Search by Drug Name or NDC
NDC 59779-0787-15 CVS Health Sinus Relief 0.0005 g/mL Details
CVS Health Sinus Relief 0.0005 g/mL
CVS Health Sinus Relief is a NASAL SPRAY in the HUMAN OTC DRUG category. It is labeled and distributed by CVS Pharmacy,Inc.. The primary component is OXYMETAZOLINE HYDROCHLORIDE.
MedlinePlus Drug Summary
Oxymetazoline nasal spray is used to relieve nasal discomfort caused by colds, allergies, and hay fever. It is also used to relieve sinus congestion and pressure. Oxymetazoline nasal spray should not be used to treat children younger than 6 years of age unless it is recommended by a doctor. Children 6 to 12 years of age should use oxymetazoline nasal spray carefully and under adult supervision. Oxymetazoline is in a class of medications called nasal decongestants. It works by narrowing the blood vessels in the nasal passages.
Related Packages: 59779-0787-15Last Updated: 12/01/2022
MedLinePlus Full Drug Details: Oxymetazoline Nasal Spray
Product Information
| NDC | 59779-0787 |
|---|---|
| Product ID | 59779-787_3e15a1f9-c93e-4cdb-865c-68bfa63d3323 |
| Associated GPIs | |
| GCN Sequence Number | n/a |
| GCN Sequence Number Description | n/a |
| HIC3 | n/a |
| HIC3 Description | n/a |
| GCN | n/a |
| HICL Sequence Number | n/a |
| HICL Sequence Number Description | n/a |
| Brand/Generic | n/a |
| Proprietary Name | CVS Health Sinus Relief |
| Proprietary Name Suffix | n/a |
| Non-Proprietary Name | oxymetazoline hydrochloride |
| Product Type | HUMAN OTC DRUG |
| Dosage Form | SPRAY |
| Route | NASAL |
| Active Ingredient Strength | 0.0005 |
| Active Ingredient Units | g/mL |
| Substance Name | OXYMETAZOLINE HYDROCHLORIDE |
| Labeler Name | CVS Pharmacy,Inc. |
| Pharmaceutical Class | Imidazolines [CS], Increased Sympathetic Activity [PE], Vasoconstriction [PE], Vasoconstrictor [EPC] |
| DEA Schedule | n/a |
| Marketing Category | OTC MONOGRAPH FINAL |
| Application Number | part341 |
| Listing Certified Through | 2024-12-31 |
Package
Package Images
NDC 59779-0787-15 (59779078715)
| NDC Package Code | 59779-787-15 |
|---|---|
| Billing NDC | 59779078715 |
| Package | 1 BOTTLE, SPRAY in 1 CARTON (59779-787-15) / 15 mL in 1 BOTTLE, SPRAY |
| Marketing Start Date | 2015-04-21 |
| NDC Exclude Flag | N |
| Pricing Information | N/A |
Standard Product Labeling (SPL)/Prescribing Information SPL 3e15a1f9-c93e-4cdb-865c-68bfa63d3323 Details
Uses
Warnings
Ask a doctor before use if you have
- •
- heart disease
- •
- high blood pressure
- •
- thyroid disease
- •
- diabetes
- •
- trouble urinating due to enlarged prostate gland
Directions
Inactive ingredients
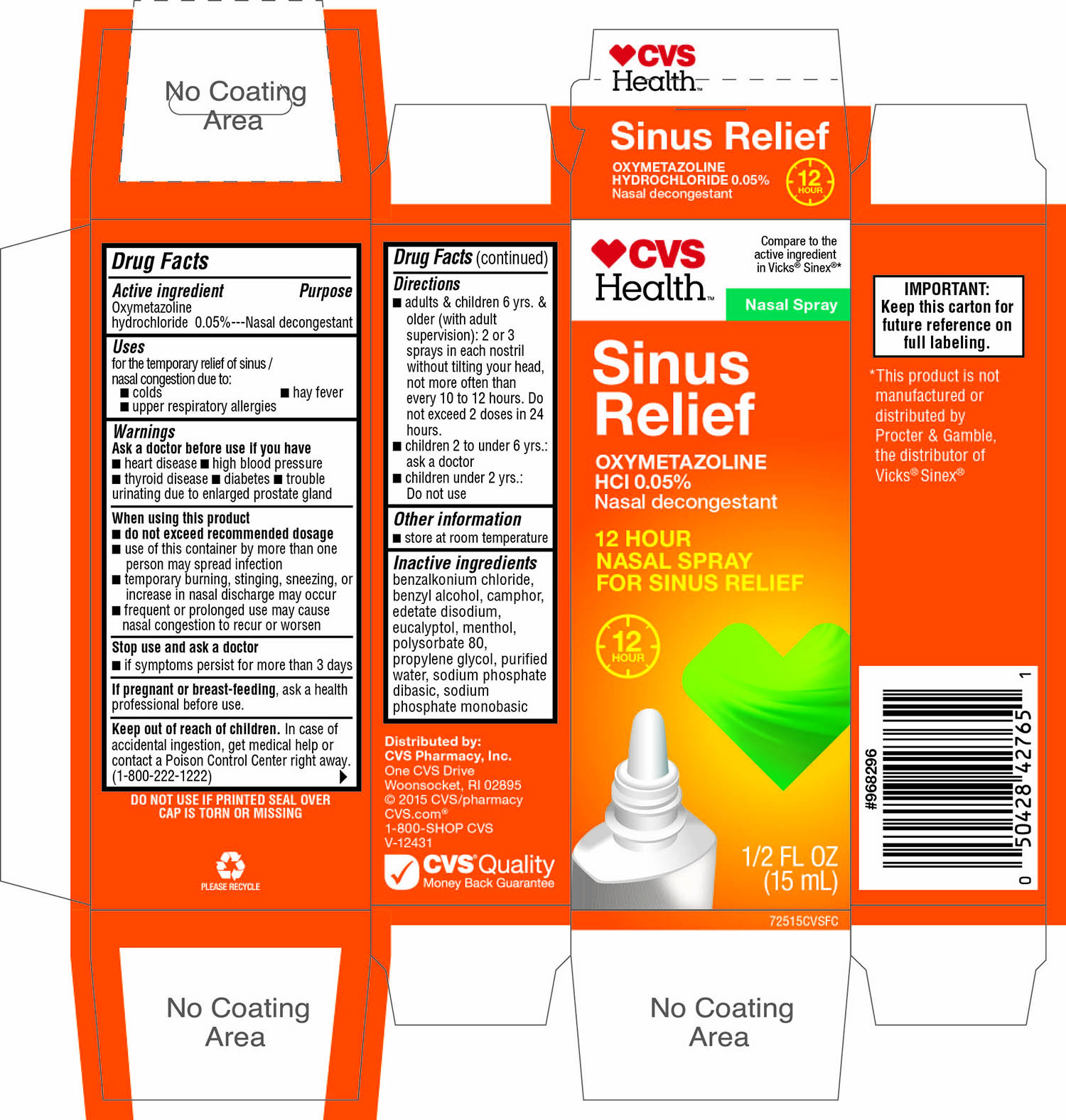
Principal Display Panel
CVS Health ™
Compare to the active ingredient in Vicks® Sinex® *
Nasal Spray
Sinus Relief
OXYMETAZOLINE HCl 0.05%
Nasal decongestant
12 HOUR
NASAL SPRAY FOR SINUS RELIEF
12 Hour
1/2 FL OZ (15 mL)
DO NOT USE IF PRINTED SEAL OVER CAP IS TORN OR MISSNG
IMPORTANT: Keep this carton for future reference on full labeling.
*This product is not manufactured or distributed by Procter & Gamble, the distributor of Vicks®Sinex®
Distributed by:
CVS Pharmacy, Inc.
One CVS Drive
Woonsocket, RI 02895
© 2015 CVS/pharmacy
CVS.com®
1-800-SHOP CVS
V-12431
CVS Quality
Money Back Guarantee
INGREDIENTS AND APPEARANCE
| CVS HEALTH SINUS RELIEF
oxymetazoline hydrochloride spray |
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
| Labeler - CVS Pharmacy,Inc. (062312574) |