Search by Drug Name or NDC
NDC 59212-0200-30 Uroxatral 10 mg/1 Details
Uroxatral 10 mg/1
Uroxatral is a ORAL TABLET, EXTENDED RELEASE in the HUMAN PRESCRIPTION DRUG category. It is labeled and distributed by Concordia Pharmaceuticals Inc.. The primary component is ALFUZOSIN HYDROCHLORIDE.
MedlinePlus Drug Summary
Alfuzosin is used in men to treat symptoms of an enlarged prostate (benign prostatic hyperplasia or BPH), which include difficulty urinating (hesitation, dribbling, weak stream, and incomplete bladder emptying), painful urination, and urinary frequency and urgency. Alfuzosin is in a class of medications called alpha blockers. It works by relaxing the muscles in the prostate and bladder to allow urine to flow more easily.
Related Packages: 59212-0200-30Last Updated: 12/01/2022
MedLinePlus Full Drug Details: Alfuzosin
Product Information
| NDC | 59212-0200 |
|---|---|
| Product ID | 59212-200_9f852c9b-350d-4d1a-a54a-85151ccad544 |
| Associated GPIs | 56852010107530 |
| GCN Sequence Number | 045052 |
| GCN Sequence Number Description | alfuzosin HCl TAB ER 24H 10 MG ORAL |
| HIC3 | Q9B |
| HIC3 Description | BENIGN PROSTATIC HYPERTROPHY/MICTURITION AGENTS |
| GCN | 92024 |
| HICL Sequence Number | 010802 |
| HICL Sequence Number Description | ALFUZOSIN HCL |
| Brand/Generic | Brand |
| Proprietary Name | Uroxatral |
| Proprietary Name Suffix | n/a |
| Non-Proprietary Name | Alfuzosin HCl |
| Product Type | HUMAN PRESCRIPTION DRUG |
| Dosage Form | TABLET, EXTENDED RELEASE |
| Route | ORAL |
| Active Ingredient Strength | 10 |
| Active Ingredient Units | mg/1 |
| Substance Name | ALFUZOSIN HYDROCHLORIDE |
| Labeler Name | Concordia Pharmaceuticals Inc. |
| Pharmaceutical Class | Adrenergic alpha-Antagonists [MoA], alpha-Adrenergic Blocker [EPC] |
| DEA Schedule | n/a |
| Marketing Category | NDA |
| Application Number | NDA021287 |
| Listing Certified Through | 2024-12-31 |
Package
Package Images


NDC 59212-0200-30 (59212020030)
| NDC Package Code | 59212-200-30 |
|---|---|
| Billing NDC | 59212020030 |
| Package | 30 TABLET, EXTENDED RELEASE in 1 BOTTLE (59212-200-30) |
| Marketing Start Date | 2019-01-18 |
| NDC Exclude Flag | N |
| Pricing Information | N/A |
Standard Product Labeling (SPL)/Prescribing Information SPL 9fc7f119-f36b-44cf-945d-940160f3afe3 Details
HIGHLIGHTS OF PRESCRIBING INFORMATION
UROXATRAL® (alfuzosin HCl) extended-release tablets
Initial U.S. Approval: 2003
INDICATIONS AND USAGE
UROXATRAL is an alpha adrenergic antagonist, indicated for the treatment of signs and symptoms of benign prostatic hyperplasia. (1)
Important Limitations of Use:
UROXATRAL is not indicated for treatment of hypertension. (1.1)
UROXATRAL is not indicated for use in the pediatric population. (1.1, 8.4, 12.3)
DOSAGE AND ADMINISTRATION
DOSAGE FORMS AND STRENGTHS
Extended-release tablet: 10 mg (3)
CONTRAINDICATIONS
WARNINGS AND PRECAUTIONS
- Postural hypotension/syncope: Care should be taken in patients with symptomatic hypotension or who have had a hypotensive response to other medications or are concomitantly treated with antihypertensive medication or nitrates (5.1)
- Use with caution in patients with severe renal impairment (creatinine clearance <30 mL/min) (5.2, 8.6, 12.3)
- Use with caution in patients with mild hepatic impairment (5.3, 8.7, 12.3)
- Should not be used in combination with other alpha adrenergic antagonists (5.4, 7.2)
- Prostate carcinoma should be ruled out prior to treatment (5.5)
- Intraoperative Floppy Iris Syndrome (IFIS) during cataract surgery may require modifications to the surgical technique (5.6)
- Discontinue UROXATRAL if symptoms of angina pectoris appear or worsen (5.8)
- Use with caution in patients with a history of QT prolongation or who are taking medications which prolong the QT interval (5.9, 12.2)
ADVERSE REACTIONS
Most common adverse reactions in clinical studies (incidence ≥2% and at a higher incidence than placebo): dizziness, upper respiratory tract infection, headache, fatigue. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Concordia Pharmaceuticals at 1-877-370-1142 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 5/2020
Table Of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
1.1 Important Limitations of Use
2 DOSAGE AND ADMINISTRATION
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Postural Hypotension
5.2 Patients with Renal Impairment
5.3 Patients with Hepatic Impairment
5.4 Drug-Drug Interactions
5.5 Prostatic Carcinoma
5.6 Intraoperative Floppy Iris Syndrome (IFIS)
5.7 Priapism
5.8 Coronary Insufficiency
5.9 Patients with Congenital or Acquired QT Prolongation
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Post-Marketing Experience
7 DRUG INTERACTIONS
7.1 CYP3A4 Inhibitors
7.2 Alpha Adrenergic Antagonists
7.3 Antihypertensive Medication and Nitrates
7.4 PDE5 Inhibitors
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Renal Impairment
8.7 Hepatic Impairment
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
17.1 Hypotension/Syncope
17.2 Intraoperative Floppy Iris Syndrome
17.3 Priapism
17.4 Instructions of Use
- *
- Sections or subsections omitted from the full prescribing information are not listed.
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
UROXATRAL is contraindicated for use:
- in patients with moderate or severe hepatic impairment (Childs-Pugh categories B and C), since alfuzosin blood levels are increased in these patients [see Use in Specific Populations (8.7) and Clinical Pharmacology (12.3)].
- with potent CYP3A4 inhibitors such as ketoconazole, itraconazole, and ritonavir, since alfuzosin blood levels are increased [see Drug Interactions (7.1) and Clinical Pharmacology (12.3)].
- in patients with known hypersensitivity, such as urticaria and angioedema, to alfuzosin hydrochloride or any component of UROXATRAL tablets [see Adverse Reactions (6.2)]
5 WARNINGS AND PRECAUTIONS
5.1 Postural Hypotension
Postural hypotension with or without symptoms (e.g., dizziness) may develop within a few hours following administration of UROXATRAL. As with other alpha adrenergic antagonists, there is a potential for syncope. Patients should be warned of the possible occurrence of such events and should avoid situations where injury could result should syncope occur. There may be an increased risk of hypotension/postural hypotension and syncope when taking UROXATRAL concomitantly with anti-hypertensive medication and nitrates. Care should be taken when UROXATRAL is administered to patients with symptomatic hypotension or patients who have had a hypotensive response to other medications.
5.2 Patients with Renal Impairment
Caution should be exercised when UROXATRAL is administered in patients with severe renal impairment (creatinine clearance < 30 mL/min) [see Use in Specific Populations (8.6) and Clinical Pharmacology (12.3)].
5.3 Patients with Hepatic Impairment
UROXATRAL is contraindicated for use in patients with moderate or severe hepatic impairment [see Contraindications (4), Use in Specific Populations (8.7) and Clinical Pharmacology (12.3)]. Although the pharmacokinetics of UROXATRAL have not been studied in patients with mild hepatic impairment, caution should be exercised when UROXATRAL is administered to such patients [see Use in Specific Populations (8.7) and Clinical Pharmacology (12.3)].
5.4 Drug-Drug Interactions
Potent CYP3A4 Inhibitors: UROXATRAL is contraindicated for use with potent CYP3A4 inhibitors (e.g. ketoconazole, itraconazole, ritonavir) since alfuzosin blood levels are increased [see Contraindications (4), Drug Interactions (7.1) and Clinical Pharmacology (12.3)].
Other alpha adrenergic antagonists: UROXATRAL is an alpha adrenergic antagonist and should not be used in combination with other alpha adrenergic antagonist [see Drug Interactions (7.2)].
Phosphodiesterase-5 (PDE5) Inhibitors: PDE5-inhibitors are also vasodilators. Caution is advised for concomitant use of PDE5-inhibitors and UROXATRAL, as this combination can potentially cause symptomatic hypotension [see Drug Interactions (7.4)].
5.5 Prostatic Carcinoma
Carcinoma of the prostate and benign prostatic hyperplasia (BPH) cause many of the same symptoms. These two diseases frequently coexist. Therefore, patients thought to have BPH should be examined to rule out the presence of carcinoma of the prostate prior to starting treatment with UROXATRAL.
5.6 Intraoperative Floppy Iris Syndrome (IFIS)
IFIS has been observed during cataract surgery in some patients on or previously treated with alpha adrenergic antagonists. This variant of small pupil syndrome is characterized by the combination of a flaccid iris that billows in response to intraoperative irrigation currents, progressive intraoperative miosis despite preoperative dilation with standard mydriatic drugs, and potential prolapse of the iris toward the phacoemulsification incisions. The patient's ophthalmologist should be prepared for possible modifications to their surgical technique, such as the utilization of iris hooks, iris dilator rings, or viscoelastic substances.
There does not appear to be a benefit of stopping alpha adrenergic antagonist therapy prior to cataract surgery.
5.7 Priapism
Rarely (probably less than 1 in 50,000), alfuzosin, like other alpha adrenergic antagonists, has been associated with priapism (persistent painful penile erection unrelated to sexual activity). Because this condition can lead to permanent impotence if not properly treated, patients should be advised about the seriousness of the condition [see Adverse Reactions (6.2) and Patient Counseling Information [17.3]).
5.8 Coronary Insufficiency
If symptoms of angina pectoris should appear or worsen, UROXATRAL should be discontinued.
5.9 Patients with Congenital or Acquired QT Prolongation
Use with caution in patients with acquired or congenital QT prolongation or who are taking medications that prolong the QT interval [see Clinical Pharmacology (12.2)].
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in clinical practice.
The incidence of adverse reactions has been ascertained from 3 placebo-controlled clinical trials involving 1,608 men where daily doses of 10 and 15 mg alfuzosin were evaluated. In these 3 trials, 473 men received UROXATRAL (alfuzosin HCl) 10 mg extended-release tablets. In these trials, 4% of patients taking UROXATRAL (alfuzosin HCl) 10 mg extended-release tablets withdrew from the trial due to adverse reactions, compared with 3% in the placebo group.
Table 1 summarizes adverse reactions that occurred in ≥2% of patients receiving UROXATRAL, and at a higher incidence than that of the placebo group. In general, the adverse reactions seen in long-term use were similar in type and frequency to the events described below for the 3-month trials.
| Adverse Reaction | Placebo (n=678) | UROXATRAL (n=473) |
| Dizziness | 19 (2.8%) | 27 (5.7%) |
| Upper respiratory tract infection | 4 (0.6%) | 14 (3.0%) |
| Headache | 12 (1.8%) | 14 (3.0%) |
| Fatigue | 12 (1.8%) | 13 (2.7%) |
The following adverse reactions, reported by between 1% and 2% of patients receiving UROXATRAL and occurring more frequently than with placebo, are listed alphabetically by body system and by decreasing frequency within body system:
Body as a whole: pain
Gastrointestinal system: abdominal pain, dyspepsia, constipation, nausea
Reproductive system: impotence
Respiratory system: bronchitis, sinusitis, pharyngitis
Signs and Symptoms of Orthostasis in Clinical Trials: The adverse reactions related to orthostasis that occurred in the double-blind phase 3 trials with alfuzosin 10 mg are summarized in Table 2. Approximately 20% to 30% of patients in these trials were taking antihypertensive medication.
| Symptoms | Placebo (n=678) | UROXATRAL (n=473) |
| Dizziness | 19 (2.8%) | 27 (5.7%) |
| Hypotension or postural hypotension | 0 | 2 (0.4%) |
| Syncope | 0 | 1 (0.2%) |
Testing for blood pressure changes or orthostatic hypotension was conducted in three controlled studies. Decreased systolic blood pressure (≤90 mm Hg, with a decrease ≥20 mm Hg from baseline) was observed in none of the 674 placebo patients and 1 (0.2%) of the 469 UROXATRAL patients. Decreased diastolic blood pressure (≤50 mm Hg, with a decrease ≥15 mm Hg from baseline) was observed in 3 (0.4%) of the placebo patients and in 4 (0.9%) of the UROXATRAL patients. A positive orthostatic test (decrease in systolic blood pressure of ≥20 mm Hg upon standing from the supine position) was seen in 52 (7.7%) of placebo patients and in 31 (6.6%) of the UROXATRAL patients.
6.2 Post-Marketing Experience
The following adverse reactions have been identified during post approval use of UROXATRAL. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
General disorders: edema
Cardiac disorders: tachycardia, chest pain, angina pectoris in patients with pre-existing coronary artery disease, atrial fibrillation
Gastrointestinal disorders: diarrhea, vomiting
Hepatobiliary disorders: hepatocellular and cholestatic liver injury (including cases with jaundice leading to drug discontinuation)
Respiratory system disorders: rhinitis
Reproductive system disorders: priapism
Skin and subcutaneous tissue disorders: rash, pruritis, urticaria, angioedema, toxic epidermal necrolysis
Vascular disorders: flushing
Blood and lymphatic system disorders: thrombocytopenia
During cataract surgery, a variant of small pupil syndrome known as Intraoperative Floppy Iris Syndrome (IFIS) has been reported in some patients on or previously treated with alpha adrenergic antagonists [see Warnings and Precautions (5.6)].
7 DRUG INTERACTIONS
7.1 CYP3A4 Inhibitors
UROXATRAL is contraindicated for use with potent CYP3A4 inhibitors such as ketoconazole, itraconazole, or ritonavir, since alfuzosin blood levels are increased [see Contraindications (4), Warnings and Precautions (5.4) and Clinical Pharmacology (12.3)].
7.2 Alpha Adrenergic Antagonists
The pharmacokinetic and pharmacodynamic interactions between UROXATRAL and other alpha adrenergic antagonists have not been determined. However, interactions may be expected, and UROXATRAL should not be used in combination with other alpha adrenergic antagonists [see Warnings and Precautions (5.4)].
7.3 Antihypertensive Medication and Nitrates
There may be an increased risk of hypotension/postural hypotension and syncope when taking UROXATRAL concomitantly with anti-hypertensive medication and nitrates [see Warnings and Precautions (5.1)].
7.4 PDE5 Inhibitors
Caution is advised when alpha adrenergic antagonists, including UROXATRAL, are co-administered with PDE5 inhibitors. Alpha adrenergic antagonists and PDE5 inhibitors are both vasodilators that can lower blood pressure. Concomitant use of these two drug classes can potentially cause symptomatic hypotension [see Warnings and Precautions (5.4)].
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
Uroxatral is not indicated for use in women.
There are no adequate data on the developmental risk associated with use of UROXATRAL in pregnant women.
Based on findings from animal studies, alfuzosin administered during the period of organogenesis was not teratogenic, embryotoxic or fetotoxic at upto 1200 times the MRHD of 10 mg via AUC in rats and 3 times in rabbit, via body surface area.
In the U.S. general population, the estimated background risk of major birth defects and miscarriages in clinically recognized pregnancies is 2 to 4% and 15 to 20% respectively.
Data
Animal data
Alfuzosin was not teratogenic, embryotoxic or fetotoxic in rats at plasma exposure levels (based on AUC of unbound drug) up to 1200 times (maternal oral dose of 250 mg/kg/day) the maximum recommended human dose (MRHD) of 10 mg. In rabbits administered up to 3 times the MRHD (based on body surface area) (maternal oral dose of 100 mg/kg/day) no embryofetal toxicity or teratogenicity was observed. Gestation was slightly prolonged in rats at exposure levels (based on AUC of unbound drug) approximately 12 times (greater than 5 mg/kg/day oral maternal dose) the MRHD, but difficulties with parturition were not observed.
8.2 Lactation
Risk Summary
UROXATRAL is not indicated for use in women. There are no data on presence of UROXATRAL in human milk, the effect on breastfed child, or effect on milk production.
8.4 Pediatric Use
UROXATRAL is not indicated for use in the pediatric population.
Efficacy of alfuzosin hydrochloride was not demonstrated in a randomized, double-blind, placebo-controlled, efficacy and safety trial conducted in 172 patients ages 2 to 16 years with elevated detrusor leak point pressure (LPP≥40 cm H2O) of neurologic origin treated with alfuzosin hydrochloride using pediatric formulations. The trial included a 12-week efficacy phase followed by a 40-week safety extension period. No statistically significant difference in the proportion of patients achieving a detrusor leak point pressure of <40 cm H20 was observed between the alfuzosin and placebo groups.
During the placebo-controlled trial, the adverse reactions reported in ≥2% of patients treated with alfuzosin and at a higher incidence than in the placebo group were: pyrexia, headache, respiratory tract infection, cough, epistaxis and diarrhea. The adverse reactions reported for the whole 12-month trial period, which included the open-label extension, were similar in type and frequency to the reactions observed during the 12-week period.
Alfuzosin hydrochloride was not studied in patients below the age of 2.
8.5 Geriatric Use
Of the total number of subjects in clinical studies of UROXATRAL, 48% were 65 years of age and over, whereas 11% were 75 and over. No overall differences in safety or effectiveness were observed between these subjects and younger subjects, but greater sensitivity of some older individuals cannot be ruled out [see Clinical Pharmacology (12.3)]
8.6 Renal Impairment
Systemic exposure was increased by approximately 50% in pharmacokinetic studies of patients with mild, moderate, and severe renal impairment [see Clinical Pharmacology (12.3)]. In phase 3 studies, the safety profile of patients with mild (n=172) or moderate (n=56) renal impairment was similar to the patients with normal renal function in those studies. Safety data are available in only a limited number of patients (n=6) with creatinine clearance below 30 mL/min; therefore, caution should be exercised when UROXATRAL is administered in patients with severe renal impairment [see Warnings and Precautions (5.2)].
8.7 Hepatic Impairment
The pharmacokinetics of UROXATRAL have not been studied in patients with mild hepatic impairment. UROXATRAL is contraindicated for use in patients with moderate or severe hepatic impairment [see Contraindications (4), Warnings and Precautions (5.3) and Clinical Pharmacology (12.3)].
10 OVERDOSAGE
Should overdose of UROXATRAL lead to hypotension, support of the cardiovascular system is of first importance. Restoration of blood pressure and normalization of heart rate may be accomplished by keeping the patient in the supine position. If this measure is inadequate, then the administration of intravenous fluids should be considered. If necessary, vasopressors should then be used, and the renal function should be monitored and supported as needed. Alfuzosin is 82% to 90% protein bound; therefore, dialysis may not be of benefit.
11 DESCRIPTION
Each UROXATRAL extended-release tablet contains 10 mg alfuzosin hydrochloride as the active ingredient. Alfuzosin hydrochloride is a white to off-white crystalline powder that melts at approximately 240°C. It is freely soluble in water, sparingly soluble in alcohol, and practically insoluble in dichloromethane.
Alfuzosin hydrochloride is (R,S)-N-[3-[(4-amino-6,7-dimethoxy-2-quinazolinyl) methylamino] propyl] tetrahydro-2-furancarboxamide hydrochloride. The empirical formula of alfuzosin hydrochloride is C19H27N5O4•HCl. The molecular weight of alfuzosin hydrochloride is 425.9. Its structural formula is:

The tablet also contains the following inactive ingredients: colloidal silicon dioxide (NF), ethylcellulose (NF), hydrogenated castor oil (NF), hydroxypropyl methylcellulose (USP), magnesium stearate (NF), mannitol (USP), microcrystalline cellulose (NF), povidone (USP), and yellow ferric oxide (NF).
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Alfuzosin is a selective antagonist of post-synaptic alpha1-adrenoreceptors, which are located in the prostate, bladder base, bladder neck, prostatic capsule, and prostatic urethra.
12.2 Pharmacodynamics
Alfuzosin exhibits selectivity for alpha adrenergic receptors in the lower urinary tract. Blockade of these adrenoreceptors can cause smooth muscle in the bladder neck and prostate to relax, resulting in an improvement in urine flow and a reduction in symptoms of BPH.
Cardiac Electrophysiology
The effect of 10 mg and 40 mg alfuzosin on QT interval was evaluated in a double-blind, randomized, placebo and active-controlled (moxifloxacin 400 mg), 4-way crossover single dose study in 45 healthy white male subjects aged 19 to 45 years. The QT interval was measured at the time of peak alfuzosin plasma concentrations. The 40 mg dose of alfuzosin was chosen because this dose achieves higher blood levels than those achieved with the co-administration of UROXATRAL and ketoconazole 400 mg. Table 3 summarizes the effect on uncorrected QT and mean corrected QT interval (QTc) with different methods of correction (Fridericia, population-specific and subject-specific correction methods) at the time of peak alfuzosin plasma concentrations. No single one of these correction methodologies is known to be more valid. The mean change of heart rate associated with a 10 mg dose of alfuzosin in this study was 5.2 beats/minute and 5.8 beats/minute with 40 mg alfuzosin. The change in heart rate with moxifloxacin was 2.8 beats/minute.
|
||||
| Drug/Dose | QT | Fridericia method | Population-specific method | Subject-specific method |
| Alfuzosin 10 mg | -5.8 (-10.2, -1.4) | 4.9 (0.9, 8.8) | 1.8 (-1.4, 5.0) | 1.8 (-1.3, 5.0) |
| Alfuzosin 40 mg | -4.2 (-8.5, 0.2) | 7.7 (1.9, 13.5) | 4.2 (-0.6, 9.0) | 4.3 (-0.5, 9.2) |
| Moxifloxacin *400 mg | 6.9 (2.3, 11.5) | 12.7 (8.6, 16.8) | 11.0 (7.0, 15.0) | 11.1 (7.2, 15.0) |
The QT effect appeared greater for 40 mg compared to 10 mg alfuzosin. The effect of the highest alfuzosin dose (four times the therapeutic dose) studied did not appear as large as that of the active control moxifloxacin at its therapeutic dose. This study, however, was not designed to make direct statistical comparisons between the drugs or the dose levels. There has been no signal of Torsade de Pointes in the extensive post-marketing experience with alfuzosin outside the United States.
A separate post-marketing QT study evaluated the effect of the co-administration of 10 mg alfuzosin with a drug of similar QT effect size. In this study, the mean placebo-subtracted QTcF increase of alfuzosin 10 mg alone was 1.9 msec (upperbound 95% CI, 5.5 msec). The concomitant administration of the two drugs showed an increased QT effect when compared with either drug alone. This QTcF increase [5.9 msec (UB 95% CI, 9.4 msec)] was not more than additive. Although this study was not designed to make direct statistical comparisons between drugs, the QT increase with both drugs given together appeared to be lower than the QTcF increase seen with the positive control moxifloxacin 400 mg [10.2 msec (UB 95% CI, 13.8 msec)]. The clinical impact of these QTc changes is unknown.
12.3 Pharmacokinetics
The pharmacokinetics of UROXATRAL have been evaluated in adult healthy male volunteers after single and/or multiple administration with daily doses ranging from 7.5 mg to 30 mg, and in patients with BPH at doses from 7.5 mg to 15 mg.
Absorption
The absolute bioavailability of UROXATRAL 10 mg tablets under fed conditions is 49%. Following multiple dosing of 10 mg UROXATRAL under fed conditions, the time to maximum concentration is 8 hours. Cmax and AUC0–24 are 13.6 (SD = 5.6) ng/mL and 194 (SD = 75) ng∙h/mL, respectively. UROXATRAL exhibits linear kinetics following single and multiple dosing up to 30 mg. Steady-state plasma levels are reached with the second dose of UROXATRAL administration. Steady-state alfuzosin plasma concentrations are 1.2- to 1.6-fold higher than those observed after a single administration.
Effect of Foods
As illustrated in Figure 1, the extent of absorption is 50% lower under fasting conditions. Therefore, UROXATRAL should be taken with food and with the same meal each day [see Dosage and Administration (2)].

Figure 1 - Mean (SEM) Alfuzosin Plasma Concentration-Time Profiles after a Single Administration of UROXATRAL 10 mg tablets to 8 Healthy Middle-Aged Male Volunteers in Fed and Fasted States
Distribution
The volume of distribution following intravenous administration in healthy male middle-aged volunteers was 3.2 L/kg. Results of in vitro studies indicate that alfuzosin is moderately bound to human plasma proteins (82% to 90%), with linear binding over a wide concentration range (5 to 5,000 ng/mL).
Metabolism
Alfuzosin undergoes extensive metabolism by the liver, with only 11% of the administered dose excreted unchanged in the urine. Alfuzosin is metabolized by three metabolic pathways: oxidation, O-demethylation, and N-dealkylation. The metabolites are not pharmacologically active. CYP3A4 is the principal hepatic enzyme isoform involved in its metabolism.
Excretion
Following oral administration of 14C-labeled alfuzosin solution, the recovery of radioactivity after 7 days (expressed as a percentage of the administered dose) was 69% in feces and 24% in urine. Following oral administration of UROXATRAL 10 mg tablets, the apparent elimination half-life is 10 hours.
Specific Populations
Geriatric Use: In a pharmacokinetic assessment during phase 3 clinical studies in patients with BPH, there was no relationship between peak plasma concentrations of alfuzosin and age. However, trough levels were positively correlated with age. The concentrations in subjects ≥75 years of age were approximately 35% greater than in those below 65 years of age.
Renal Impairment: The Pharmacokinetic profiles of UROXATRAL 10 mg tablets in subjects with normal renal function (CLCR>80 mL/min), mild impairment (CLCR 60 to 80 mL/min), moderate impairment (CLCR 30 to 59 mL/min), and severe impairment (CLCR <30 mL/min) were compared. These clearances were calculated by the Cockcroft-Gault formula. Relative to subjects with normal renal function, the mean Cmax and AUC values were increased by approximately 50% in patients with mild, moderate, or severe renal impairment [see Warnings and Precautions (5.2) and Use in Specific Populations (8.6)].
Hepatic Impairment: The pharmacokinetics of UROXATRAL have not been studied in patients with mild hepatic impairment. In patients with moderate or severe hepatic insufficiency (Child-Pugh categories B and C), the plasma apparent clearance (CL/F) was reduced to approximately one-third to one-fourth that observed in healthy subjects. This reduction in clearance results in three to four-fold higher plasma concentrations of alfuzosin in these patients compared to healthy subjects. Therefore, UROXATRAL is contraindicated in patients with moderate to severe hepatic impairment [see Contraindications (4), Warnings and Precautions (5.3) and Use in Specific Populations (8.7)].
Pediatric Use: UROXATRAL tablets are not indicated for use in the pediatric population [see Indications and Usage (1.1) and Use in Specific Populations (8.4)].
Drug-Drug Interactions
Metabolic Interactions
CYP3A4 is the principal hepatic enzyme isoform involved in the metabolism of alfuzosin.
Potent CYP3A4 Inhibitors
Repeated oral administration of 400 mg/day of ketoconazole, a potent inhibitor of CYP3A4, increased alfuzosin Cmax by 2.3-fold and AUClast by 3.2-fold, following a single 10 mg dose of alfuzosin.
In another study, repeated oral administration of a lower (200 mg/day) dose of ketoconazole increased alfuzosin Cmax by 2.1-fold and AUClast by 2.5-fold, following a single 10 mg dose of alfuzosin.
Therefore, UROXATRAL is contraindicated for co-administration with potent inhibitors of CYP3A4 (e.g., ketoconazole, itraconazole, or ritonavir) because of increased alfuzosin exposure[see Contraindications (4), Warnings and Precautions (5.4) and Drug Interactions (7.1)].
Moderate CYP3A4 Inhibitors
Diltiazem: Repeated co-administration of 240 mg/day of diltiazem, a moderately-potent inhibitor of CYP3A4, with 7.5 mg/day (2.5 mg three times daily) alfuzosin (equivalent to the exposure with UROXATRAL) increased the Cmax and AUC0–24 of alfuzosin 1.5- and 1.3-fold, respectively. Alfuzosin increased the Cmax and AUC0–12 of diltiazem 1.4-fold. Although no changes in blood pressure were observed in this study, diltiazem is an antihypertensive medication and the combination of UROXATRAL and antihypertensive medications has the potential to cause hypotension in some patients [see Warnings and Precautions (5.1)].
In human liver microsomes, at concentrations that are achieved at the therapeutic dose, alfuzosin did not inhibit CYP1A2, 2A6, 2C9, 2C19, 2D6 or 3A4 isoenzymes. In primary culture of human hepatocytes, alfuzosin did not induce CYP1A, 2A6 or 3A4 isoenzymes.
Other Interactions
Warfarin: Multiple dose administration of an immediate release tablet formulation of alfuzosin 5 mg twice daily for six days to six healthy male volunteers did not affect the pharmacological response to a single 25 mg oral dose of warfarin.
Digoxin: Repeated co-administration of UROXATRAL 10 mg tablets and digoxin 0.25 mg/day for 7 days did not influence the steady-state pharmacokinetics of either drug.
Cimetidine: Repeated administration of 1 g/day cimetidine increased both alfuzosin Cmax and AUC values by 20%.
Atenolol: Single administration of 100 mg atenolol with a single dose of 2.5 mg of an immediate release alfuzosin tablet in eight healthy young male volunteers increased alfuzosin Cmax and AUC values by 28% and 21%, respectively. Alfuzosin increased atenolol Cmax and AUC values by 26% and 14%, respectively. In this study, the combination of alfuzosin with atenolol caused significant reductions in mean blood pressure and in mean heart rate. [see Warnings and Precautions (5.1)].
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
There was no evidence of a drug-related increase in the incidence of tumors in mice following dietary administration of 100 mg/kg/day alfuzosin for 98 weeks (13 and 15 times the maximum recommended human dose [MRHD] of 10 mg based on AUC of unbound drug), in females and males, respectively. The highest dose tested in female mice may not have constituted a maximally tolerated dose. Likewise, there was no evidence of a drug-related increase in the incidence of tumors in rats following dietary administration of 100 mg/kg/day alfuzosin for 104 weeks (53 and 37 times the MRHD in females and males, respectively).
Alfuzosin showed no evidence of mutagenic effect in the Ames and mouse lymphoma assays, and was free of any clastogenic effects in the Chinese hamster ovary cell and in vivo mouse micronucleus assays. Alfuzosin treatment did not induce DNA repair in a human cell line.
There was no evidence of reproductive organ toxicity when male rats were administered oral doses of several hundred times (250 mg/kg/day for 26 weeks) the MRHD of alfuzosin. No impairment of fertility was observed following oral (gavage) administration to male rats at doses of up to 125 mg/kg/day for 70 days. Estrous cycling was inhibited in rats and dogs at approximately 12 and 18 times the MRHD respectively (doses of 25 mg/kg and 20 mg/kg, respectively), but did not result in impaired fertility in female rats.
14 CLINICAL STUDIES
Three randomized placebo-controlled, double-blind, parallel-arm, 12-week trials were conducted with the 10 mg daily dose of alfuzosin. In these three trials, 1,608 patients [mean age 64.2 years, range 49–92 years; Caucasian (96.1%), Black (1.6%), Asian (1.1%), Other (1.2%)] were randomized and 473 patients received UROXATRAL 10 mg daily. Table 4 provides the results of the three trials that evaluated the 10 mg dose.
There were two primary efficacy variables in these three studies. The International Prostate Symptom Score (IPSS, or AUA Symptom Score) consists of seven questions that assess the severity of both irritative (frequency, urgency, nocturia) and obstructive (incomplete emptying, stopping and starting, weak stream, and pushing or straining) symptoms, with possible scores ranging from 0 to 35 with higher numerical scores on the IPSS total symptom score representing greater severity of symptoms. The second efficacy variable was peak urinary flow rate. The peak flow rate was measured just prior to the next dose in study 2 and on average at 16 hours post-dosing in trials 1 and 3.
There was a statistically significant reduction from baseline to last assessment (Week 12) in the IPSS total symptom score versus placebo in all three studies, indicating a reduction in symptom severity (Table 5 and Figures 2, 3, and 4).
Table 4 - Mean Change (SD) from Baseline to week 12 in International Prostate Symptom Score in Three Randomized, Controlled, Double Blind Trials
|
||||||
|
Symptom Score | Trial 1 | Trial 2 | Trial 3 | |||
| Placebo (n=167) | UROXATRAL 10 mg (n=170) | Placebo (n=152) | UROXATRAL 10 mg (n=137) | Placebo (n=150) | UROXATRAL 10 mg (n=151) |
|
| Total symptom score | ||||||
| Baseline | 18.2 (6.4) | 18.2 (6.3) | 17.7 (4.1) | 17.3 (3.5) | 17.7 (5.0) | 18.0 (5.4) |
| Change*
| -1.6 (5.8) | -3.6 (4.8) | -4.9 (5.9) | -6.9 (4.9) | -4.6 (5.8) | -6.5 (5.2) |
| p-value | 0.001 | 0.002 | 0.007 |
|||
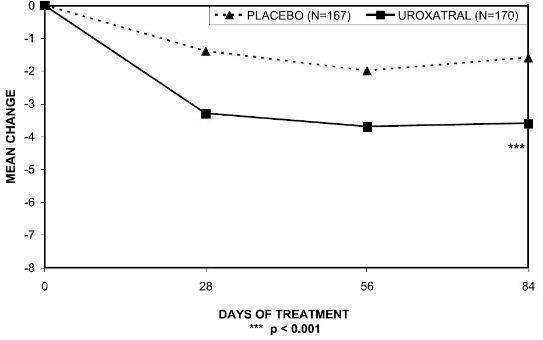
Figure 2 — Mean Change from Baseline in IPSS Total Symptom Score: Trial 1

Figure 3 — Mean Change from Baseline in IPSS Total Symptom Score: Trial 2

Figure 4 — Mean Change from Baseline in IPSS Total Symptom Score: Trial 3
Peak urinary flow rate was increased statistically significantly from baseline to last assessment (Week 12) versus placebo in trials 1 and 2 (Table 5 and Figures 5, 6, and 7).
|
||||||
| Trial 1 | Trial 2 | Trial 3 |
||||
| Placebo (n=167) | UROXATRAL 10 mg (n=170) | Placebo (n=147) | UROXATRAL 10 mg (n=136) | Placebo (n=150) | UROXATRAL 10 mg (n=151) |
|
| Mean Peak flow rate | ||||||
| Baseline | 10.2 (4.0) | 9.9 (3.9) | 9.2 (2.0) | 9.4 (1.9) | 9.3 (2.6) | 9.5 (3.0) |
| Change*
| 0.2 (3.5) | 1.7 (4.2) | 1.4 (3.2) | 2.3 (3.6) | 0.9 (3.0) | 1.5 (3.3) |
| p-value | 0.0004 | 0.03 | 0.22 |
|||

Figure 5 — Mean Change from Baseline in Peak Urine Flow Rate (mL/s): Trial 1

Figure 6 — Mean Change from Baseline in Peak Urine Flow Rate (mL/s): Trial 2

Figure 7 — Mean Change from Baseline in Peak Urine Flow Rate (mL/s): Trial 3
Mean total IPSS decreased at the first scheduled observation at Day 28 and mean peak flow rate increased starting at the first scheduled observation at Day 14 in trials 2 and 3 and Day 28 in trial 1.
16 HOW SUPPLIED/STORAGE AND HANDLING
UROXATRAL is supplied as follows:
Package NDC Number
Bottles of 100 with child-resistant closure, NDC 59212-200-10
Bottles of 30 with child-resistant closure, NDC 59212-200-30
UROXATRAL (alfuzosin HCl) extended-release tablet 10 mg is available as a round, three-layer tablet: one white layer between two yellow layers, debossed with X10.
17 PATIENT COUNSELING INFORMATION
See FDA-approved patient labeling.
17.1 Hypotension/Syncope
Patients should be told about the possible occurrence of symptoms related to postural hypotension, such as dizziness, when beginning UROXATRAL, and they should be cautioned about driving, operating machinery, or performing hazardous tasks during this period. This is important for those with low blood pressure or who are taking antihypertensive medications or nitrates [see Warnings and Precautions (5.1)].
17.2 Intraoperative Floppy Iris Syndrome
Patients should be instructed to tell their ophthalmologist about their use of UROXATRAL before cataract surgery or other procedures involving the eyes, even if the patient is no longer taking UROXATRAL [see Warnings and Precautions (5.6)].
17.3 Priapism
Patients should be advised about the possibility of priapism resulting from treatment with UROXATRAL and medications in the same class. Although this reaction is extremely rare, but if not brought to immediate medical attention, can lead to permanent erectile dysfunction (impotence) [see Warnings and Precautions (5.7)].
SPL UNCLASSIFIED SECTION
PATIENT INFORMATION
UROXATRAL® (yoo-ROX-uh-trahl)
(Alfuzosin hydrochloride extended-release tablets)
Read the Patient Information that comes with UROXATRAL before you start using it and each time you get a refill. There may be new information. This leaflet does not take the place of talking with your doctor about your condition or your treatment. You and your doctor should talk about all your medicines, including UROXATRAL, now and at your regular checkups.
What is the most important information I should know about UROXATRAL?
UROXATRAL can cause serious side effects, including a sudden drop in blood pressure, especially when you start treatment. This may cause you to faint, or to feel dizzy or lightheaded.
- Your risk of having this problem may be increased if you take UROXATRAL with certain other medicine that lowers blood pressure:
- medicines for high blood pressure
- a nitrate medicine for angina
- Do not drive, operate machinery, or do any dangerous activities until you know how UROXATRAL affects you. This is especially important if you already have a problem with low blood pressure or take medicines to treat high blood pressure.
- If you begin to feel dizzy or lightheaded, lie down with your legs and feet up. If your symptoms do not improve call your doctor.
See the section "What are the possible side effects of UROXATRAL?" for more information about side effects.
What is UROXATRAL?
UROXATRAL is a prescription medicine that is called an "alpha-blocker". UROXATRAL is used in adult men to treat the symptoms of benign prostatic hyperplasia (BPH). UROXATRAL may help to relax the muscles in the prostate and the bladder which may lessen the symptoms of BPH and improve urine flow.
Before prescribing UROXATRAL, your doctor may examine your prostate gland and do a blood test called a prostate specific antigen (PSA) test to check for prostate cancer. Prostate cancer and BPH can cause the same symptoms. Prostate cancer needs a different treatment.
UROXATRAL is not for use in women or children.
Some medicines called "alpha-blockers" are used to treat high blood pressure. UROXATRAL is not for the treatment of high blood pressure.
Who should not take UROXATRAL?
Do not take UROXATRAL if you:
- have certain liver problems
- take antifungal medicines like ketoconazole or itraconazole (Sporanox)
- take anti-HIV medicines like ritonavir (Norvir, Kaletra)
- are allergic to alfuzosin hydrochloride or any of the ingredients in UROXATRAL.
See the end of this leaflet for a complete list of ingredients in UROXATRAL.
Before taking UROXATRAL, tell your doctor if you:
- have liver problems
- have kidney problems
- have had low blood pressure, especially after taking another medicine. Signs of low blood pressure are fainting, dizziness, and lightheadedness.
- have a heart problem called angina
- or any family members have a rare heart condition known as congenital prolongation of the QT interval.
Tell your doctor about all the medicines you take, including prescription and non-prescription medicines, vitamins and herbal supplements. Some of your other medicines may affect the way UROXATRAL works and cause serious side effects. See "What is the most important information I should know about UROXATRAL?"
Especially tell your doctor if you take:
- another alpha blocker medicine
- a medicine to treat high blood pressure
- a medicine to treat angina
- a medicine to treat erectile dysfunction (ED)
- the antifungal medicines like ketoconazole or itraconazole (Sporanox)
- the anti-HIV medicine like ritonavir (Norvir, Kaletra)
Ask your doctor or pharmacist if you are not sure if your medicine is one of those listed above.
What you need to know while taking UROXATRAL (alfuzosin HCl) tablets
- If you have an eye surgery for cataract (clouding of the eye) planned, tell your ophthalmologist that you are using UROXATRAL or have previously been treated with an alpha-blocker.
How do I take UROXATRAL?
- UROXATRAL comes in child-resistant package.
- Take UROXATRAL exactly as your doctor prescribes it.
- Take UROXATRAL after the same meal each day. Do not take it on an empty stomach.
- Swallow the UROXATRAL tablet whole. Do not crush, split, or chew UROXATRAL tablets.
- If you take too much UROXATRAL call your local poison control center or emergency room right away.
What are the possible side effects of UROXATRAL?
UROXATRAL can cause serious side effects, including:
- See "What is the most important information I should know about UROXATRAL?"
- A painful erection that will not go away. UROXATRAL can cause a painful erection (priapism), which cannot be relieved by having sex. If this happens, get medical help right away. If priapism is not treated, you may not be able to get an erection in the future.
The most common side effects with UROXATRAL are:
- dizziness
- headache
- tiredness
Call your doctor if you get any side effect that bothers you.
These are not all the side effects of UROXATRAL. For more information ask your doctor or pharmacist.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
How do I store UROXATRAL?
- Store UROXATRAL between 59°F and 86°F (15°C and 30°C).
- Protect from light and moisture.
Keep UROXATRAL and all medicines out of the reach of children.
General information about UROXATRAL:
Medicines are sometimes prescribed for conditions that are not mentioned in patient information leaflets. Do not use UROXATRAL for a condition for which it was not prescribed. Do not give UROXATRAL to other people, even if they have the same symptoms you have. It may harm them.
This leaflet summarizes the most important information about UROXATRAL. If you would like more information, talk with your doctor. You can ask your doctor or pharmacist for information about UROXATRAL that is written for health professionals.
What are the ingredients of UROXATRAL?
Active Ingredient: alfuzosin hydrochloride
Inactive Ingredients: colloidal silicon dioxide (NF), ethylcellulose (NF), hydrogenated castor oil (NF), hydroxypropyl methylcellulose (USP), magnesium stearate (NF), mannitol (USP), microcrystalline cellulose (NF), povidone (USP), and yellow ferric oxide (NF).
Mfd. for: Concordia Pharmaceuticals
Distributed by:
Amdipharm Limited,
17 Northwood House
Dublin 9, Ireland
©2015. All rights reserved.
PRINCIPAL DISPLAY PANEL - 30 TABLETS BOTTLE
PRINCIPAL DISPLAY PANEL - 100 TABLETS BOTTLE
INGREDIENTS AND APPEARANCE
| UROXATRAL
alfuzosin hcl tablet, extended release |
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
| Labeler - Concordia Pharmaceuticals Inc. (815240092) |