Search by Drug Name or NDC
NDC 59310-0311-06 ArmonAir Digihaler 232 ug/1 Details
ArmonAir Digihaler 232 ug/1
ArmonAir Digihaler is a RESPIRATORY (INHALATION) POWDER, METERED in the HUMAN PRESCRIPTION DRUG category. It is labeled and distributed by Teva Respiratory, LLC. The primary component is FLUTICASONE PROPIONATE.
MedlinePlus Drug Summary
Fluticasone oral inhalation is used to prevent difficulty breathing, chest tightness, wheezing, and coughing caused by asthma in adults and children. It is in a class of medications called corticosteroids. Fluticasone works by decreasing swelling and irritation in the airways to allow for easier breathing.
Related Packages: 59310-0311-06Last Updated: 12/01/2022
MedLinePlus Full Drug Details: Fluticasone Oral Inhalation
Product Information
| NDC | 59310-0311 |
|---|---|
| Product ID | 59310-311_8fd236af-c81c-46e9-819e-b37891da2662 |
| Associated GPIs | 44400033208028 |
| GCN Sequence Number | 081485 |
| GCN Sequence Number Description | fluticasone propionate AER PW BAS 232 MCG INHALATION |
| HIC3 | B6M |
| HIC3 Description | GLUCOCORTICOIDS, ORALLY INHALED |
| GCN | 48615 |
| HICL Sequence Number | 007873 |
| HICL Sequence Number Description | FLUTICASONE PROPIONATE |
| Brand/Generic | Brand |
| Proprietary Name | ArmonAir Digihaler |
| Proprietary Name Suffix | n/a |
| Non-Proprietary Name | Fluticasone Propionate |
| Product Type | HUMAN PRESCRIPTION DRUG |
| Dosage Form | POWDER, METERED |
| Route | RESPIRATORY (INHALATION) |
| Active Ingredient Strength | 232 |
| Active Ingredient Units | ug/1 |
| Substance Name | FLUTICASONE PROPIONATE |
| Labeler Name | Teva Respiratory, LLC |
| Pharmaceutical Class | Corticosteroid Hormone Receptor Agonists [MoA], Corticosteroid [EPC] |
| DEA Schedule | n/a |
| Marketing Category | NDA |
| Application Number | NDA208798 |
| Listing Certified Through | 2024-12-31 |
Package
Package Images








NDC 59310-0311-06 (59310031106)
| NDC Package Code | 59310-311-06 |
|---|---|
| Billing NDC | 59310031106 |
| Package | 1 POUCH in 1 CARTON (59310-311-06) / 1 INHALER in 1 POUCH / 60 POWDER, METERED in 1 INHALER |
| Marketing Start Date | 2020-09-15 |
| NDC Exclude Flag | N |
| Pricing Information | N/A |
Standard Product Labeling (SPL)/Prescribing Information SPL 5cea205f-e6d4-45e8-bfcc-a12a6907d5bb Details
HIGHLIGHTS OF PRESCRIBING INFORMATION
ARMONAIR DIGIHALER (fluticasone propionate) inhalation powder, for oral inhalation
Initial U.S. Approval: 1994
INDICATIONS AND USAGE
DOSAGE AND ADMINISTRATION
For oral inhalation only. (2.1)
- Starting dosage is based on prior asthma therapy and disease severity. (2.2)
- Treatment of asthma in patients 12 years and older: 1 inhalation of ArmonAir Digihaler 55 mcg, 113 mcg, or 232 mcg twice daily (2.2).
- Do not use with a spacer or volume holding chamber. (2.2)
ArmonAir Digihaler contains a built-in electronic module which detects, records, and stores data on inhaler events for transmission to the mobile App. Use of the App is not required for administration of medication to the patient. (2.4)
DOSAGE FORMS AND STRENGTHS
Inhalation powder containing 55 mcg, 113 mcg, or 232 mcg of fluticasone propionate per actuation.
ArmonAir Digihaler contains a built-in electronic module. (3)
CONTRAINDICATIONS
WARNINGS AND PRECAUTIONS
- Localized infections: Candida albicans infection of the mouth and throat may occur. Monitor patients periodically. Advise the patient to rinse his/her mouth with water without swallowing after inhalation. (5.1)
- Deterioration of asthma and acute episodes: Do not use for relief of acute symptoms. Patients require immediate re-evaluation during rapidly deteriorating asthma. (5.2)
- Immunosuppression: Potential worsening of existing tuberculosis, fungal, bacterial, viral, parasitic infections or ocular herpes simplex. Use with caution in patients with these infections. More serious or even fatal course of chickenpox or measles can occur in susceptible patients. (5.3)
- Transferring patients from systemic corticosteroids: Risk of impaired adrenal function when transferring from systemic corticosteroids. Taper patients slowly from systemic corticosteroids if transferring to ArmonAir Digihaler. (5.4)
- Hypercorticism and adrenal suppression: May occur with very high dosages or at the regular dosage in susceptible individuals. If such changes occur, discontinue ArmonAir Digihaler slowly. (5.5)
- Decreases in bone mineral density: Monitor patients with major risk factors for decreased bone mineral content. (5.7)
- Monitor growth of pediatric patients. (5.8)
- Close monitoring for glaucoma and cataracts is warranted. (5.9)
- Paradoxical bronchospasm: Discontinue ArmonAir Digihaler and institute alternative therapy if paradoxical bronchospasm occurs. (5.10)
ADVERSE REACTIONS
Most common adverse reactions (reported in greater than or equal to 3% of subjects) are: upper respiratory tract infection, nasopharyngitis, oral candidiasis, headache, and cough. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Teva Respiratory, LLC at 1-888-483-8279 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS
Strong cytochrome P450 3A4 inhibitors (e.g., ritonavir, ketoconazole): Use not recommended. May increase risk of systemic corticosteroid effects. (7.1)
USE IN SPECIFIC POPULATIONS
Hepatic impairment: Monitor for systemic corticosteroid effects. (8.6)
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 9/2022
Table Of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Important Administration Instructions
2.2 Recommended Dosage
2.3 Storing and Cleaning the Inhaler
2.4 Dose Counter and Storage of Inhaler Events Data
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
4.1 Status Asthmaticus
4.2 Hypersensitivity
5 WARNINGS AND PRECAUTIONS
5.1 Local Effects of Inhaled Corticosteroids
5.2 Acute Asthma Episodes
5.3 Immunosuppression
5.4 Transferring Patients from Systemic Corticosteroid Therapy
5.5 Hypercorticism and Adrenal Suppression
5.6 Hypersensitivity Reactions, Including Anaphylaxis
5.7 Reduction in Bone Mineral Density
5.8 Effect on Growth
5.9 Glaucoma and Cataracts
5.10 Paradoxical Bronchospasm
5.11 Drug Interactions with Strong Cytochrome P450 3A4 Inhibitors
5.12 Eosinophilic Conditions and Churg-Strauss Syndrome
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Postmarketing Experience
7 DRUG INTERACTIONS
7.1 Inhibitors of Cytochrome P450 3A4
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Hepatic Impairment
8.7 Renal Impairment
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
14.1 Dose-Ranging Trials in Patients with Asthma
14.2 Trials in the Maintenance Treatment of Asthma
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
16.2 Storage and Handling
17 PATIENT COUNSELING INFORMATION
- *
- Sections or subsections omitted from the full prescribing information are not listed.
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Important Administration Instructions
Administer ArmonAir Digihaler as 1 inhalation twice daily (approximately 12 hours apart at the same time every day) by the orally inhaled route. Advise the patient to rinse his/her mouth with water without swallowing after each dose.
- ArmonAir Digihaler does not require priming.
- Do not use ArmonAir Digihaler with a spacer or volume holding chamber.
- Do not use ArmonAir Digihaler by other routes.
- Do not use more than 2 times every 24 hours. The safety and efficacy of ArmonAir Digihaler when administered in excess of recommended dosages have not been established.
If symptoms arise between doses, an inhaled short-acting beta2-agonist should be used for immediate relief.
2.2 Recommended Dosage
The recommended starting dosage for ArmonAir Digihaler is based on asthma severity and current asthma drug therapy and strength. For example:
- For patients with greater asthma severity, use the higher doses: either
- 113 mcg (113 mcg of fluticasone propionate) administered twice daily; or
- 232 mcg (232 mcg of fluticasone propionate) administered twice daily.
- For patients not taking inhaled corticosteroids (ICS), with less severe asthma, select 55 mcg (55 mcg of fluticasone propionate), administered twice daily.
- For patients switching to ArmonAir Digihaler from another ICS: select the low (55 mcg), medium (113 mcg), or high (232 mcg) dose strength of ArmonAir Digihaler based on the strength of the previous ICS product and disease severity.
The maximum benefit may not be achieved for up to 2 weeks or longer after starting treatment. Individual patients will experience a variable time to onset and degree of symptom relief. For patients who do not respond adequately to the starting dose of ArmonAir Digihaler after 2 weeks of therapy, increasing the dose may provide additional asthma control. The highest recommended dose is 232 mcg twice daily.
If a previously effective dosage regimen of ArmonAir Digihaler fails to provide adequate control of asthma, the therapeutic regimen should be re-evaluated and additional therapeutic options (e.g., replacing the current strength of ArmonAir Digihaler with a higher strength, or adding additional controller therapies) should be considered. After asthma stability has been achieved, it is desirable to titrate to the lowest effective dosage to reduce the possibility of side effects.
2.3 Storing and Cleaning the Inhaler
- Keep the inhaler in a cool dry place.
- Routine maintenance is not required. If the mouthpiece needs cleaning, gently wipe the mouthpiece with a dry cloth or tissue as needed.
- Never wash or put any part of the inhaler in water.
2.4 Dose Counter and Storage of Inhaler Events Data
The ArmonAir Digihaler inhaler has a dose counter.
- The number 60 is displayed (prior to use).
- The dose counter will count down each time the mouthpiece is opened and closed [see Patient Counseling Information (17)].
ArmonAir Digihaler contains a built-in electronic module which detects, records, and stores data on inhaler events, including peak inspiratory flow rate (L/min), for transmission to the mobile App where inhaler events are categorized. Use of the App is not required for administration of fluticasone propionate to the patient. There is no evidence the use of the App leads to improved clinical outcomes, including safety and effectiveness [see How Supplied/Storage and Handling (16.2)].
3 DOSAGE FORMS AND STRENGTHS
Inhalation Powder: ArmonAir Digihaler is an inhalation-driven, multidose dry powder inhaler (MDPI) with an electronic module, for oral inhalation that meters 55 mcg, 113 mcg, or 232 mcg of fluticasone propionate from the device reservoir and delivers 51 mcg, 103 mcg, or 210 mcg of fluticasone propionate, respectively, from the mouthpiece per actuation. The ArmonAir Digihaler is a white inhaler with a green cap in a sealed foil pouch with desiccant. ArmonAir Digihaler contains a built-in electronic module [see How Supplied/Storage and Handling (16)].
4 CONTRAINDICATIONS
4.1 Status Asthmaticus
ArmonAir Digihaler is contraindicated in the primary treatment of status asthmaticus or other acute episodes of asthma where intensive measures are required [see Warnings and Precautions (5.2)].
5 WARNINGS AND PRECAUTIONS
5.1 Local Effects of Inhaled Corticosteroids
In clinical trials, the development of localized infections of the mouth and pharynx with Candida albicans has occurred in subjects treated with fluticasone propionate MDPI. When such an infection develops, it should be treated with appropriate local or systemic (i.e., oral) antifungal therapy while treatment with ArmonAir Digihaler continues, but at times therapy with ArmonAir Digihaler may need to be interrupted. Advise the patient to rinse his/her mouth with water without swallowing following inhalation to help reduce the risk of oropharyngeal candidiasis.
5.2 Acute Asthma Episodes
ArmonAir Digihaler is not indicated for the relief of acute symptoms, i.e., as rescue therapy for the treatment of acute episodes of bronchospasm. An inhaled, short-acting beta2-agonist, not ArmonAir Digihaler, should be used to relieve acute symptoms such as shortness of breath. When prescribing ArmonAir Digihaler, the physician must provide the patient with an inhaled, short-acting beta2-agonist (e.g., albuterol) for treatment of acute symptoms, despite regular twice-daily use of ArmonAir Digihaler. Instruct patients to contact their physicians immediately if episodes of asthma not responsive to bronchodilators occur during the course of treatment with ArmonAir Digihaler. During such episodes, patients may require therapy with oral corticosteroids.
5.3 Immunosuppression
Persons who are using drugs that suppress the immune system are more susceptible to infections than healthy individuals.
Chickenpox and measles, for example, can have a more serious or even fatal course in susceptible children or adults using corticosteroids. In such patients who have not had these diseases or who have not been properly immunized, particular care should be taken to avoid exposure. How the dose, route and duration of corticosteroid administration affect the risk of developing a disseminated infection is not known. The contribution of the underlying disease and/or prior corticosteroid treatment to the risk is also not known. If a patient is exposed to chickenpox, prophylaxis with varicella-zoster immune globulin (VZIG) or pooled intravenous immunoglobulin (IVIG) may be indicated. If a patient is exposed to measles, prophylaxis with pooled intramuscular immunoglobulin (IG) may be indicated. (See the respective package inserts for complete VZIG and IG prescribing information.) If chickenpox develops, treatment with antiviral agents may be considered.
Inhaled corticosteroids should be used with caution, if at all, in patients with active or quiescent tuberculosis infections of the respiratory tract; untreated systemic fungal, bacterial, viral, or parasitic infections; or ocular herpes simplex.
5.4 Transferring Patients from Systemic Corticosteroid Therapy
Particular care is needed for patients who are transferred from systemically active corticosteroids to inhaled corticosteroids because deaths due to adrenal insufficiency have occurred in patients with asthma during and after transfer from systemic corticosteroids to less systemically available inhaled corticosteroids. After withdrawal from systemic corticosteroids, a number of months are required for recovery of hypothalamic‑pituitary‑adrenal (HPA) function.
Patients who have been previously maintained on 20 mg or more of prednisone (or its equivalent) may be most susceptible, particularly when their systemic corticosteroids have been almost completely withdrawn. During this period of HPA suppression, patients may exhibit signs and symptoms of adrenal insufficiency when exposed to trauma, surgery, or infection (particularly gastroenteritis) or other conditions associated with severe electrolyte loss. Although ArmonAir Digihaler may improve control of asthma symptoms during these episodes, in recommended doses it supplies less than normal physiological amounts of corticosteroid systemically and does NOT provide the mineralocorticoid that is necessary for coping with these emergencies.
During periods of stress or a severe asthmatic attack, patients who have been withdrawn from systemic corticosteroids should be instructed to resume oral corticosteroids (in large doses) immediately and to contact their physician for further instruction. These patients should also be instructed to carry a medical identification warning card indicating that they may need supplementary systemic corticosteroids during periods of stress or a severe asthma attack.
Patients requiring systemic corticosteroids should be weaned slowly from systemic corticosteroid use after transferring to ArmonAir Digihaler. Prednisone reduction can be accomplished by reducing the daily prednisone dose by 2.5 mg on a weekly basis during therapy with ArmonAir Digihaler. Lung function (mean forced expiratory volume in 1 second [FEV1] or morning peak expiratory flow [AM PEF]), beta‑agonist use, and asthma symptoms should be carefully monitored during withdrawal of systemic corticosteroids. In addition to monitoring asthma signs and symptoms, patients should be observed for signs and symptoms of adrenal insufficiency, such as fatigue, lassitude, weakness, nausea and vomiting, and hypotension.
Transfer of patients from systemic corticosteroid therapy to ArmonAir Digihaler may unmask allergic conditions previously suppressed by the systemic corticosteroid therapy (e.g., rhinitis, conjunctivitis, eczema, arthritis, eosinophilic conditions).
During withdrawal from oral corticosteroids, some patients may experience symptoms of systemically active corticosteroid withdrawal (e.g., joint and/or muscular pain, lassitude, depression) despite maintenance or even improvement of respiratory function.
5.5 Hypercorticism and Adrenal Suppression
ArmonAir Digihaler will often help control asthma symptoms with less suppression of HPA function than therapeutically equivalent oral doses of prednisone. Since ArmonAir Digihaler is absorbed into the circulation and can be systemically active at higher doses, the beneficial effects of ArmonAir Digihaler in minimizing HPA dysfunction may be expected only when recommended dosages are not exceeded and individual patients are titrated to the lowest effective dose. A relationship between plasma levels of fluticasone propionate and inhibitory effects on stimulated cortisol production has been shown after 4 weeks of treatment with fluticasone propionate inhalation aerosol. Since individual sensitivity to effects on cortisol production exists, physicians should consider this information when prescribing ArmonAir Digihaler.
Because of the possibility of significant systemic absorption of inhaled corticosteroids, patients treated with ArmonAir Digihaler should be observed carefully for any evidence of systemic corticosteroid effects. Particular care should be taken in observing patients postoperatively or during periods of stress for evidence of inadequate adrenal response.
It is possible that systemic corticosteroid effects such as hypercorticism and adrenal suppression (including adrenal crisis) may appear in a small number of patients who are sensitive to these effects. If such effects occur, the dosage of ArmonAir Digihaler should be reduced slowly, consistent with accepted procedures for reducing systemic corticosteroids, and for management of asthma symptoms.
5.6 Hypersensitivity Reactions, Including Anaphylaxis
Immediate hypersensitivity reactions (e.g., urticaria, angioedema, rash, bronchospasm, hypotension), including anaphylaxis, may occur after administration of ArmonAir Digihaler. There have been reports of anaphylactic reactions in patients with severe milk protein allergy after inhalation of other powder products containing lactose; therefore, patients with severe milk protein allergy should not use ArmonAir Digihaler [see Contraindications (4.2)].
5.7 Reduction in Bone Mineral Density
Decreases in bone mineral density (BMD) have been observed with long-term administration of products containing inhaled corticosteroids. The clinical significance of small changes in BMD with regard to long‑term consequences, such as fracture, is unknown. Patients with major risk factors for decreased bone mineral content, such as prolonged immobilization, family history of osteoporosis, or chronic use of drugs that can reduce bone mass (e.g., anticonvulsants, oral corticosteroids) should be monitored and treated with established standards of care.
5.8 Effect on Growth
Orally inhaled corticosteroids, including ArmonAir Digihaler, may cause a reduction in growth velocity when administered to pediatric patients. Monitor the growth of pediatric patients receiving ArmonAir Digihaler routinely (e.g., via stadiometry). To minimize the systemic effects of orally inhaled corticosteroids, including ArmonAir Digihaler, titrate each patient’s dosage to the lowest dosage that effectively controls his/her symptoms [see Dosage and Administration (2) and Use in Specific Populations (8.4)].
5.9 Glaucoma and Cataracts
Glaucoma, increased intraocular pressure, and cataracts have been reported in patients following the long-term administration of inhaled corticosteroids, including fluticasone propionate. Therefore, close monitoring is warranted in patients with a change in vision or with a history of increased intraocular pressure, glaucoma, and/or cataracts.
5.10 Paradoxical Bronchospasm
As with other inhaled medicines, bronchospasm may occur with an immediate increase in wheezing after dosing. If bronchospasm occurs following dosing with ArmonAir Digihaler, it should be treated immediately with an inhaled, short-acting bronchodilator; ArmonAir Digihaler should be discontinued immediately; and alternative therapy should be instituted.
5.11 Drug Interactions with Strong Cytochrome P450 3A4 Inhibitors
The use of strong cytochrome P450 3A4 (CYP3A4) inhibitors (e.g., ritonavir, atazanavir, clarithromycin, indinavir, itraconazole, nefazodone, nelfinavir, saquinavir, ketoconazole, telithromycin) with ArmonAir Digihaler is not recommended because increased systemic corticosteroid adverse effects may occur [see Drug Interactions (7.1) and Clinical Pharmacology (12.3)].
5.12 Eosinophilic Conditions and Churg-Strauss Syndrome
In rare cases, patients on inhaled fluticasone propionate may present with systemic eosinophilic conditions. Some of these patients have clinical features of vasculitis consistent with Churg‑Strauss syndrome, a condition that is often treated with systemic corticosteroid therapy. These events usually, but not always, have been associated with the reduction and/or withdrawal of oral corticosteroid therapy following the introduction of fluticasone propionate. Cases of serious eosinophilic conditions have also been reported with other inhaled corticosteroids in this clinical setting. Physicians should be alert to eosinophilia, vasculitic rash, worsening pulmonary symptoms, cardiac complications, and/or neuropathy presenting in their patients. A causal relationship between fluticasone propionate and these underlying conditions has not been established.
6 ADVERSE REACTIONS
Systemic and local corticosteroid use may result in the following:
- Candida albicans infection [see Warnings and Precautions (5.1)]
- Immunosuppression [see Warnings and Precautions (5.3)]
- Hypercorticism and adrenal suppression [see Warnings and Precautions (5.5)]
- Reduction in bone mineral density [see Warnings and Precautions (5.7)]
- Growth effects in pediatrics [see Warnings and Precautions (5.8)]
- Glaucoma and cataracts [see Warnings and Precautions (5.9)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The safety of ArmonAir Digihaler has been established from adequate and well-controlled studies of fluticasone propionate inhalation powder [ARMONAIR RESPICLICK, hereafter referred to as fluticasone propionate multidose dry powder inhaler (MDPI)].
In two placebo-controlled, 12-week, clinical studies (Trial 1 and Trial 2) [see Clinical Studies (14)] a total of 822 adolescent and adult patients with persistent symptomatic asthma despite ICS or ICS/LABA therapy were treated twice daily with either placebo; or fluticasone propionate MDPI 55 mcg, 113 mcg, or 232 mcg. Sixty percent of patients were female and 80% of patients were white. The average duration of exposure was 82 days in the fluticasone propionate MDPI groups compared with 75 days in the placebo group. Table 1 displays the incidence of most common adverse reactions in pooled Trials 1 and 2.
Table 1: Adverse Reactions with ≥ 3% Incidence with Fluticasone Propionate MDPI, and More Common than Placebo in Subjects with Asthma
|
Adverse Reaction |
Fluticasone Propionate MDPI 55 mcg (n=129) % |
Fluticasone Propionate MDPI 113 mcg (n=274) % |
Fluticasone Propionate MDPI 232 mcg (n=146) % |
Placebo (n=273) % |
|
URTI |
5.4 |
4.7 |
5.5 |
4.8 |
|
Nasopharyngitis |
5.4 |
5.8 |
4.8 |
4.4 |
|
Oral candidiasis* |
3.1 |
2.9 |
4.8 |
0.7 |
|
Headache |
1.6 |
7.3 |
4.8 |
4.4 |
|
Cough |
1.6 |
1.8 |
3.4 |
2.6 |
* Oral candidiasis includes oropharyngeal candidiasis, oral fungal infection, oropharyngitis fungal
URTI = upper respiratory tract infection
Other adverse reactions not previously listed (and occurring in <3% of patients and in three or more patients on fluticasone propionate MDPI) that were reported more frequently by patients with asthma treated with fluticasone propionate MDPI compared with patients treated with placebo include the following:
Oropharyngeal pain, hypertension, rhinitis allergic, influenza, pyrexia, dizziness, respiratory tract infection, muscle spasms, rhinitis, epistaxis, ligament sprain, musculoskeletal pain, pain in extremity, throat irritation, and vomiting.
Long Term Safety Study: This was a 26-week, open label study of 674 patients previously treated with inhaled corticosteroids who were treated twice daily with fluticasone propionate MDPI 113 mcg or 232 mcg; fluticasone propionate/salmeterol MDPI (AIRDUO RESPICLICK hereafter referred to as fluticasone propionate/salmeterol MDPI) 113/14 mcg or 232/14 mcg; fluticasone propionate aerosol 110 mcg or 220 mcg; or fluticasone propionate and salmeterol inhalation powder 250/50 mcg or 500/50 mcg. The types of adverse reactions among fluticasone propionate MDPI treatments were similar to those reported above in placebo-controlled studies.
6.2 Postmarketing Experience
In addition to adverse reactions reported from clinical trials, the following adverse reactions have been identified during post‑approval use of fluticasone propionate. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure. These events have been chosen for inclusion due to either their seriousness, frequency of reporting, or causal connection to fluticasone propionate or a combination of these factors.
Ear, Nose, and Throat: Aphonia, facial and oropharyngeal edema, and throat soreness.
Endocrine and Metabolic: Cushingoid features, growth velocity reduction in children/adolescents, hyperglycemia, and osteoporosis.
Eye: Cataracts, blurred vision, and central serous chorioretinopathy.
Immune System Disorders: Immediate and delayed hypersensitivity reactions, including anaphylaxis, rash, angioedema, and bronchospasm, have been reported. Anaphylactic reactions in patients with severe milk protein allergy have been reported.
Infections and Infestations: Esophageal candidiasis.
Psychiatry: Agitation, aggression, anxiety, depression, and restlessness. Behavioral changes, including hyperactivity and irritability, have been reported very rarely and primarily in children.
Respiratory: Asthma exacerbation, bronchospasm, chest tightness, dyspnea, immediate bronchospasm, pneumonia, and wheeze.
Skin: Contusions and ecchymoses.
7 DRUG INTERACTIONS
7.1 Inhibitors of Cytochrome P450 3A4
Fluticasone propionate is a substrate of CYP3A4. The use of strong CYP3A4 inhibitors (e.g., ritonavir, atazanavir, clarithromycin, indinavir, itraconazole, nefazodone, nelfinavir, saquinavir, ketoconazole, telithromycin) with ArmonAir Digihaler is not recommended because increased systemic corticosteroid adverse effects may occur.
Ritonavir: A drug interaction trial with fluticasone propionate aqueous nasal spray in healthy subjects has shown that ritonavir (a strong CYP3A4 inhibitor) can significantly increase plasma fluticasone propionate exposure, resulting in significantly reduced serum cortisol concentrations [see Clinical Pharmacology (12.3)]. During postmarketing use, there have been reports of clinically significant drug interactions in patients receiving fluticasone propionate and ritonavir, resulting in systemic corticosteroid effects including Cushing’s syndrome and adrenal suppression.
Ketoconazole: Coadministration of orally inhaled fluticasone propionate (1,000 mcg) and ketoconazole (200 mg once daily) resulted in a 1.9‑fold increase in plasma fluticasone propionate exposure and a 45% decrease in plasma cortisol area under the curve (AUC), but had no effect on urinary excretion of cortisol.
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
There are no randomized clinical studies of ArmonAir Digihaler in pregnant women. There are clinical considerations with the use of ArmonAir Digihaler in pregnant women [see Clinical Considerations]. In animals, teratogenicity characteristic of corticosteroids, decreased fetal body weight, and/or skeletal variations in rats, mice, and rabbits were observed with subcutaneously administered maternal toxic doses of fluticasone propionate less than the maximum recommended human daily inhaled dose (MRHDID) on a mcg/m2 basis [see Data]. However, fluticasone propionate administered via inhalation to rats decreased fetal body weight, but did not induce teratogenicity at a maternal toxic dose approximately 2 times the MRHDID on a mcg/m2 basis [see Data]. Experience with oral corticosteroids suggests that rodents are more prone to teratogenic effects from corticosteroids than humans. The estimated risk of major birth defects and miscarriage for the indicated population is unknown. In the U.S. general population, the estimated risk of major birth defects and miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively.
Clinical Considerations
Disease‑Associated Maternal and/or Embryo/Fetal Risk
In women with poorly or moderately controlled asthma, there is an increased risk of several perinatal adverse outcomes such as preeclampsia in the mother and prematurity, low birth weight, and small for gestational age for the neonate. Pregnant women with asthma should be closely monitored and medication adjusted as necessary to maintain optimal asthma control.
Data
Animal Data
In embryo/fetal development studies with pregnant rats and mice dosed by the subcutaneous route throughout the period of organogenesis, fluticasone propionate was teratogenic in both species. Omphalocele, decreased body weight, and skeletal variations were observed in rat fetuses, in the presence of maternal toxicity, at a dose approximately 2 times the MRHDID (on a mcg/m2 basis with a maternal subcutaneous dose of 100 mcg/kg/day). The rat no observed adverse effect level (NOAEL) was observed at approximately 0.6 times the MRHDID (on a mcg/m2 basis with a maternal subcutaneous dose of 30 mcg/kg/day). Cleft palate and fetal skeletal variations were observed in mouse fetuses at a dose approximately 0.5 times the MRHDID (on a mcg/m2 basis with a maternal subcutaneous dose of 45 mcg/kg/day). The mouse NOAEL was observed with a dose approximately 0.16 times the MRHDID (on a mcg/m2 basis with a maternal subcutaneous dose of 15 mcg/kg/day).
In an embryo/fetal development study with pregnant rats dosed by the inhalation route throughout the period of organogenesis, fluticasone propionate produced decreased fetal body weights and skeletal variations, in the presence of maternal toxicity, at a dose approximately 0.5 times the MRHDID (on a mcg/m2 basis with a maternal inhalation dose of 25.7 mcg/kg/day); however, there was no evidence of teratogenicity. The NOAEL was observed with a dose approximately 0.1 times the MRHDID (on a mcg/m2 basis with a maternal inhalation dose of 5.5 mcg/kg/day).
In an embryofetal development study in pregnant rabbits that were dosed by the subcutaneous route throughout organogenesis, fluticasone propionate produced reductions of fetal body weights, in the presence of maternal toxicity at doses approximately 0.02 times the MRHDID and higher (on a mcg/m2 basis with a maternal subcutaneous dose of 0.57 mcg/kg/day). Teratogenicity was evident based upon a finding of cleft palate for 1 fetus at a dose approximately 0.2 times the MRHDID (on a mcg/m2 basis with a maternal subcutaneous dose of 4 mcg/kg/day). The NOAEL was observed in rabbit fetuses with a dose approximately 0.004 times the MRHDID (on a mcg/m2 basis with a maternal subcutaneous dose of 0.08 mcg/kg/day).
Fluticasone propionate crossed the placenta following subcutaneous administration to mice and rats and oral administration to rabbits.
In a pre- and post-natal development study in pregnant rats dosed from late gestation through delivery and lactation (Gestation Day 17 to Postpartum Day 22), fluticasone propionate was not associated with decreases in pup body weight, and had no effects on developmental landmarks, learning, memory, reflexes, or fertility at doses up to approximate equivalence to the MRHDID (on a mcg/m2 basis with maternal subcutaneous doses up to 50 mcg/kg/day).
8.2 Lactation
Risk Summary
There are no available data on the presence of fluticasone propionate in human milk, the effects on the breastfed child, or the effects on milk production. Other corticosteroids have been detected in human milk. However, fluticasone propionate concentrations in plasma after inhaled therapeutic doses are low and therefore concentrations in human breast milk are likely to be correspondingly low [see Clinical Pharmacology (12.3)]. The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for ArmonAir Digihaler and any potential adverse effects on the breastfed child from ArmonAir Digihaler or from the underlying maternal condition.
Data
Animal Data
Subcutaneous administration of tritiated fluticasone propionate at a dose in lactating rats approximately 0.2 times the MRHDID for adults (on a mcg/m2 basis) resulted in measurable levels in milk.
8.4 Pediatric Use
The safety and effectiveness of ArmonAir Digihaler in the maintenance treatment of asthma as prophylactic therapy in pediatric patients 12 years of age and older has been established. Use of ArmonAir Digihaler for this indication was supported by evidence from two adequate and well-controlled trials in pediatric patients 12 years old and older with persistent symptomatic asthma despite ICS or ICS/LABA therapy (Trials 1 and 2) [see Clinical Studies (14)]. In these trials, 50 adolescents received fluticasone propionate MDPI one inhalation twice daily.
The safety and effectiveness of ArmonAir Digihaler in pediatric patients below the age of 12 years have not been established.
Inhaled corticosteroids, including fluticasone propionate, may cause a reduction in growth velocity when administered to pediatric patients [see Warnings and Precautions (5.8)]. A reduction of growth velocity in children or teenagers may occur as a result of poorly controlled asthma or from use of corticosteroids, including inhaled corticosteroids. The effects of long-term treatment of children and adolescents with inhaled corticosteroids, including fluticasone propionate, on final adult height are not known.
8.5 Geriatric Use
No overall differences in safety or efficacy were observed in data collected in 135 subjects aged 65 years and older versus younger subjects who were treated with ArmonAir Digihaler in placebo-controlled Phase 2 and 3 studies.
8.6 Hepatic Impairment
Formal pharmacokinetic studies using ArmonAir Digihaler have not been conducted in patients with hepatic impairment. Since fluticasone propionate is predominantly cleared by hepatic metabolism [see Clinical Pharmacology (12.3)], impairment of liver function may lead to accumulation of fluticasone propionate in plasma. Therefore, patients with hepatic impairment should be closely monitored.
10 OVERDOSAGE
Chronic overdosage may result in signs/symptoms of hypercorticism [see Warnings and Precautions (5.5)]. Inhalation by healthy volunteers of a single dose of 4,000 mcg of fluticasone propionate inhalation powder or single doses of 1,760 or 3,520 mcg of fluticasone propionate CFC inhalation aerosol was well tolerated. Fluticasone propionate given by inhalation aerosol at dosages of 1,320 mcg twice daily for 7 to 15 days to healthy human volunteers was also well tolerated. Repeat oral doses up to 80 mg daily for 10 days in healthy volunteers and repeat oral doses up to 20 mg daily for 42 days in subjects were well tolerated. Adverse reactions were of mild or moderate severity, and incidences were similar in active and placebo treatment groups.
11 DESCRIPTION
The active component of ArmonAir Digihaler 55 mcg, ArmonAir Digihaler 113 mcg, and ArmonAir Digihaler 232 mcg is fluticasone propionate, a corticosteroid having the chemical name S-(fluoromethyl) 6α,9-difluoro-11ß,17-dihydroxy-16α-methyl-3-oxoandrosta-1,4-diene-17ß-carbothioate, 17-propionate, and the following chemical structure:
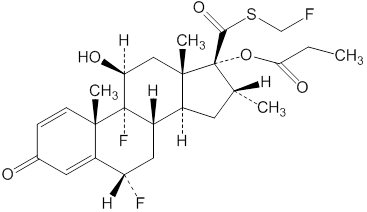
Fluticasone propionate is a white powder with a molecular weight of 500.6, and the empirical formula is C25H31F3O5S. It is practically insoluble in water, freely soluble in dimethyl sulfoxide and dimethylformamide, and slightly soluble in methanol and 95% ethanol.
ArmonAir Digihaler is a multidose dry powder inhaler (MDPI) with an electronic module, for oral inhalation only. It contains a formulation blend of fluticasone propionate and alpha lactose monohydrate (which may contain milk proteins). The opening of the mouthpiece cover meters 11.5 mg of the formulation from the device reservoir, which contains 55 mcg, 113 mcg, or 232 mcg of fluticasone propionate. Patient inhalation through the mouthpiece causes the deagglomeration and aerosolization of the drug particles as the formulation moves through the cyclone component of the device. This is followed by dispersion into the airstream.
Under standardized in vitro test conditions, the ArmonAir Digihaler inhaler delivers 51 mcg, 103 mcg, or 210 mcg of fluticasone propionate with lactose from the mouthpiece when tested at a flow rate of 88 L/min for 1.4 seconds.
The amount of drug delivered to the lung will depend on patient factors such as inspiratory flow profiles. In adult subjects (N=50, aged 18 to 45 years) with asthma, mean peak inspiratory flow (PIF) through the MDPI inhaler was 108.28 L/min (range: 70.37 to 129.24 L/min). In adolescent subjects (N=50, aged 12 to 17 years) with asthma, mean peak inspiratory flow (PIF) through the MDPI inhaler was 106.72 L/min (range: 73.64 to 125.51 L/min).
ArmonAir Digihaler includes a QR code (on the top of the inhaler), and contains a built-in electronic module which automatically detects, records and stores data on inhaler events, including peak inspiratory flow rate (L/min). ArmonAir Digihaler may pair with and transmit data to the mobile App where inhaler events are categorized.
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Fluticasone propionate is a synthetic trifluorinated corticosteroid with anti-inflammatory activity. Fluticasone propionate has been shown in vitro to exhibit a binding affinity for the human glucocorticoid receptor that is 18 times that of dexamethasone, almost twice that of beclomethasone-17-monopropionate (BMP), the active metabolite of beclomethasone dipropionate, and over 3 times that of budesonide. Data from the McKenzie vasoconstrictor assay in humans are consistent with these results. The clinical significance of these findings is unknown.
Inflammation is an important component in the pathogenesis of asthma. Corticosteroids have been shown to have a wide range of actions on multiple cell types (e.g., mast cells, eosinophils, neutrophils, macrophages, and lymphocytes) and mediators (e.g., histamine, eicosanoids, leukotrienes, and cytokines) involved in inflammation. These anti-inflammatory actions of corticosteroids contribute to their efficacy in the treatment of asthma.
Though effective for the treatment of asthma, corticosteroids do not affect asthma symptoms immediately. Individual patients will experience a variable time of onset and degree of symptom relief. Maximum benefit may not be achieved for 1 to 2 weeks or longer after starting treatment. When corticosteroids are discontinued, asthma stability may persist for several days or longer.
Trials in subjects with asthma have shown a favorable ratio between topical anti-inflammatory activity and systemic corticosteroid effects with recommended doses of orally inhaled fluticasone propionate. This is explained by a combination of a relatively high local anti-inflammatory effect, negligible oral systemic availability (<1%), and the minimal pharmacological activity of the only metabolite detected in man.
12.2 Pharmacodynamics
Hypothalamic Pituitary Adrenal Axis Effects (HPA)
The potential systemic effects of ArmonAir Digihaler on the HPA axis were not fully studied, but other clinical trials evaluated the systemic effects of fluticasone propionate inhalation powder on the HPA axis in healthy subjects and in subjects with asthma.
There are no data regarding serum cortisol from controlled trials using ArmonAir Digihaler in healthy subjects or subjects with asthma.
12.3 Pharmacokinetics
Absorption
Fluticasone propionate acts locally in the lung; therefore, plasma levels do not predict therapeutic effect. Trials using oral dosing of labeled and unlabeled drug have demonstrated that the oral systemic bioavailability of fluticasone propionate was negligible (<1%), primarily due to incomplete absorption and presystemic metabolism in the gut and liver. In contrast, the majority of the fluticasone propionate delivered to the lung was systemically absorbed.
Following fluticasone propionate MDPI administration, the peak plasma concentration of fluticasone propionate occurs at approximately 1 hour after inhalation.
The mean peak concentration following a 232 mcg single oral inhalation of fluticasone propionate MDPI to patients 12 years and older with persistent asthma was 73 pg/mL.
Distribution
Following intravenous administration, the initial disposition phase for fluticasone propionate was rapid and consistent with its high lipid solubility and tissue binding. The volume of distribution averaged 4.2 L/kg.
The percentage of fluticasone propionate bound to human plasma proteins averages 99%. Fluticasone propionate is weakly and reversibly bound to erythrocytes and is not significantly bound to human transcortin.
Elimination
Terminal half-life estimate of fluticasone propionate following oral inhalation administration of fluticasone propionate MDPI was approximately 11.2 hours.
Metabolism
The total clearance of fluticasone propionate is high (average, 1,093 mL/min), with renal clearance accounting for less than 0.02% of the total. The only circulating metabolite detected in man is the 17β carboxylic acid derivative of fluticasone propionate, which is formed through the CYP3A4 pathway. This metabolite has less affinity (approximately 1/2,000) than the parent drug for the glucocorticoid receptor of human lung cytosol in vitro and negligible pharmacological activity in animal studies. Other metabolites detected in vitro using cultured human hepatoma cells have not been detected in man.
Excretion
Less than 5% of a radiolabeled oral dose of fluticasone propionate was excreted in the urine as metabolites, with the remainder excreted in the feces as parent drug and metabolites.
Specific Populations
Age: No pharmacokinetic studies have been performed with ArmonAir Digihaler in children or geriatric patients. A subgroup analysis was conducted to compare patients aged 12-17 (n=16) and ≥18 (n=23) years following administration of 232 mcg fluticasone propionate MDPI. No overall differences in fluticasone propionate pharmacokinetics were observed.
Sex: A subgroup analysis was conducted to compare male (n=22) and female (n=17) patients following administration of 232 mcg fluticasone propionate MDPI. No overall differences in fluticasone propionate pharmacokinetics were observed.
Renal Impairment: The effect of renal impairment on the pharmacokinetics of ArmonAir Digihaler has not been evaluated.
Hepatic Impairment: Formal pharmacokinetic studies using ArmonAir Digihaler have not been conducted in patients with hepatic impairment. However, since fluticasone propionate is predominantly cleared by hepatic metabolism, impairment of liver function may lead to accumulation of fluticasone propionate in plasma.
Drug Interaction Studies: In vitro and in vivo drug interaction studies have not been conducted with ArmonAir Digihaler. Known clinically significant drug interactions are outlined in Drug Interactions (7).
Inhibitors of Cytochrome P450 3A4: Ritonavir: Fluticasone propionate is a substrate of CYP3A4. Coadministration of fluticasone propionate and the strong CYP3A4 inhibitor ritonavir is not recommended based upon a multiple-dose, crossover drug interaction trial in 18 healthy subjects. Fluticasone propionate aqueous nasal spray (200 mcg once daily) was coadministered for 7 days with ritonavir (100 mg twice daily). Plasma fluticasone propionate concentrations following fluticasone propionate aqueous nasal spray alone were undetectable (<10 pg/mL) in most subjects, and when concentrations were detectable, peak levels (Cmax) averaged 11.9 pg/mL (range: 10.8 to 14.1 pg/mL) and AUC0-τ averaged 8.43 pg•h/mL (range: 4.2 to 18.8 pg•h/mL). Fluticasone propionate Cmax and AUC0-τ increased to 318 pg/mL (range: 110 to 648 pg/mL) and 3,102.6 pg•h/mL (range: 1,207.1 to 5,662.0 pg•h/mL), respectively, after coadministration of ritonavir with fluticasone propionate aqueous nasal spray. This significant increase in plasma fluticasone propionate exposure resulted in a significant decrease (86%) in serum cortisol AUC.
Ketoconazole: In a placebo-controlled crossover trial in 8 healthy adult volunteers, coadministration of a single dose of orally inhaled fluticasone propionate (1,000 mcg) with multiple doses of ketoconazole (200 mg) to steady state resulted in increased plasma fluticasone propionate exposure, a reduction in plasma cortisol AUC, and no effect on urinary excretion of cortisol.
Following orally inhaled fluticasone propionate alone, AUC2-last averaged 1.559 ng•h/mL (range: 0.555 to 2.906 ng•h/mL) and AUC2-∞ averaged 2.269 ng•h/mL (range: 0.836 to 3.707 ng•h/mL). Fluticasone propionate AUC2-last and AUC2-∞ increased to 2.781 ng•h/mL (range: 2.489 to 8.486 ng•h/mL) and 4.317 ng•h/mL (range: 3.256 to 9.408 ng•h/mL), respectively, after coadministration of ketoconazole with orally inhaled fluticasone propionate. This increase in plasma fluticasone propionate concentration resulted in a decrease (45%) in serum cortisol AUC.
Erythromycin: In a multiple-dose drug interaction trial, coadministration of orally inhaled fluticasone propionate (500 mcg twice daily) and erythromycin (333 mg 3 times daily) did not affect fluticasone propionate pharmacokinetics.
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Fluticasone propionate demonstrated no tumorigenic potential in mice at oral doses up to 1,000 mcg/kg (approximately 10 times the MRHDID for adults on a mcg/m2 basis) for 78 weeks or in rats at inhalation doses up to 57 mcg/kg (approximately equivalent to the MRHDID for adults on a mcg/m2 basis) for 104 weeks.
Fluticasone propionate did not induce gene mutation in prokaryotic or eukaryotic cells in vitro. No significant clastogenic effect was seen in cultured human peripheral lymphocytes in vitro or in the in vivo mouse micronucleus test.
Fertility and reproductive performance were unaffected in male and female rats at subcutaneous doses up to 50 mcg/kg (approximately equivalent to the MRHDID for adults on a mcg/m2 basis).
14 CLINICAL STUDIES
The safety and efficacy of fluticasone propionate inhalation powder [ARMONAIR RESPICLICK, hereafter referred to as fluticasone propionate MDPI] was evaluated in 2130 patients with asthma. The development program included 2 confirmatory trials of 12 weeks duration, a 26 week safety trial and two dose-ranging trials of 12 weeks duration. The efficacy of Armonair Digihaler is based primarily on the dose-ranging trials and the confirmatory trials described below.
14.1 Dose-Ranging Trials in Patients with Asthma
Six doses of fluticasone propionate ranging from 16 mcg to 434 mcg (expressed as metered doses) administered twice daily via MDPI were evaluated in 2 randomized, double-blind, placebo-controlled 12 week trials in patients with asthma.
- Trial 201 was conducted in patients whose asthma was uncontrolled at baseline and had been treated by short-acting beta2-agonist (SABA) alone or in combination with non-corticosteroid asthma medication. Low dose inhaled corticosteroid (ICS)-treated patients may have been included after a minimum of 2 weeks washout. This trial contained an open-label active comparator fluticasone propionate inhalation powder 100 mcg administered twice daily.
- Trial 202 was conducted in patients whose asthma was uncontrolled at baseline and had been treated with high dose ICS with or without a LABA. This study contained an open-label active comparator fluticasone propionate inhalation powder 250 mcg twice daily.
The trials were dose-ranging trials of fluticasone propionate MDPI and not designed to provide comparative effectiveness data and should not be interpreted as evidence of superiority/inferiority to fluticasone propionate inhalation powder. The metered doses for fluticasone multidose dry powder inhaler (16, 28, 59, 118, 225, 434 mcg) used in Trial 201 and Trial 202 (see Figure 1) are slightly different from the metered doses for the comparator products (fluticasone inhalation powder) and the Phase 3 investigational products which are the basis of the proposed commercial labeled claim (55, 113, 232 mcg for fluticasone). The changes in doses between Phase 2 and 3 resulted from optimization of the manufacturing process.
Figure 1 Baseline Adjusted Least Square Mean Change in Trough Morning FEV1 (L) over 12 weeks (FAS)a

FAS = full analysis set;
a Trials were not designed to provide comparative effectiveness data and should not be interpreted as superiority/inferiority to fluticasone propionate inhalation powder.
14.2 Trials in the Maintenance Treatment of Asthma
Adult and Adolescent Patients Aged 12 Years and Older:
Two 12-week randomized, double-blind, placebo-controlled, parallel-group, global Phase 3 clinical trials were conducted in 1375 adult and adolescent patients (aged 12 years and older, with baseline FEV1 40% to 85% of predicted normal) with asthma that was not optimally controlled on their current therapy. Patients were randomized to receive 1 inhalation twice a day of fluticasone propionate and salmeterol inhalation powder (referred to as fluticasone propionate/salmeterol MDPI), fluticasone propionate MDPI, or placebo. Other maintenance asthma therapies were discontinued at randomization.
Trial 1: In this trial, adolescent and adult patients with persistent symptomatic asthma despite low-dose or mid-dose inhaled corticosteroid (ICS) or ICS/LABA therapy were included. After completing a run-in period where patients were treated with beclomethasone dipropionate inhalation aerosol 40 mcg twice daily and a single blind placebo MDPI, the patients who met the randomization criteria were randomized to 1 inhalation twice a day of the following treatments:
- Placebo MDPI (n=130)
- Fluticasone propionate MDPI 55 mcg (n=129)
- Fluticasone propionate MDPI 113 mcg (n=130)
- Fluticasone propionate/salmeterol MDPI 55/14 mcg (n=129), or
- Fluticasone propionate/salmeterol MDPI 113/14 mcg (n=129)
Baseline FEV1 measurements were similar across treatments: fluticasone propionate MDPI 55 mcg 2.134 L, fluticasone propionate MDPI 113 mcg 2.166 L, and placebo 2.188 L.
The primary endpoints for this trial were the change from baseline in trough FEV1 at week 12 for all patients and standardized baseline-adjusted FEV1 AUEC0-12h at week 12 analyzed for a subset of 312 patients who performed postdose serial spirometry.
Patients receiving fluticasone propionate MDPI 55 mcg and fluticasone propionate MDPI 113 mcg had significantly greater improvements in trough FEV1 compared with the placebo group:
- Fluticasone propionate MDPI 55 mcg: LS mean change of 0.172 L at 12 weeks
- Fluticasone propionate MDPI 113 mcg: LS mean change of 0.204 L at 12 weeks
- Placebo: LS mean change of 0.053 L at 12 weeks
The estimated mean differences between:
-
Fluticasone propionate MDPI 55 mcg compared to placebo was 0.119 L (95% CI: 0.025, 0.212).
-
Fluticasone propionate MDPI 113 mcg compared to placebo was 0.151 L (95% CI: 0.057, 0.244).
In addition, the mean FEV1 results at each visit are displayed in Figure 2.
Figure 2: Mean Change from Baseline in Trough FEV1 at Each Visit by Treatment Group Trial 1 (FAS)

FAS = full analysis set; FEV1 = forced expiratory volume in 1 second
Supportive evidence of efficacy for fluticasone propionate MDPI compared with placebo was derived from secondary endpoints such as the weekly average of daily trough morning peak expiratory flow and the total daily use of rescue medication. The Asthma Quality of Life Questionnaire (AQLQ) for patients age ≥ 18 years or the pediatric AQLQ (PAQLQ) for patients aged 12-17 were assessed in Trial 1. The responder rate for both measures was defined as an improvement in score of 0.5 or more as threshold. In Trial 1, the responder rates for patients receiving fluticasone propionate MDPI 55 mcg and fluticasone propionate MDPI 113 mcg were 46% and 45%, respectively, compared to 40% for patients receiving placebo, with odds ratios of 1.23 (95% CI: 0.74, 2.06) and 1.25 (95% CI: 0.75, 2.08), respectively.
Improvements in FEV1 for both fluticasone propionate MDPI dose groups were sustained over the 12 hours of testing at week 12 (Figure 3). No diminution in the 12 hour bronchodilator effect was observed with fluticasone propionate MDPI as assessed by FEV1 following 12 weeks of therapy.
Figure 3: Serial Spirometry: Mean Change from Baseline in FEV1 (L) at Week 12 by Time Point and Treatment Group Trial 1 (FAS; Serial Spirometry Subset)
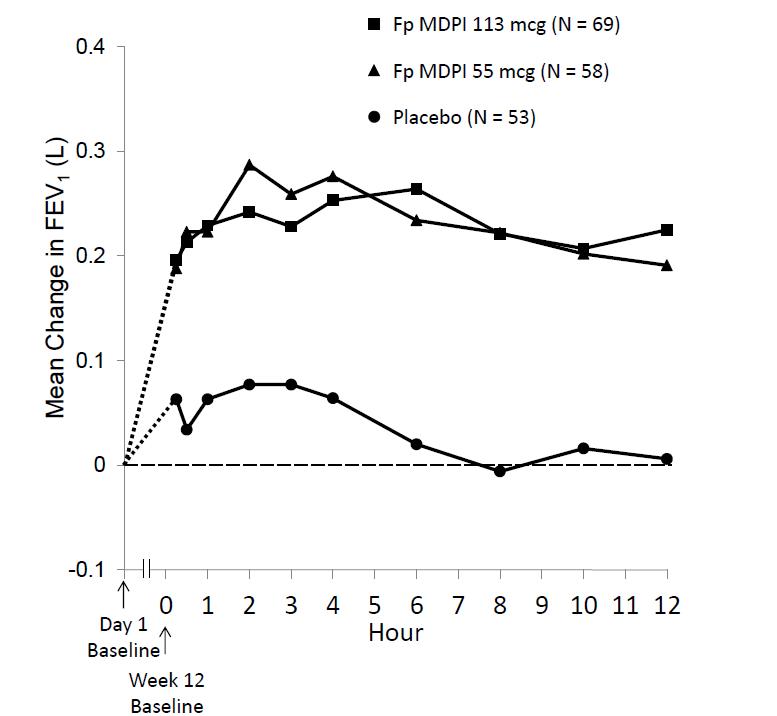
FAS = full analysis set; FEV1 = forced expiratory volume in 1 second
Trial 2: In this trial, adolescent and adult patients with persistent symptomatic asthma despite inhaled corticosteroid (ICS) or ICS/LABA therapy were included. After completing a run-in period where patients were treated with single-blind fluticasone propionate MDPI 55 mcg twice daily replacing their current asthma therapy, patients who met the randomization criteria were randomized to 1 inhalation twice a day of the following treatments:
- Placebo MDPI (n=145)
- Fluticasone propionate MDPI 113 mcg (n=146)
- Fluticasone propionate MDPI 232 mcg (n=146)
- Fluticasone propionate/salmeterol MDPI 113/14 mcg (n=145)
- Fluticasone propionate/salmeterol MDPI 232/14 mcg (n=146)
Baseline FEV1 measurements were similar across treatments, as follows: fluticasone propionate MDPI 113 mcg 2.069 L, fluticasone propionate MDPI 232 mcg 2.075 L, and placebo 2.141 L.
The primary endpoints for this trial were the change from baseline in trough FEV1 at week 12 for all patients and standardized baseline-adjusted FEV1 AUEC0-12h at week 12 analyzed for a subset of 312 patients who performed postdose serial spirometry.
Efficacy results in this trial were similar to those observed in Trial 1. Patients receiving fluticasone propionate MDPI 113 mcg and fluticasone propionate MDPI 232 mcg had significantly greater improvements in trough FEV1 compared with the placebo group:
- Fluticasone propionate MDPI 113 mcg: LS mean change of 0.119 L at 12 weeks
- Fluticasone propionate MDPI 232 mcg: LS mean change of 0.179 L at 12 weeks
- Placebo: LS mean change of ‑0.004 L at 12 weeks
Estimated mean differences between:
-
Fluticasone propionate MDPI 113 mcg compared to placebo was 0.123 L (95% CI: 0.038, 0.208).
-
Fluticasone propionate MDPI 232 mcg compared to placebo was 0.183 L (95% CI: 0.098, 0.268).
In addition, the mean FEV1 results at each visit are displayed in Figure 4.
Figure 4: Mean Change from Baseline in Trough FEV1 at Each Visit by Treatment Group Trial 2 (FAS)

FAS = full analysis set; FEV1 = forced expiratory volume in 1 second
Supportive evidence of efficacy for fluticasone propionate MDPI compared with placebo was derived from secondary endpoints such as the weekly average of daily trough morning peak expiratory flow and total daily use of rescue medication. There were fewer withdrawals due to worsening asthma in patients treated with fluticasone propionate MDPI than with placebo. The AQLQ (patients age ≥ 18 years) or the PAQLQ (patients aged 12-17) were assessed in Trial 2. The responder rates for patients receiving fluticasone propionate MDPI 113 mcg and fluticasone propionate MDPI 232 mcg were 38% and 44%, respectively, compared to 27% for patients receiving placebo, with odds ratios of 1.75 (95% CI:1.05, 2.93) and 2.12 (95% CI: 1.27, 3.53), respectively.
Improvements in FEV1 for both fluticasone propionate MDPI dose groups were sustained over the 12 hours of testing at week 12 (Figure 5). No diminution in the 12 hour bronchodilator effect was observed with fluticasone propionate MDPI as assessed by FEV1 following 12 weeks of therapy.
Figure 5: Serial Spirometry: Mean Change from Baseline in FEV1 (L) at Week 12 by Time Point and Treatment Group Trial 2 (FAS; Serial Spirometry Subset)

FAS = full analysis set; FEV1 = forced expiratory volume in 1 second
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
ArmonAir Digihaler is supplied in the following three strengths as a white multidose dry-powder inhaler (MDPI) with an electronic module. Each inhaler has a green cap and is packaged individually in a foil pouch in a carton. Each inhaler contains 0.9g of the formulation and provides 60 actuations:
|
STRENGTH |
NDC CODE |
|
ArmonAir Digihaler 55 mcg (low) |
NDC 59310-114-06 |
|
ArmonAir Digihaler 113 mcg (medium) |
NDC 59310-200-06 |
|
ArmonAir Digihaler 232 mcg (high) |
NDC 59310-311-06 |
Each ArmonAir Digihaler inhaler has a dose counter attached to the actuator. Patients should never try to alter the numbers for the dose counter. Discard the inhaler when the counter displays 0, 30 days after opening the foil pouch or after the expiration date on the product, whichever comes first. The labeled amount of medication in each actuation cannot be assured after the counter displays 0, even though the inhaler is not completely empty and will continue to operate [see Patient Counseling Information (17)].
16.2 Storage and Handling
Store at room temperature (between 15ºF and 25ºC; 59ºF and 77ºF) in a dry place; excursions permitted from 59ºF to 86ºF (15ºC to 30ºC). Avoid exposure to extreme heat, cold, or humidity.
Keep out of reach of children.
ArmonAir Digihaler should be stored inside the unopened, moisture‑protective foil pouch and removed from the pouch immediately before initial use. Discard ArmonAir Digihaler 30 days after opening the foil pouch or when the counter reads “0”, whichever comes first. The inhaler is not reusable. Do not attempt to take the inhaler apart.
ArmonAir Digihaler includes a QR code, and contains a built-in electronic module which automatically detects, records, and stores data on inhaler events, including peak inspiratory flow rate (L/min). ArmonAir Digihaler may pair with and transmit data to the mobile App via Bluetooth® wireless technology where inhaler events are categorized.
ArmonAir Digihaler contains a lithium-manganese dioxide battery and should be disposed of in accordance with state and local regulations.
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Patient Information and Instructions for Use).
Patients should be given the following information:
Local Effects
Inform patients that localized infections with Candida albicans occurred in the mouth and pharynx in some patients. If oropharyngeal candidiasis develops, treat it with appropriate local or systemic (i.e., oral) antifungal therapy while still continuing therapy with ArmonAir Digihaler, but at times therapy with ArmonAir Digihaler may need to be temporarily interrupted under close medical supervision. Rinsing the mouth with water without swallowing after inhalation is advised to help reduce the risk of thrush.
Status Asthmaticus and Acute Asthma Symptoms
Inform patients that ArmonAir Digihaler is not a bronchodilator and is not intended for use as rescue medicine for acute asthma exacerbations. Advise patients to treat acute asthma symptoms with an inhaled, short‑acting beta2‑agonist such as albuterol. Instruct the patient to contact their physicians immediately if there is deterioration of their asthma.
Immunosuppression
Warn patients who are on immunosuppressant doses of corticosteroids to avoid exposure to chickenpox or measles and, if exposed, to consult their physicians without delay. Inform patients of potential worsening of existing tuberculosis; fungal, bacterial, viral, or parasitic infections; or ocular herpes simplex.
Hypercorticism and Adrenal Suppression
Advise patients that ArmonAir Digihaler may cause systemic corticosteroid effects of hypercorticism and adrenal suppression. Additionally, instruct patients that deaths due to adrenal insufficiency have occurred during and after transfer from systemic corticosteroids. Patients should taper slowly from systemic corticosteroids if transferring to ArmonAir Digihaler.
Immediate Hypersensitivity Reactions
Advise patients that immediate hypersensitivity reactions (e.g., urticaria, angioedema, rash, bronchospasm, and hypotension), including anaphylaxis, may occur after administration of ArmonAir Digihaler. Patients should discontinue ArmonAir Digihaler if such reactions occur and contact their healthcare provider or get emergency medical help. There have been reports of anaphylactic reactions in patients with severe milk protein allergy after inhalation of powder products containing lactose; therefore, patients with severe milk protein allergy should not take ArmonAir Digihaler.
Reduction in Bone Mineral Density (BMD)
Advise patients who are at an increased risk for decreased BMD that the use of corticosteroids may pose an additional risk.
Reduced Growth Velocity
Inform patients that orally inhaled corticosteroids, including ArmonAir Digihaler, may cause a reduction in growth velocity when administered to pediatric patients. Physicians should closely follow the growth of adolescents taking corticosteroids by any route.
Ocular Effects
Long-term use of inhaled corticosteroids may increase the risk of some eye problems (cataracts or glaucoma); consider regular eye examinations.
Pregnancy
Inform patients who are pregnant or nursing that they should contact their physician about the use of ArmonAir Digihaler.
Use Daily for Best Effect
Patients should use ArmonAir Digihaler at regular intervals as directed. The daily dosage of ArmonAir Digihaler should not exceed 1 inhalation twice a day. Advise patients, if they miss a dose, to take their next dose at the same time they normally do and to not take 2 doses at one time. Individual patients will experience a variable time to onset and degree of symptom relief and the full benefit may not be achieved until treatment has been administered for 1 to 2 weeks or longer. Patients should not increase the prescribed dosage but should contact their physicians if symptoms do not improve or if the condition worsens. Instruct patients to not stop use of ArmonAir Digihaler abruptly. Patients should contact their physicians immediately if they discontinue use of ArmonAir Digihaler.
Use of ArmonAir Digihaler and Mobile App
Direct the patient to the Instructions for Use (IFU) for information on how to set up the App and use the inhaler. Advise the patient that pairing of the inhaler to the App, having Bluetooth turned on, or being near their smartphone is not required for delivery of the medication from the inhaler or for normal use of the product.
Dose Counter
Instruct patients that the ArmonAir Digihaler inhaler has a dose counter that displays the number of actuations (inhalations) left in the inhaler. When the patient receives a new inhaler, the number 60 will be displayed. The dose counter will count down each time the mouthpiece is opened and closed. The dose counter window displays the number of actuations (inhalations) left in the inhaler in units of two (e.g., 60, 58, 56, etc.). When the dose counter reaches 20, the color of the numbers will change to red to remind the patient to contact their pharmacist or healthcare provider for a refill of their medication. When the dose counter reaches 0, the patient should stop using the inhaler, and it should be disposed of in accordance with state and local regulations.
Caring for and Storing the Inhaler
Instruct patients to not open their inhaler unless they are taking a dose. Repeated opening and closing the cover without taking medication will waste medication and may damage the inhaler.
Advise patients to keep their inhaler dry and clean at all times. Never wash or put any part of the inhaler in water. Patients should replace inhaler if washed or placed in water.
Advise patients to immediately replace inhaler if mouthpiece cover is damaged or broken.
Gently wipe the mouthpiece with a dry cloth or tissue as needed.
Instruct patients to store the inhaler at room temperature and to avoid exposure to extreme heat, cold, or humidity.
Instruct patients to never take the inhaler apart.
Instruct patients to discard ArmonAir Digihaler when the dose counter displays 0, 30 days after opening the foil pouch or after the expiration date on the product, whichever comes first.
Rx only
Distributed by:
Teva Pharmaceuticals USA, Inc.
Parsippany, NJ 07054
©2020 Teva Respiratory, LLC. All rights reserved.
The Bluetooth® word mark and logos are registered trademarks owned by Bluetooth SIG, Inc. and any use of such marks by Teva Respiratory, LLC is under license.
United States Patent Nos. 6701917, 6718972, 6748947, 6871646, 7540282, 8006690, 8651103, 8714149, 8978966, 9216260, 9463288, 9616024, 9731087, 9782550, 9782551, 10022510, 10124131, 10195375.
ARMDH-001
Rev. February 2020

PATIENT INFORMATION
|
ARMONAIR® DIGIHALERTM (ar´ moe nayr di´ji haye´´ ler) (fluticasone propionate) inhalation powder 55 mcg ARMONAIR DIGIHALER (ar´ moe nayr di´ji haye´´ ler) (fluticasone propionate) inhalation powder 113 mcg ARMONAIR DIGIHALER (ar´ moe nayr di´ji haye´´ ler) (fluticasone propionate) inhalation powder 232 mcg |
|
What is ARMONAIR DIGIHALER? ARMONAIR DIGIHALER is a prescription inhaled corticosteroid (ICS) medicine for the long-term treatment of asthma in people aged 12 years and older.
|
|
Do not use ARMONAIR DIGIHALER:
|
|
Before using ARMONAIR DIGIHALER, tell your healthcare provider about all of your medical conditions, including if you:
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. ARMONAIR DIGIHALER and certain other medicines may affect each other causing serious side effects. Especially tell your healthcare provider if you take antifungal or anti-HIV medicines. Know the medicines you take. Keep a list of them to show to your healthcare provider and pharmacist when you get a new medicine. |
|
How should I use ARMONAIR DIGIHALER? Read the step-by-step instructions for using ARMONAIR DIGIHALER at the end of this Patient Information leaflet.
|
|
What are the possible side effects with ARMONAIR DIGIHALER? ARMONAIR DIGIHALER can cause serious side effects, including:
Common side effects of ARMONAIR DIGIHALER include:
These are not all the possible side effects with ARMONAIR DIGIHALER. Call your healthcare provider for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088. |
|
How should I store ARMONAIR DIGIHALER?
|
|
General information about the safe and effective use of ARMONAIR DIGIHALER. Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use ARMONAIR DIGIHALER for a condition for which it was not prescribed. Do not give your ARMONAIR DIGIHALER to other people, even if they have the same symptoms that you have. It may harm them. You can ask your pharmacist or healthcare provider for information about ARMONAIR DIGIHALER that was written for health professionals. |
|
What are the ingredients in ARMONAIR DIGIHALER?
Active ingredient: fluticasone propionate The Bluetooth® word mark and logos are registered trademarks owned by the Bluetooth Distributed by: Teva Pharmaceuticals USA, Inc. Parsippany, NJ 07054 ©2020 Teva Respiratory, LLC. All rights reserved. ARMDHPL-001 Revised: 02/2020  For more information, go to www.ARMONAIRDIGIHALER.com or call 1-888-603-0788. |
This Patient Information has been approved by the U.S. Food and Drug Administration.
Instructions for Use
ARMONAIR® DIGIHALERTM (ar´ moe nayr di´ji haye´´ ler)
(fluticasone propionate) inhalation powder 55 mcg
ARMONAIR DIGIHALER (ar´ moe nayr di´ji haye´´ ler)
(fluticasone propionate) inhalation powder 113 mcg
ARMONAIR DIGIHALER (ar´ moe nayr di´ji haye´´ ler)
(fluticasone propionate) inhalation powder 232 mcg
for oral inhalation use
Your ARMONAIR DIGIHALER Inhaler
When you are ready to use ARMONAIR DIGIHALER for the first time, remove the ARMONAIR DIGIHALER inhaler from the foil pouch.
There are 3 main parts of your ARMONAIR DIGIHALER inhaler including the:
- white inhaler with the mouthpiece. See Figure A.
- green cap that covers the mouthpiece of the inhaler. See Figure A.
- electronic module. See Figure A.
There is an electronic module built into the top of the inhaler that records and stores information about inhaler events. The electronic module sends information through Bluetooth® wireless technology to a mobile application (App). The electronic module does not control or interfere with delivery of the medicine through the inhaler.
There is a dose counter in the back of the inhaler with a viewing window that shows you how many doses of medicine you have left. See Figure A.

Figure A
- Your ARMONAIR DIGIHALER inhaler contains 60 doses (inhalations). See Figure B.
- The dose counter shows the number of doses you have left in your inhaler.
- When there are 20 doses left, the color of the numbers on the dose counter will change to red and you should refill your prescription or ask your healthcare provider for another prescription.
- When the dose counter displays ‘0’ your inhaler is empty and you should stop using the inhaler and throw it away. See Figure B.

Figure B
Important:
- Always close the cap after each inhalation so your inhaler will be ready for you to take your next dose. Do not open the cap unless you are ready for your next dose.
- You will hear a “click” sound when the cap is opened fully. If you do not hear the “click” sound the inhaler may not be activated to give you a dose of medicine.
- ARMONAIR DIGIHALER does not have an activation button or medicine canister. When you open the cap, a dose of ARMONAIR DIGIHALER will be activated for delivery of the medicine.
- ARMONAIR DIGIHALER does not need to be wirelessly connected to the mobile application (App) in order for it to work and for you to take your medicine.
- Do not use a spacer or volume holding chamber with ARMONAIR DIGIHALER. ARMONAIR DIGIHALER does not need priming.
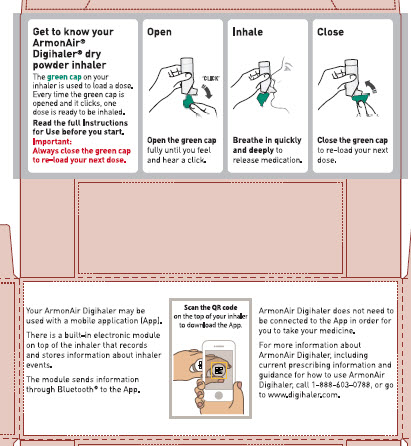
Using your ARMONAIR DIGIHALER inhaler
Important: Make sure the green cap is closed before you start using your inhaler.
Step 1. Open

Figure C
- Hold the inhaler upright and open the green cap all the way back until it “clicks”. See Figure C.
- Each time you open the green cap and it clicks, a dose of ARMONAIR DIGIHALER is ready to be inhaled.
- Do not open the green cap until you are ready to take a dose of ARMONAIR DIGIHALER.
Remember:
- For correct use of ARMONAIR DIGIHALER, hold the inhaler upright as you open the green cap. See Figure D.
- Do not hold the inhaler in any other way as you open the green cap.

Figure D
Step 2. Inhale

Figure E
- Before you inhale, breathe out through your mouth away from the inhaler and push as much air from your lungs as you can. See Figure E.
- Do not breathe out into the inhaler mouthpiece.

Figure F
- Put the mouthpiece in your mouth and close your lips tightly around it. See Figure F.
- Breathe in quickly and deeply through your mouth, to deliver the dose of medicine to your lungs.

Figure G
- Do not block the vent above the mouthpiece with your lips or fingers. See Figure G.
- Remove the inhaler from your mouth.
- Hold your breath for about 10 seconds or for as long as you comfortably can.
- Your ARMONAIR DIGIHALER inhaler delivers your dose of medicine as a very fine powder that you may or may not taste or feel. Do not take an extra dose from the inhaler even if you do not taste or feel the medicine.
Step 3. Close

Figure H
- Close the green cap after each inhalation so that the inhaler will be ready for your next dose. See Figure H.
- Rinse your mouth with water without swallowing after each inhalation.
How should I store ARMONAIR DIGIHALER?
-
Store ARMONAIR DIGIHALER at room temperature between 59ºF and 77ºF (15ºC and 25ºC).
-
Store the ARMONAIR DIGIHALER in a dry place. Avoid exposure to extreme heat, cold, or humidity.
-
Store ARMONAIR DIGIHALER in the unopened foil pouch and only open when ready for use.
-
Keep the green cap on the inhaler closed during storage.
-
Keep your ARMONAIR DIGIHALER inhaler dry and clean at all times.
-
Keep your ARMONAIR DIGIHALER inhaler and all medicines out of the reach of children.
Cleaning your ARMONAIR DIGIHALER inhaler
- Do not wash or put any part of your ARMONAIR DIGIHALER inhaler in water. Replace your inhaler if washed or placed in water.
- ARMONAIR DIGIHALER contains a powder and must be kept clean and dry at all times.
- You can clean the mouthpiece if needed using a dry cloth or tissue. Routine cleaning is not required.
- Do not take the ARMONAIR DIGIHALER inhaler apart.
Replacing your ARMONAIR DIGIHALER inhaler
- Immediately replace your inhaler if the mouthpiece cover is damaged or broken. Never take the inhaler apart.
- The counter on the back of your inhaler shows how many doses you have left.
- When there are 20 doses left, the color of the numbers on the dose counter will change to red and you should refill your prescription or ask your healthcare provider for another prescription.
- When the counter displays ‘0’ your ARMONAIR DIGIHALER inhaler is empty and you should stop using it.
- Throw away ARMONAIR DIGIHALER 30 days after removing it from the foil pouch for the first time, when the dose counter displays ‘0’, or after the expiration date on the package, whichever comes first.
- ARMONAIR DIGIHALER contains a lithium – manganese dioxide battery and should be thrown away (disposed of) in accordance with state and local regulations.
Important information
- Do not open the green cap unless you are taking a dose. Repeatedly opening and closing the cap without inhaling a dose will waste the medicine and may damage your inhaler.
- Your ARMONAIR DIGIHALER inhaler contains dry powder so it is important that you do not blow or breathe into it.
Support
- For instructions on setting up the App, go to www.ArmonAir.com or call Teva at 1-888-603-0788.
- If you have any questions about ARMONAIR DIGIHALER or how to use your inhaler, go to www.ArmonAirDigihaler.com, or call 1-888-603-0788.
This device complies with part 15 of the FCC Rules. Operation is subject to the following two conditions: (1) This device may not cause harmful interference, and (2) this device must accept any interference received, including interference that may cause undesired operation. Changes or modifications not expressly approved by Teva could void the user’s authority to operate the equipment.
These Instructions for Use have been approved by the U.S. Food and Drug Administration.
The Bluetooth® word mark and logos are registered trademarks owned by the Bluetooth
SIG, Inc. and any use of such marks by Teva Respiratory, LLC is under license.
Distributed by: Teva Pharmaceuticals USA, Inc. Parsippany, NJ 07054
©2020 Teva Respiratory, LLC. All rights reserved.

ARMDHIFU-001
Rev. 02/2020
NDC 59310-114-06 55 mcg

Trade Carton 55 mcg

55 mcg inner carton
NDC 59310-114-06
Rx Only
ArmonAir®
Digihaler® 55
(fluticasone propionate 55 mcg) Inhalation Powder
55 mcg
With Dose Counter
FOR ORAL INHALATION ONLY
60 METERED INHALATIONS
0.9 g NET CONTENTS
Refer to enclosed Patient Leaflet and Instructions for Use for detailed information on product use and handling.
Rev. 01/2022
NDC 59310-200-06 113 mcg

Carton 113 mcg

113 mcg inner carton
NDC 59310-200-06
Rx Only
ArmonAir®
Digihaler® 113
(fluticasone propionate 113 mcg) Inhalation Powder
113 mcg
With Dose Counter
FOR ORAL INHALATION ONLY
60 METERED INHALATIONS
0.9 g NET CONTENTS
Refer to enclosed Patient Leaflet and Instructions for Use for detailed information on product use and handling.
Rev 01/2022
NDC 59310-311-06 232 mcg

232 mcg carton

232 inner carton
NDC 59310-311-06
Rx Only
ArmonAir®
Digihaler® 232
(fluticasone propionate 232 mcg) Inhalation Powder
232 mcg
With Dose Counter
FOR ORAL INHALATION ONLY
60 METERED INHALATIONS
0.9 g NET CONTENTS
Refer to enclosed Patient Leaflet and Instructions for Use for detailed information on product use and handling.
Rev 01/2022
NDC 59310-515-08 113 mcg Sample

Sample Carton 113 mcg

Sample 113 mcg inner carton
NDC 59310-200-06
Rx Only
ArmonAir® Digihaler® 113
(fluticasone propionate 113 mcg) Inhalation Powder
SAMPLE NOT FOR SALE
113 mcg
With Dose Counter
FOR ORAL INHALATION ONLY
60 METERED INHALATIONS
0.9 g NET CONTENTS
Refer to enclosed Patient Leaflet and Instructions for Use for detailed information on product use and handling.
Rev 01/2022
NDC 59310-525-08 232 mcg Sample

Sample Carton 232 mcg

Inner Carton Sample 232 mcg
NDC 59310-311-06
Rx Only
ArmonAir® Digihaler® 232
(fluticasone propionate 232 mcg) Inhalation Powder
SAMPLE NOT FOR SALE
232 mcg
With Dose Counter
FOR ORAL INHALATION ONLY
60 METERED INHALATIONS
0.9 g NET CONTENTS
Refer to enclosed Patient Leaflet and Instructions for Use for detailed information on product use and handling.
Rev 01/2022
INGREDIENTS AND APPEARANCE
| ARMONAIR DIGIHALER
fluticasone propionate powder, metered |
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
| ARMONAIR DIGIHALER
fluticasone propionate powder, metered |
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
| ARMONAIR DIGIHALER
fluticasone propionate powder, metered |
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
| ARMONAIR DIGIHALER
fluticasone propionate powder, metered |
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
| ARMONAIR DIGIHALER
fluticasone propionate powder, metered |
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
| ARMONAIR DIGIHALER
fluticasone propionate powder, metered |
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
| Labeler - Teva Respiratory, LLC (176697907) |