Search by Drug Name or NDC
NDC 58602-0602-07 Diclofenac Sodium 10 mg/g Details
Diclofenac Sodium 10 mg/g
Diclofenac Sodium is a TOPICAL GEL in the HUMAN OTC DRUG category. It is labeled and distributed by Aurohealth LLC. The primary component is DICLOFENAC SODIUM.
MedlinePlus Drug Summary
Diclofenac topical gel (Solaraze) is used to treat actinic keratosis (flat, scaly growths on the skin caused by too much sun exposure). Diclofenac is in a class of medications called nonsteroidal anti-inflammatory drugs (NSAIDs). The way diclofenac gel works to treat actinic keratosis is not known. Diclofenac is also available as a liquid (Pennsaid) and a gel (Voltaren) that are applied to the skin to treat arthritis pain. This monograph only gives information about diclofenac gel (Solaraze) for actinic keratosis. If you are using either of the products for osteoarthritis, read the monograph entitled diclofenac topical (osteoarthritis pain).
Related Packages: 58602-0602-07Last Updated: 12/01/2022
MedLinePlus Full Drug Details: Diclofenac Topical (actinic keratosis)
Product Information
| NDC | 58602-0602 |
|---|---|
| Product ID | 58602-602_49961909-3aca-40b3-8424-668c1791c01d |
| Associated GPIs | |
| GCN Sequence Number | n/a |
| GCN Sequence Number Description | n/a |
| HIC3 | n/a |
| HIC3 Description | n/a |
| GCN | n/a |
| HICL Sequence Number | n/a |
| HICL Sequence Number Description | n/a |
| Brand/Generic | n/a |
| Proprietary Name | Diclofenac Sodium |
| Proprietary Name Suffix | n/a |
| Non-Proprietary Name | Diclofenac |
| Product Type | HUMAN OTC DRUG |
| Dosage Form | GEL |
| Route | TOPICAL |
| Active Ingredient Strength | 10 |
| Active Ingredient Units | mg/g |
| Substance Name | DICLOFENAC SODIUM |
| Labeler Name | Aurohealth LLC |
| Pharmaceutical Class | Anti-Inflammatory Agents, Non-Steroidal [CS], Cyclooxygenase Inhibitors [MoA], Decreased Prostaglandin Production [PE], Nonsteroidal Anti-inflammatory Drug [EPC] |
| DEA Schedule | n/a |
| Marketing Category | ANDA |
| Application Number | ANDA204306 |
| Listing Certified Through | 2023-12-31 |
Package
Package Images
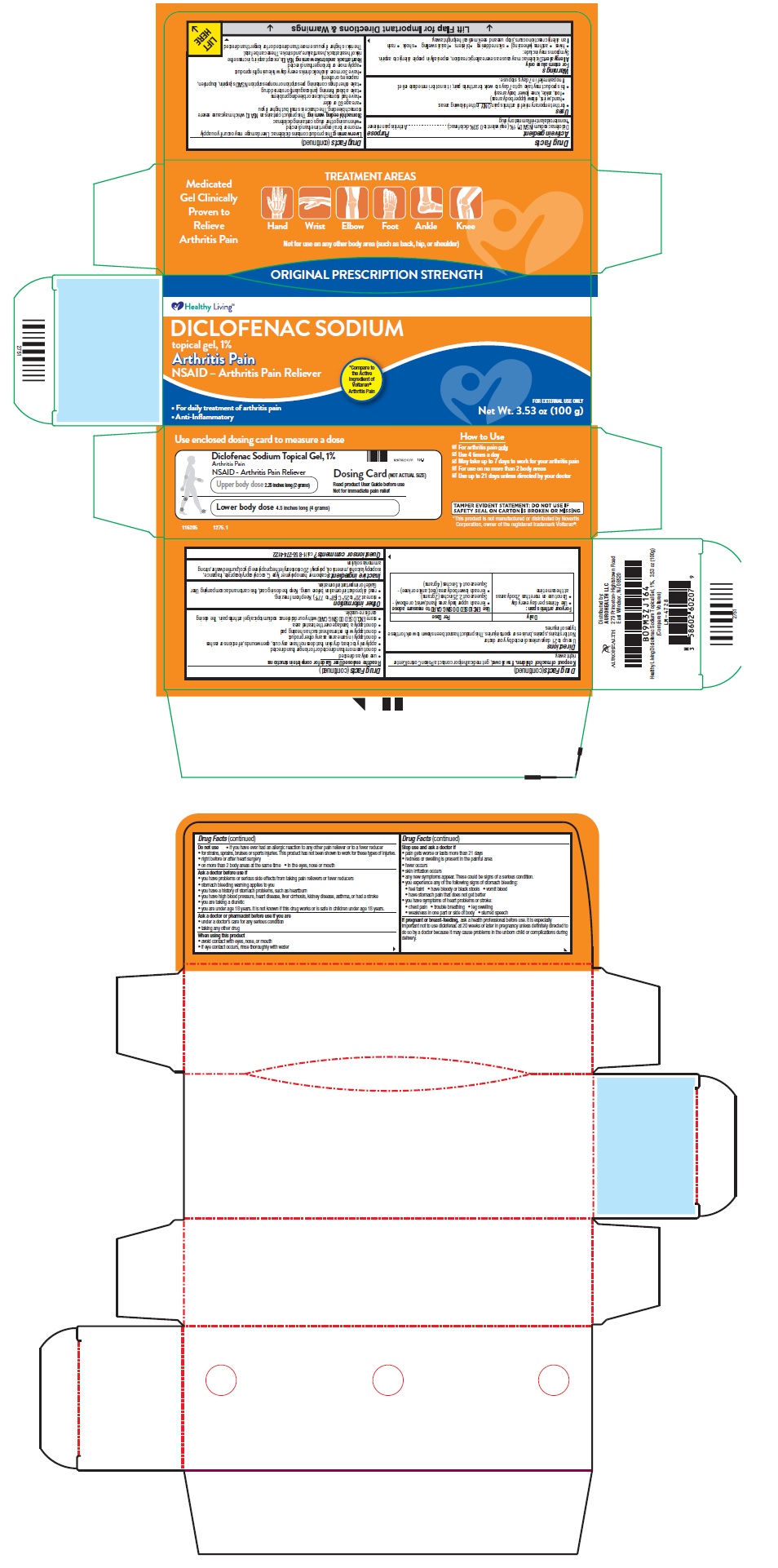
NDC 58602-0602-07 (58602060207)
| NDC Package Code | 58602-602-07 |
|---|---|
| Billing NDC | 58602060207 |
| Package | 1 TUBE in 1 CARTON (58602-602-07) / 100 g in 1 TUBE |
| Marketing Start Date | 2022-03-07 |
| NDC Exclude Flag | N |
| Pricing Information | N/A |
Standard Product Labeling (SPL)/Prescribing Information SPL 49961909-3aca-40b3-8424-668c1791c01d Details
Drug Facts
Uses
Warnings
For external use only
Allergy alert: Diclofenac may cause a severe allergic reaction, especially in people allergic to aspirin.
Symptoms may include:
- hives
- asthma (wheezing)
- skin reddening
- blisters
- facial swelling
- shock
- rash
If an allergic reaction occurs, stop use and seek medical help right away.
Liver warning: This product contains diclofenac. Liver damage may occur if you apply
- more or for a longer time than directed
- when using other drugs containing diclofenac
Stomach bleeding warning: This product contains an NSAID, which may cause severe stomach bleeding. The chance is small but higher if you
- are age 60 or older
- have had stomach ulcers or bleeding problems
- take a blood thinning (anticoagulant) or steroid drug
- take other drugs containing prescription or nonprescription NSAIDs (aspirin, ibuprofen, naproxen, or others)
- have 3 or more alcoholic drinks every day while using this product
- apply more or for longer than directed
Heart attack and stroke warning: NSAIDs, except aspirin, increase the risk of heart attack, heart failure, and stroke. These can be fatal. The risk is higher if you use more than directed or for longer than directed.
Do not use
- if you have ever had an allergic reaction to any other pain reliever or to a fever reducer
- for strains, sprains, bruises or sports injuries. This product has not been shown to work for these types of injuries.
- right before or after heart surgery
- on more than 2 body areas at the same time
- in the eyes, nose or mouth
Ask a doctor before use if
- you have problems or serious side effects from taking pain relievers or fever reducers
- stomach bleeding warning applies to you
- you have a history of stomach problems, such as heartburn
- you have high blood pressure, heart disease, liver cirrhosis, kidney disease, asthma, or had a stroke
- you are taking a diuretic
- you are under age 18 years. It is not known if this drug works or is safe in children under age 18 years.
Ask a doctor or pharmacist before use if you are
When using this product
Stop use and ask a doctor if
- pain gets worse or lasts more than 21 days
- redness or swelling is present in the painful area
- fever occurs
- skin irritation occurs
- any new symptoms appear. These could be signs of a serious condition.
- you experience any of the following signs of stomach bleeding:
- feel faint
- have bloody or black stools
- vomit blood
- have stomach pain that does not get better
- you have symptoms of heart problems or stroke:
- chest pain
- trouble breathing
- leg swelling
- weakness in one part or side of body
- slurred speech
If pregnant or breast-feeding,
Keep out of reach of children.
Directions
Use up to 21 days unless directed by your doctor
Not for strains, sprains, bruises or sports injuries. This product has not been shown to work for these types of injuries.
| Daily
| Per Dose
|
For your arthritis pain:
| Use ENCLOSED DOSING CARD to measure a dose
|
Read the enclosed User Guide for complete instructions:
- use only as directed
- do not use more than directed or for longer than directed
- apply only to clean, dry skin that does not have any cuts, open wounds, infections or rashes
- do not apply in same area as any other product
- do not apply with external heat such as heating pad
- do not apply a bandage over the treated area
- store ENCLOSED DOSING CARD with your diclofenac sodium topical gel arthritis pain. The dosing card is re-usable.
Other information
Inactive ingredients
carbomer homopolymer Type C, cocoyl caprylocaprate, fragrance, isopropyl alcohol, mineral oil, polyoxyl 20 cetostearyl ether, propylene glycol, purified water, strong ammonia solution
Questions or comments? call 1-855-274-4122
Distributed by:
AUROHEALTH LLC
279 Princeton-Hightstown Road
East Windsor, NJ 08520
SPL UNCLASSIFIED SECTION
DICLOFENAC SODIUM TOPICAL GEL, 1%
Arthritis Pain
NSAID – Arthritis Pain Reliever
User Guide
How to use diclofenac sodium topical gel arthritis pain, with answers to frequently asked questions
Quick Start
How to get started right away with diclofenac sodium topical gel arthritis pain
Understanding Diclofenac Sodium Topical Gel Arthritis Pain
Diclofenac sodium topical gel arthritis pain (diclofenac sodium topical gel, 1% NSAID - arthritis pain reliever) is an arthritis pain relief medication that you apply to your skin.
Before You Use Diclofenac Sodium Topical Gel Arthritis Pain
Read the Drug Facts Label, which appears on the carton.

Remove the dosing card from the inside of the carton. You should always use the dosing card to measure out the correct dose of diclofenac sodium topical gel arthritis pain.
The first time you use a tube of diclofenac sodium topical gel arthritis pain:

1. Take the cap off the tube. Open the safety seal by firmly pressing the indent on the top of the cap onto the foil seal on the tube.

2. Do not open the safety seal with scissors or other sharp objects.

3. After use, put the cap back on the end of the tube.

Using Diclofenac Sodium Topical Gel Arthritis Pain
How often to use diclofenac sodium topical gel arthritis pain
Use 4 times a day for best results.
For example:

Where to apply diclofenac sodium topical gel arthritis pain
Diclofenac sodium topical gel arthritis pain can be used on up to 2 body areas from the following list: a hand, a wrist, an elbow, a foot, an ankle, a knee. Do not use on more than 2 body areas at once.
Some examples include:

Measuring the correct amount using the dosing card

For each upper body area (a hand, a wrist, or an elbow):
Squeeze gel from the tube equal to the length shown on the upper body section of the dosing card (2.25 inches).

For each lower body area (a foot, an ankle, or a knee):
Squeeze gel from the tube equal to the length shown on the lower body section of the dosing card (4.5 inches).
Lost Your Dosing Card? Don’t Worry!
Call 1-855-274-4122 to get a free replacement.
Applying Diclofenac Sodium Topical Gel Arthritis Pain
Gently rub diclofenac sodium topical gel arthritis pain into the skin using your hand. Apply 4 times a day for best results. Do not use on more than 2 body areas at once.
Apply only to clean, dry skin that doesn’t have any cuts, open wounds, infections or rashes. Do not apply in the same area as any other medicines or products that are applied to the skin.

After You Use Diclofenac Sodium Topical Gel Arthritis Pain

Wash your hands after applying diclofenac sodium topical gel arthritis pain.
If the treated area is the hands wait up to 1 hour to wash the hands.

Wash the dosing card with water. Store dosing card with your diclofenac sodium topical gel arthritis pain and keep out of reach of children.

Wait ten minutes before covering the treated areas with clothes or gloves.
Avoid:
- showering or bathing for at least 1 hour after use
- exposing the treated area to sunlight or artificial light (such as in tanning booths)
- use in same area as other products applied to the skin
- applying a bandage over the treated area
- eye, nose, or mouth contact
- if eye contact occurs, rinse thoroughly with water
Facts you need to know
Important information about diclofenac sodium topical gel arthritis pain
Do not use diclofenac sodium topical gel arthritis pain...
- if you have ever had an allergic reaction to any other pain reliever or to a fever reducer
- for strains, sprains, bruises or sports injuries. This product has not been shown to work for these types of injuries.
- right before or after heart surgery
- on more than 2 body areas at the same time
- in the eyes, nose or mouth
Ask a doctor before use if...
- you have problems or serious side effects from taking pain relievers or fever reducers
- stomach bleeding warning applies to you
- you have a history of stomach problems such as heartburn
- you have high blood pressure, heart disease, liver cirrhosis, kidney disease, asthma, or had a stroke
- you are taking a diuretic
- you are under age 18 years. It is not known if this drug works or is safe in children under age 18 years.
Ask a doctor or pharmacist before use if...
- you are under a doctor’s care for any serious condition
- you are taking any other drug
- you are pregnant or breast-feeding
It is especially important not to use diclofenac at 20 weeks or later in pregnancy unless definitely directed to do so by a doctor because it may cause problems in the unborn child or complications during delivery.
Warnings to consider before use…
Allergy alert
Diclofenac may cause a severe allergic reaction, especially in people allergic to aspirin. Symptoms may include:
- hives
- asthma (wheezing)
- skin reddening
- rash
- blisters
- facial swelling
- shock
If an allergic reaction occurs, stop use and seek medical help right away.
Liver warning
This product contains diclofenac. Liver damage may occur if you apply
- more or for a longer time than directed
- when using other drugs containing diclofenac
Stomach bleeding warning
This product contains an NSAID, which may cause stomach bleeding. The chance is small but higher if you
- are age 60 or older
- have had stomach ulcers or bleeding problems
- take a blood thinning (anticoagulant) or steroid drug
- take other drugs containing prescription or nonprescription NSAIDs (aspirin, ibuprofen, naproxen, or others)
- have 3 or more alcoholic drinks every day while using this product
- apply more or for longer than directed
Heart attack and stroke warning
NSAIDs, except aspirin, increase the risk of heart attack, heart failure, and stroke. This can be fatal. The risk is higher if you use more than directed or for longer than directed.
Follow these important guidelines when using diclofenac sodium topical gel arthritis pain...
- use only as directed
- apply only to clean, dry skin that does not have any cuts, open wounds, infections or rashes
- do not apply in same area as any other product used on the skin
- do not apply with external heat such as heating pad
- do not apply a bandage over the treated area
- do not get gel in eyes, nose or mouth
- store ENCLOSED DOSING CARD with your diclofenac sodium topical gel arthritis pain. The dosing card is re-usable.
Keep out of reach of children.
If swallowed, get medical help or contact a Poison Control Center right away.
Stop using diclofenac sodium topical gel arthritis pain and ask a doctor if...
- pain gets worse or lasts more than 21 days
- redness or swelling is present in the painful area
- fever occurs
- skin irritation occurs
- any new symptoms appear. These could be signs of a serious condition.
- you experience any of the following signs of stomach bleeding:
- feel faint
- have bloody or black stools
- vomit blood
- have stomach pain that does not get better
- you have symptoms of heart problems or stroke:
- chest pain
- trouble breathing
- leg swelling
- weakness in one part or side of body
- slurred speech
Questions and Answers
Answers to questions you may ask about diclofenac sodium topical gel arthritis pain
Can I use diclofenac sodium topical gel arthritis pain for the immediate relief of pain like that associated with sprains, strains, bruises or sports injuries?
No.
Do not use for strains, sprains, bruises, or sports injuries. This product has not been shown to work for these types of injuries. It’s intended only for the temporary relief of arthritis pain.
How quickly will diclofenac sodium topical gel arthritis pain work for arthritis pain?
Diclofenac sodium topical gel arthritis pain may take up to 7 days to work for your arthritis pain; it is not for immediate relief. With 4 times-a-day use, you may start to feel relief within a few days. You should feel significant pain relief within 7 days of continuous use. If no pain relief in 7 days, stop use.
What is diclofenac sodium topical gel arthritis pain used for?
Diclofenac sodium topical gel arthritis pain can be used for the temporary relief of arthritis pain in the hands, wrists, elbows, feet, ankles, and knees. Osteoarthritis (OA) is the most common form of arthritis. It mainly affects a type of tissue called cartilage, which cushions the joints and prevents the bones from rubbing against each other.
With OA, cartilage begins to break down and wear away, resulting in joint pain and stiffness. OA occurs more frequently as you get older. Pain develops slowly and can gradually worsen over time.

How does diclofenac sodium topical gel arthritis pain work?
Diclofenac sodium, the medicine in diclofenac sodium topical gel arthritis pain, is a nonsteroidal anti-inflammatory drug (NSAID). NSAIDS are used to treat pain from medical conditions such as arthritis. Diclofenac sodium topical gel arthritis pain works similarly to oral NSAIDs like ibuprofen or naproxen by temporarily blocking the production of pain signaling chemicals called prostaglandins. However, when taking a pill, the medication is distributed to the site of pain through the bloodstream. Compared to oral diclofenac, only 6% of the medicine in diclofenac sodium topical gel arthritis pain is absorbed in the bloodstream. It works by penetrating through your skin at the application site to deliver arthritis pain relief.
How does over-the-counter diclofenac sodium topical gel arthritis pain differ from prescription diclofenac sodium topical gel?
Over-the-counter diclofenac sodium topical gel arthritis pain is the same strength and formula as original full prescription strength diclofenac sodium topical gel but is available without a prescription.
Apply diclofenac sodium topical gel arthritis pain 4 times a day to ensure you get the full effect of the medicine.
Can I apply diclofenac sodium topical gel arthritis pain to upper and lower body areas at the same time?
Yes.
You can use diclofenac sodium topical gel arthritis pain on up to 2 body areas at the same time. The areas can include the foot, ankle, knee, hand, wrist, and elbow.
Some correct examples include:

Do not use diclofenac sodium topical gel arthritis pain on more than 2 body areas at once.
Can I apply diclofenac sodium topical gel arthritis pain without measuring it out on the dosing card first?
No.
Use the dosing card to make sure you are getting the correct amount of medicine.
Can I use diclofenac sodium topical gel arthritis pain on my spine, hips, or shoulders?
No.
The use of diclofenac sodium topical gel arthritis pain on the spine, hips, and shoulders has not been studied.
Can I take any other pain medications with diclofenac sodium topical gel arthritis pain?
Ask your doctor or pharmacist before using diclofenac sodium topical gel arthritis pain if you are taking any other pain reliever or are planning on taking any other pain reliever, especially prescription or nonprescription NSAIDs (aspirin, ibuprofen, naproxen, or others).
Can I take any other pain medications during the first 7 days I use diclofenac sodium topical gel arthritis pain?
Taking other pain medications is generally not recommended. Speak with your doctor or pharmacist before taking other pain medications when using diclofenac sodium topical gel arthritis pain.
How long can I use diclofenac sodium topical gel arthritis pain?
Use diclofenac sodium topical gel arthritis pain 4 times a day every day for up to 21 days for treatment of arthritis pain or as directed by your doctor.
Diclofenac sodium topical gel arthritis pain should be part of an overall program for managing your arthritis pain. The program should also include activities such as appropriate movement or exercise, specific lifestyle changes, and weight maintenance. Regular visits and discussions with your doctor can help incorporate diclofenac sodium topical gel arthritis pain into a complete program to help manage your arthritis pain.
What do I do if I get diclofenac sodium topical gel arthritis pain in my eyes?
If eye contact occurs, rinse thoroughly with water and consult a doctor if irritation lasts more than an hour.
How do I clean the dosing card?
Wash the dosing card with water, and store dosing card and tube out of reach of children.
What if I miss a dose of diclofenac sodium topical gel arthritis pain?
If you forget to apply diclofenac sodium topical gel arthritis pain, don’t double your dose. Just apply the next dose as scheduled.
How should I store diclofenac sodium topical gel arthritis pain?
- Store at 20° to 25°C (68° to 77°F).
- Do not freeze diclofenac sodium topical gel arthritis pain.
- Store the dosing card with your diclofenac sodium topical gel arthritis pain.
- Keep out of reach of children.
Additional Questions?
Call 1-855-274-4122.
Distributed by:
Aurohealth LLC
279 Princeton Hightstown Rd,
East Windsor, NJ 08520
Revised: 11/2021
PACKAGE LABEL-PRINCIPAL DISPLAY PANEL - 1% w/w Tube Carton Label (100 g tube)
INGREDIENTS AND APPEARANCE
| DICLOFENAC SODIUM
diclofenac gel |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Aurohealth LLC (078728447) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Mylan Institutional Inc. | 965542777 | ANALYSIS(58602-602) , MANUFACTURE(58602-602) | |