incidence of testicular Leydig cell adenomas was observed at 1824 times the human exposure (250 mg/kg/day). In mice at 184 times the human exposure, estimated (25 mg/kg/day) and in rats at 312 times the human exposure (≥40 mg/kg/day) an increase in the incidence of Leydig cell hyperplasia was observed. A positive correlation between the proliferative changes in the Leydig cells and an increase in serum LH levels (2- to 3-fold above control) has been demonstrated in both rodent species treated with high doses of finasteride. No drug-related Leydig cell changes were seen in either rats or dogs treated with finasteride for 1 year at 240 and 2800 times (20 mg/kg/day and 45 mg/kg/day, respectively), or in mice treated for 19 months at 18.4 times the human exposure, estimated (2.5 mg/kg/day).
No evidence of mutagenicity was observed in an in vitro bacterial mutagenesis assay, a mammalian cell mutagenesis assay, or in an in vitro alkaline elution assay. In an in vitro chromosome aberration assay, using Chinese hamster ovary cells, there was a slight increase in chromosome aberrations. In an in vivo chromosome aberration assay in mice, no treatment-related increase in chromosome aberration was observed with finasteride at the maximum tolerated dose of 250 mg/kg/day (1824 times the human exposure) as determined in the carcinogenicity studies.
In sexually mature male rabbits treated with finasteride at 4344 times the human exposure (80 mg/kg/day) for up to 12 weeks, no effect on fertility, sperm count, or ejaculate volume was seen. In sexually mature male rats treated with 488 times the human exposure (80 mg/kg/day), there were no significant effects on fertility after 6 or 12 weeks of treatment; however, when treatment was continued for up to 24 or 30 weeks, there was an apparent decrease in fertility, fecundity, and an associated significant decrease in the weights of the seminal vesicles and prostate. All these effects were reversible within 6 weeks of discontinuation of treatment. No drug-related effect on testes or on mating performance has been seen in rats or rabbits. This decrease in fertility in finasteride-treated rats is secondary to its effect on accessory sex organs (prostate and seminal vesicles) resulting in failure to form a seminal plug. The seminal plug is essential for normal fertility in rats but is not relevant in man.
14 CLINICAL STUDIES
14.1 Studies in Men
The efficacy of finasteride was demonstrated in men (88% Caucasian) with mild to moderate androgenetic alopecia (male pattern hair loss) between 18 and 41 years of age. In order to prevent seborrheic dermatitis which might confound the assessment of hair growth in these studies, all men, whether treated with finasteride or placebo, were instructed to use a specified, medicated, tar-based shampoo (Neutrogena T/Gel ® Shampoo) during the first 2 years of the studies.
There were three double-blind, randomized, placebo-controlled studies of 12-month duration. The two primary endpoints were hair count and patient self-assessment; the two secondary endpoints were investigator assessment and ratings of photographs. In addition, information was collected regarding sexual function (based on a self-administered questionnaire) and non-scalp body hair growth. The three studies were conducted in 1879 men with mild to moderate, but not complete, hair loss. Two of the studies enrolled men with predominantly mild to moderate vertex hair loss (n=1553). The third enrolled men having mild to moderate hair loss in the anterior mid-scalp area with or without vertex balding (n=326).
Studies in Men with Vertex Baldness
Of the men who completed the first 12 months of the two vertex baldness trials, 1215 elected to continue in double-blind, placebo-controlled, 12-month extension studies. There were 547 men receiving finasteride for both the initial study and first extension periods (up to 2 years of treatment) and 60 men receiving placebo for the same periods. The extension studies were continued for 3 additional years, with 323 men on finasteride and 23 on placebo entering the fifth year of the study.
In order to evaluate the effect of discontinuation of therapy, there were 65 men who received finasteride for the initial 12 months followed by placebo in the first 12-month extension period. Some of these men continued in additional extension studies and were switched back to treatment with finasteride, with 32 men entering the fifth year of the study. Lastly, there were 543 men who received placebo for the initial 12 months followed by finasteride in the first 12-month extension period. Some of these men continued in additional extension studies receiving finasteride, with 290 men entering the fifth year of the study (see Figure 1 below).
Hair counts were assessed by photographic enlargements of a representative area of active hair loss. In these two studies in men with vertex baldness, significant increases in hair count were demonstrated at 6 and 12 months in men treated with finasteride, while significant hair loss from baseline was demonstrated in those treated with placebo. At 12 months there was a 107-hair difference from placebo (p<0.001, finasteride [n=679] vs. placebo [n=672]) within a 1-inch diameter circle (5.1 cm2). Hair count was maintained in those men taking finasteride for up to 2 years, resulting in a 138-hair difference between treatment groups (p<0.001, finasteride [n=433] vs. placebo [n=47]) within the same area. In men treated with finasteride, the maximum improvement in hair count compared to baseline was achieved during the first 2 years. Although the initial improvement was followed by a slow decline, hair count was maintained above baseline throughout the 5 years of the studies. Furthermore, because the decline in the placebo group was more rapid, the difference between treatment groups also continued to increase throughout the studies, resulting in a 277-hair difference (p<0.001, finasteride [n=219] vs. placebo [n=15]) at 5 years (see Figure 1 below).
Patients who switched from placebo to finasteride (n=425) had a decrease in hair count at the end of the initial 12-month placebo period, followed by an increase in hair count after 1 year of treatment with finasteride. This increase in hair count was less (56 hairs above original baseline) than the increase (91 hairs above original baseline) observed after 1 year of treatment in men initially randomized to finasteride. Although the increase in hair count, relative to when therapy was initiated, was comparable between these two groups, a higher absolute hair count was achieved in patients who were started on treatment with finasteride in the initial study. This advantage was maintained through the remaining 3 years of the studies. A change of treatment from finasteride to placebo (n=48) at the end of the initial 12 months resulted in reversal of the increase in hair count 12 months later, at 24 months (see Figure 1 below).
At 12 months, 58% of men in the placebo group had further hair loss (defined as any decrease in hair count from baseline), compared with 14% of men treated with finasteride. In men treated for up to 2 years, 72% of men in the placebo group demonstrated hair loss, compared with 17% of men treated with finasteride. At 5 years, 100% of men in the placebo group demonstrated hair loss, compared with 35% of men treated with finasteride.
Figure 1

Patient self-assessment was obtained at each clinic visit from a self-administered questionnaire, which included questions on their perception of hair growth, hair loss, and appearance. This self-assessment demonstrated an increase in amount of hair, a decrease in hair loss, and improvement in appearance in men treated with finasteride. Overall improvement compared with placebo was seen as early as 3 months (p<0.05), with improvement maintained over 5 years.
Investigator assessment was based on a 7-point scale evaluating increases or decreases in scalp hair at each patient visit. This assessment showed significantly greater increases in hair growth in men treated with finasteride compared with placebo as early as 3 months (p<0.001). At 12 months, the investigators rated 65% of men treated with finasteride as having increased hair growth compared with 37% in the placebo group. At 2 years, the investigators rated 80% of men treated with finasteride as having increased hair growth compared with 47% of men treated with placebo. At 5 years, the investigators rated 77% of men treated with finasteride as having increased hair growth, compared with 15% of men treated with placebo.
An independent panel rated standardized photographs of the head in a blinded fashion based on increases or decreases in scalp hair using the same 7-point scale as the investigator assessment. At 12 months, 48% of men treated with finasteride had an increase as compared with 7% of men treated with placebo. At 2 years, an increase in hair growth was demonstrated in 66% of men treated with finasteride, compared with 7% of men treated with placebo. At 5 years, 48% of men treated with finasteride demonstrated an increase in hair growth, 42% were rated as having no change (no further visible progression of hair loss from baseline) and 10% were rated as having lost hair when compared to baseline. In comparison, 6% of men treated with placebo demonstrated an increase in hair growth, 19% were rated as having no change and 75% were rated as having lost hair when compared to baseline.
A 48-week, placebo-controlled study designed to assess by phototrichogram the effect of finasteride on total and actively growing (anagen) scalp hairs in vertex baldness enrolled 212 men with androgenetic alopecia. At baseline and 48 weeks, total and anagen hair counts were obtained in a 1 cm2 target area of the scalp. Men treated with finasteride showed increases from baseline in total and anagen hair counts of 7 hairs and 18 hairs, respectively, whereas men treated with placebo had decreases of 10 hairs and 9 hairs, respectively. These changes in hair counts resulted in a between-group difference of 17 hairs in total hair count (p<0.001) and 27 hairs in anagen hair count (p<0.001), and an improvement in the proportion of anagen hairs from 62% at baseline to 68% for men treated with finasteride.
Study in Men with Hair Loss in the Anterior Mid-Scalp Area
A study of 12-month duration, designed to assess the efficacy of finasteride in men with hair loss in the anterior mid-scalp area, also demonstrated significant increases in hair count compared with placebo. Increases in hair count were accompanied by improvements in patient self-assessment, investigator assessment, and ratings based on standardized photographs. Hair counts were obtained in the anterior mid-scalp area, and did not include the area of bitemporal recession or the anterior hairline.
Summary of Clinical Studies in Men
Clinical studies were conducted in men aged 18 to 41 with mild to moderate degrees of androgenetic alopecia. All men treated with finasteride or placebo received a tar-based shampoo (Neutrogena T/Gel® Shampoo) during the first 2 years of the studies. Clinical improvement was seen as early as 3 months in the patients treated with finasteride and led to a net increase in scalp hair count and hair regrowth. In clinical studies for up to 5 years, treatment with finasteride slowed the further progression of hair loss observed in the placebo group. In general, the difference between treatment groups continued to increase throughout the 5 years of the studies.
Ethnic Analysis of Clinical Data from Men
In a combined analysis of the two studies on vertex baldness, mean hair count changes from baseline were 91 vs. -19 hairs (finasteride vs. placebo) among Caucasians (n=1185), 49 vs. -27 hairs among Blacks (n=84), 53 vs. -38 hairs among Asians (n=17), 67 vs. 5 hairs among Hispanics (n=45) and 67 vs. -15 hairs among other ethnic groups (n=20). Patient self-assessment showed improvement across racial groups with finasteride treatment, except for satisfaction of the frontal hairline and vertex in Black men, who were satisfied overall.
14.2 Study in Women
In a study involving 137 postmenopausal women with androgenetic alopecia who were treated with finasteride (n=67) or placebo (n=70) for 12 months, effectiveness could not be demonstrated. There was no improvement in hair counts, patient self-assessment, investigator assessment, or ratings of standardized photographs in the women treated with finasteride when compared with the placebo group [see Indications and Usage (1)].
16 HOW SUPPLIED/STORAGE AND HANDLING
Finasteride Tablets USP, 1 mg are white colored, octagonal, biconvex, film-coated tablets debossed with ‘J’ on one side and ‘81’ on the other side.
Bottles of 30 NDC 57237-061-30
Bottles of 90 NDC 57237-061-90
Storage and Handling
Store at 20º to 25ºC (68º to 77ºF) [see USP Controlled Room Temperature]. Keep container closed and protect from moisture.
Women should not handle crushed or broken finasteride tablets when they are pregnant or may potentially be pregnant because of the possibility of absorption of finasteride and the subsequent potential risk to a male fetus. Finasteride tablets are coated and will prevent contact with the active ingredient during normal handling, provided that the tablets are not broken or crushed [see Warnings and Precautions (5.1), Use in Specific Populations (8.1) and Patient Counseling Information (17)].
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Patient Information).
Exposure of Women — Risk to Male Fetus
Physicians should inform patients that women who are pregnant or may potentially be pregnant should not handle crushed or broken finasteride tablets because of the possibility of absorption of finasteride and the subsequent potential risk to a male fetus. Finasteride tablets are coated and will prevent contact with the active ingredient during normal handling, provided that the tablets have not been broken or crushed. If a woman who is pregnant or may potentially be pregnant comes in contact with crushed or broken finasteride tablets, the contact area should be washed immediately with soap and water [see Contraindications (4), Warnings and Precautions (5.1), Use in Specific Populations (8.1) and How Supplied/Storage and Handling (16)].
Increased Risk of High-Grade Prostate Cancer
Patients should be informed that there was an increase in high-grade prostate cancer in men treated with 5α-reductase inhibitors indicated for BPH treatment, compared to those treated with placebo in studies looking at the use of these drugs to prevent prostate cancer [see Warnings and Precautions (5.3) and Adverse Reactions (6.1)].
Sexual Adverse events
Physicians should inform the patients that finasteride may cause symptoms of sexual dysfunction such as decreased libido, erectile dysfunction, and ejaculation disorder, including decreased ejaculate volume.
Additional Instructions
Physicians should instruct their patients to promptly report any changes in their breasts such as lumps, pain or nipple discharge. Breast changes including breast enlargement, tenderness and neoplasm have been reported [see Adverse Reactions (6.1)].
Physicians should instruct their patients to read the patient package insert before starting therapy with finasteride and to read it again each time the prescription is renewed so that they are aware of current information for patients regarding finasteride.
The brands listed are trademarks of their respective owners and are not trademarks of Aurobindo Pharma Limited.
Distributed by:
Rising Health, LLC
Saddle Brook, NJ 07663
Made in India
Code: TS/DRUGS/19/1993
Revised: 08/2022
Patient Information
Finasteride Tablets, USP
(fin as' ter ide)
Finasteride tablets are for use by MEN ONLY and should NOT be used by women or children.
Read this Patient Information before you start taking finasteride tablets and each time you get a refill. There may be new information. This information does not take the place of talking with your healthcare provider about your medical condition or treatment.
What are finasteride tablets?
Finasteride tablets are a prescription medicine used for the treatment of male pattern hair loss (androgenetic alopecia).
It is not known if finasteride tablets work for a receding hairline on either side of and above your forehead (temporal area).
Finasteride tablets are not for use by women and children.
Who should not take finasteride tablets?
Do not take finasteride tablets if you:
- are pregnant or may become pregnant. Finasteride tablets may harm your unborn baby.
- Finasteride tablets are coated and will prevent contact with the medicine during handling, as long as the tablets are not broken or crushed. Females who are pregnant or who may become pregnant should not come in contact with broken or crushed finasteride tablets. If a pregnant woman comes in contact with crushed or broken finasteride tablets, wash the contact area right away with soap and water. If a woman who is pregnant comes into contact with the active ingredient in finasteride tablets, a healthcare provider should be consulted.
- If a woman who is pregnant with a male baby swallows or comes in contact with the medicine in finasteride tablets, the male baby may be born with sex organs that are not normal.
- are allergic to any of the ingredients in finasteride tablets. See the end of this leaflet for a complete list of ingredients in finasteride tablets.
What should I tell my healthcare provider before taking finasteride tablets?
Before taking finasteride tablets, tell your healthcare provider if you:
- have any other medical conditions, including problems with your prostate or liver
Tell your healthcare provider about all the medicines you take, including prescription and non-prescription medicines, vitamins, and herbal supplements.
Know the medicines you take. Keep a list of them to show your healthcare provider and pharmacist when you get a new medicine.
How should I take finasteride tablets?
- Take finasteride tablets exactly as your healthcare provider tells you to take them.
- You may take finasteride tablets with or without food.
- If you forget to take finasteride tablets, do not take an extra tablet. Just take the next tablet as usual.
Finasteride tablets will not work faster or better if you take them more than once a day.
What are the possible side effects of finasteride tablets?
- decrease in your blood Prostate Specific Antigen (PSA) levels. Finasteride tablets can affect a blood test called PSA (Prostate Specific Antigen) for the screening of prostate cancer. If you have a PSA test done you should tell your healthcare provider that you are taking finasteride tablets because finasteride tablets decreases PSA levels. Changes in PSA levels will need to be evaluated by your healthcare provider. Any increase in follow-up PSA levels from their lowest point may signal the presence of prostate cancer and should be evaluated, even if the test results are still within the normal range for men not taking finasteride tablets. You should also tell your healthcare provider if you have not been taking finasteride tablets as prescribed because this may affect the PSA test results. For more information, talk to your healthcare provider.
- There may be an increased risk of a more serious form of prostate cancer in men taking finasteride at 5 times the dose of finasteride tablets.
The most common side effects of finasteride tablets include:
- decrease in sex drive
- trouble getting or keeping an erection
- a decrease in the amount of semen
The following have been reported in general use with finasteride tablets:
- breast tenderness and enlargement. Tell your healthcare provider about any changes in your breasts such as lumps, pain or nipple discharge.
- depression;
- decrease in sex drive that continued after stopping the medication;
- allergic reactions including rash, itching, hives and swelling of the lips, tongue, throat, and face;
- problems with ejaculation that continued after stopping medication;
- testicular pain;
- difficulty in achieving an erection that continued after stopping the medication;
- male infertility and/or poor quality of semen.
- in rare cases, male breast cancer.
Tell your healthcare provider if you have any side effect that bothers you or that does not go away.
These are not all the possible side effects of finasteride tablets. For more information, ask your healthcare provider or pharmacist.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
How should I store finasteride tablets?
- Store finasteride tablets at 20º to 25ºC (68º to 77ºF).
- Keep finasteride tablets in a closed container and keep finasteride tablets dry (protect from moisture).
Keep finasteride tablets and all medicines out of the reach of children.
General information about the safe and effective use of finasteride tablets.
Medicines are sometimes prescribed for purposes other than those listed in this Patient Information leaflet. Do not use finasteride tablets for a condition for which it was not prescribed. Do not give finasteride tablets to other people, even if they have the same symptoms you have. They may harm them.
This Patient Information leaflet summarizes the most important information about finasteride tablets. If you would like more information, talk with your healthcare provider. You can ask your pharmacist or healthcare provider for information about finasteride tablets that is written for health professionals. For more information, call Rising Health, LLC at 1-833-395-6928.
What are the ingredients in finasteride tablets?
Active ingredient: finasteride.
Inactive ingredients: docusate sodium, hydroxypropyl cellulose, hypromellose, lactose monohydrate, magnesium stearate, microcrystalline cellulose, pregelatinised starch (maize), sodium starch glycolate, talc, and titanium dioxide.
This Patient Information has been approved by the U.S. Food and Drug Administration.
Distributed by:
Rising Health, LLC
Saddle Brook, NJ 07663
Made in India
Code: TS/DRUGS/19/1993
Revised: 08/2022
PACKAGE LABEL-PRINCIPAL DISPLAY PANEL - 1 mg (30 Tablet Bottle)
INGREDIENTS AND APPEARANCE
| FINASTERIDE
finasteride tablet, film coated |
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
| Labeler - Rising Health, LLC (080500961) |
| Registrant - Aurobindo Pharma Limited (650082092) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Aurobindo Pharma Limited | 918917642 | ANALYSIS(57237-061) , MANUFACTURE(57237-061) | |