Search by Drug Name or NDC
NDC 60687-0555-01 Clonazepam 1 mg/1 Details
Clonazepam 1 mg/1
Clonazepam is a ORAL TABLET in the HUMAN PRESCRIPTION DRUG category. It is labeled and distributed by American Health Packaging. The primary component is CLONAZEPAM.
MedlinePlus Drug Summary
Clonazepam is used alone or in combination with other medications to control certain types of seizures. It is also used to relieve panic attacks (sudden, unexpected attacks of extreme fear and worry about these attacks). Clonazepam is in a class of medications called benzodiazepines. It works by decreasing abnormal electrical activity in the brain.
Related Packages: 60687-0555-01Last Updated: 12/01/2022
MedLinePlus Full Drug Details: Clonazepam
Product Information
| NDC | 60687-0555 |
|---|---|
| Product ID | 60687-555_e41af6bd-28f1-d7f1-e053-2995a90a831e |
| Associated GPIs | 72100010000310 |
| GCN Sequence Number | 004561 |
| GCN Sequence Number Description | clonazepam TABLET 1 MG ORAL |
| HIC3 | H4A |
| HIC3 Description | ANTICONVULSANT - BENZODIAZEPINE TYPE |
| GCN | 17471 |
| HICL Sequence Number | 001894 |
| HICL Sequence Number Description | CLONAZEPAM |
| Brand/Generic | Generic |
| Proprietary Name | Clonazepam |
| Proprietary Name Suffix | n/a |
| Non-Proprietary Name | Clonazepam |
| Product Type | HUMAN PRESCRIPTION DRUG |
| Dosage Form | TABLET |
| Route | ORAL |
| Active Ingredient Strength | 1 |
| Active Ingredient Units | mg/1 |
| Substance Name | CLONAZEPAM |
| Labeler Name | American Health Packaging |
| Pharmaceutical Class | Benzodiazepine [EPC], Benzodiazepines [CS] |
| DEA Schedule | CIV |
| Marketing Category | ANDA |
| Application Number | ANDA074569 |
| Listing Certified Through | 2024-12-31 |
Package
Package Images




NDC 60687-0555-01 (60687055501)
| NDC Package Code | 60687-555-01 |
|---|---|
| Billing NDC | 60687055501 |
| Package | 100 BLISTER PACK in 1 BOX, UNIT-DOSE (60687-555-01) / 1 TABLET in 1 BLISTER PACK (60687-555-11) |
| Marketing Start Date | 2020-08-20 |
| NDC Exclude Flag | N |
| Pricing Information | |
| Price Per Unit | 0.03106 |
| Pricing Unit | EA |
| Effective Date | 2024-02-21 |
| NDC Description | CLONAZEPAM 1 MG TABLET |
| Pharmacy Type Indicator | C/I |
| OTC | N |
| Explanation Code | 1 |
| Classification for Rate Setting | G |
| As of Date | 2024-02-21 |
Standard Product Labeling (SPL)/Prescribing Information SPL cd7b7c4d-8dd1-4a0a-a506-a84407816bdf Details
WARNING
- Concomitant use of benzodiazepines and opioids may result in profound sedation, respiratory depression, coma, and death Reserve concomitant prescribing of these drugs for patients for whom alternative treatment options are inadequate. Limit dosages and durations to the minimum required. Follow patients for signs and symptoms of respiratory depression and sedation. (see WARNINGS and PRECAUTIONS).
- The use of benzodiazepines, including clonazepam tablets, exposes users to risks of abuse, misuse, and addiction, which can lead to overdose or death. Abuse and misuse of benzodiazepines commonly involve concomitant use of other medications, alcohol, and/or illicit substances, which is associated with an increased frequency of serious adverse outcomes. Before prescribing clonazepam tablets and throughout treatment, assess each patient’s risk for abuse, misuse, and addiction (see WARNINGS).
-
The continued use of benzodiazepines, including clonazepam tablets, may lead to clinically significant physical dependence. The risks of dependence and withdrawal increase with longer treatment duration and higher daily dose. Abrupt discontinuation or rapid dosage reduction of clonazepam tablets
after continued use may precipitate acute withdrawal reactions, which can be life-threatening. To reduce the risk of withdrawal reactions, use a gradual taper to discontinue clonazepam tablets or reduce the dosage (see DOSAGE AND ADMINISTRATION and WARNINGS).
DESCRIPTION
Each tablet, for oral administration, contains 0.5 mg, 1 mg, or 2 mg of clonazepam, USP, a benzodiazepine. Each tablet also contains corn starch, lactose monohydrate, magnesium stearate, microcrystalline cellulose, and povidone. Clonazepam tablets USP 0.5 mg contain Yellow D&C No. 10 Aluminum Lake. Clonazepam tablets USP 1 mg contain Yellow D&C No. 10 Aluminum Lake, as well as FD&C Blue No. 1 Aluminum Lake.
Chemically, clonazepam, USP is 5-( o-chlorophenyl)-1,3-dihydro-7-nitro-2 H-1,4-benzodiazepin-2-one. It is a light yellow crystalline powder. It has the following structural formula:

C 15H 10ClN 3O 3 M.W. 315.72
CLINICAL PHARMACOLOGY
Pharmacodynamics
The precise mechanism by which clonazepam exerts its antiseizure and antipanic effects is unknown, although it is believed to be related to its ability to enhance the activity of gamma aminobutyric acid (GABA), the major inhibitory neurotransmitter in the central nervous system.
Pharmacokinetics
Clonazepam is rapidly and completely absorbed after oral administration. The absolute bioavailability of clonazepam is about 90%. Maximum plasma concentrations of clonazepam are reached within 1 to 4 hours after oral administration. Clonazepam is approximately 85% bound to plasma proteins. Clonazepam is highly metabolized, with less than 2% unchanged clonazepam being excreted in the urine. Biotransformation occurs mainly by reduction of the 7-nitro group to the 4-amino derivative. This derivative can be acetylated, hydroxylated and glucuronidated. Cytochrome P-450 including CYP3A, may play an important role in clonazepam reduction and oxidation. The elimination half-life of clonazepam is typically 30 to 40 hours. Clonazepam pharmacokinetics are dose-independent throughout the dosing range. There is no evidence that clonazepam induces its own metabolism or that of other drugs in humans.
Pharmacokinetics in Demographic Subpopulations and in Disease States
Controlled studies examining the influence of gender and age on clonazepam pharmacokinetics have not been conducted, nor have the effects of renal or liver disease on clonazepam pharmacokinetics been studied. Because clonazepam undergoes hepatic metabolism, it is possible that liver disease will impair clonazepam elimination. Thus, caution should be exercised when administering clonazepam to these patients (see CONTRAINDICATIONS).
In children, clearance values of 0.42 ± 0.32 mL/min/kg (ages 2 to 18 years) and 0.88 ± 0.4 mL/min/kg (ages 7 to 12 years) were reported; these values decreased with increasing body weight. Ketogenic diet in children does not affect clonazepam concentrations.
Clinical Trials
Panic Disorder
The effectiveness of clonazepam in the treatment of panic disorder was demonstrated in two double-blind, placebo-controlled studies of adult outpatients who had a primary diagnosis of panic disorder (DSM-IIIR) with or without agoraphobia. In these studies, clonazepam was shown to be significantly more effective than placebo in treating panic disorder on change from baseline in panic attack frequency, the Clinician’s Global Impression Severity of Illness Score and the Clinician’s Global Impression Improvement Score.
Study 1 was a 9-week, fixed-dose study involving clonazepam doses of 0.5, 1, 2, 3 or 4 mg/day or placebo. This study was conducted in four phases: a 1-week placebo lead-in, a 3-week upward titration, a 6-week fixed dose, and a 7-week discontinuance phase. A significant difference from placebo was observed consistently only for the 1 mg/day group. The difference between the 1 mg dose group and placebo in reduction from baseline in the number of full panic attacks was approximately 1 panic attack per week. At endpoint, 74% of patients receiving clonazepam 1 mg/day were free of full panic attacks, compared to 56% of placebo-treated patients.
Study 2 was a 6-week, flexible-dose study involving clonazepam in a dose range of 0.5 to 4 mg/day or placebo. This study was conducted in three phases: a 1-week placebo lead-in, a 6-week optimal-dose, and a 6-week discontinuance phase. The mean clonazepam dose during the optimal dosing period was 2.3 mg/day. The difference between clonazepam and placebo in reduction from baseline in the number of full panic attacks was approximately 1 panic attack per week. At endpoint, 62% of patients receiving clonazepam were free of full panic attacks, compared to 37% of placebo-treated patients.
Subgroup analyses did not indicate that there were any differences in treatment outcomes as a function of race or gender.
INDICATIONS AND USAGE
Seizure Disorders
Clonazepam tablets are useful alone or as an adjunct in the treatment of the Lennox-Gastaut syndrome (petit mal variant), akinetic, and myoclonic seizures. In patients with absence seizures (petit mal) who have failed to respond to succinimides, clonazepam tablets may be useful.
Some loss of effect may occur during the course of clonazepam treatment (see PRECAUTIONS: Loss of Effect).
Panic Disorder
Clonazepam tablets are indicated for the treatment of panic disorder, with or without agoraphobia, as defined in DSM-V. Panic disorder is characterized by the occurrence of unexpected panic attacks and associated concern about having additional attacks, worry about the implications or consequences of the attacks, and/or a significant change in behavior related to the attacks.
The efficacy of clonazepam tablets was established in two 6 to 9-week trials in panic disorder patients whose diagnoses corresponded to the DSM-IIIR category of panic disorder (see CLINICAL PHARMACOLOGY, Clinical Trials).
Panic disorder (DSM-V) is characterized by recurrent unexpected panic attacks, i.e., a discrete period of intense fear or discomfort in which four (or more) of the following symptoms develop abruptly and reach a peak within 10 minutes: (1) palpitations, pounding heart or accelerated heart rate; (2) sweating; (3) trembling or shaking; (4) sensations of shortness of breath or smothering; (5) feeling of choking; (6) chest pain or discomfort; (7) nausea or abdominal distress; (8) feeling dizzy, unsteady, lightheaded or faint; (9) derealization (feelings of unreality) or depersonalization (being detached from oneself); (10) fear of losing control; (11) fear of dying; (12) paresthesias (numbness or tingling sensations); (13) chills or hot flushes.
The effectiveness of clonazepam tablets in long-term use, that is, for more than 9 weeks, has not been systematically studied in controlled clinical trials. The physician who elects to use clonazepam tablets for extended periods should periodically reevaluate the long-term usefulness of the drug for the individual patient (see DOSAGE AND ADMINISTRATION).
CONTRAINDICATIONS
WARNINGS
Risks from Concomitant Use with Opioids
Concomitant use of benzodiazepines, including clonazepam, and opioids may result in profound sedation, respiratory depression, coma, and death. Because of these risks, reserve concomitant prescribing of benzodiazepines and opioids for patients for whom alternative treatment options are inadequate.
Observational studies have demonstrated that concomitant use of opioid analgesics and benzodiazepines increases the risk of drug-related mortality compared to use of opioids alone. If a decision is made to prescribe clonazepam concomitantly with opioids, prescribe the lowest effective dosages and minimum durations of concomitant use, and follow patients closely for signs and symptoms of respiratory depression and sedation. Advise both patients and caregivers about the risks of respiratory depression and sedation when clonazepam is used with opioids (see PRECAUTIONS, Information for Patients and PRECAUTIONS, Drug Interactions).
Abuse, Misuse, and Addiction
The use of benzodiazepines, including clonazepam tablets, exposes users to the risks of abuse, misuse, and addiction, which can lead to overdose or death. Abuse and misuse of benzodiazepines often (but not always) involve the use of doses greater than the maximum recommended dosage and commonly involve concomitant use of other medications, alcohol, and/or illicit substances, which is associated with an increased frequency of serious adverse outcomes, including respiratory depression, overdose, or death (see DRUG ABUSE AND DEPENDENCE: Abuse).
Before prescribing clonazepam tablets and throughout treatment, assess each patient’s risk for abuse, misuse, and addiction (e.g., using a standardized screening tool). Use of clonazepam tablets, particularly in patients at elevated risk, necessitates counseling about the risks and proper use of clonazepam tablets along with monitoring for signs and symptoms of abuse, misuse, and addiction. Prescribe the lowest effective dosage; avoid or minimize concomitant use of CNS depressants and other substances associated with abuse, misuse, and addiction (e.g., opioid analgesics, stimulants); and advise patients on the proper disposal of unused drug. If a substance use disorder is suspected, evaluate the patient and institute (or refer them for) early treatment, as appropriate.
Dependence and Withdrawal Reactions
To reduce the risk of withdrawal reactions, use a gradual taper to discontinue clonazepam tablets or reduce the dosage (a patient-specific plan should be used to taper the dose) (see DOSAGE AND ADMINISTRATION: Discontinuation or Dosage Reduction of clonazepam tablets).
Patients at an increased risk of withdrawal adverse reactions after benzodiazepine discontinuation or rapid dosage reduction include those who take higher dosages, and those who have had longer durations of use.
Acute Withdrawal Reactions
The continued use of benzodiazepines, including clonazepam tablets, may lead to clinically significant physical dependence. Abrupt discontinuation or rapid dosage reduction of clonazepam tablets after continued use, or administration of flumazenil (a benzodiazepine antagonist) may precipitate acute withdrawal reactions, which can be life-threatening (e.g., seizures)
(see
DRUG ABUSE AND DEPENDENCE: Dependence).
Protracted Withdrawal Syndrome
In some cases, benzodiazepine users have developed a protracted withdrawal syndrome with withdrawal symptoms lasting weeks to more than 12 months
(see
DRUG ABUSE AND DEPENDENCE: Dependence).
Interference with Cognitive and Motor Performance
Since clonazepam produces CNS depression, patients receiving this drug should be cautioned against engaging in hazardous occupations requiring mental alertness, such as operating machinery or driving a motor vehicle. They should also be warned about the concomitant use of alcohol or other CNS-depressant drugs during clonazepam therapy (see PRECAUTIONS: Drug Interactions and PRECAUTIONS: Information for Patients).
Suicidal Behavior and Ideation
Antiepileptic drugs (AEDs), including clonazepam, increase the risk of suicidal thoughts or behavior in patients taking these drugs for any indication. Patients treated with any AED for any indication should be monitored for the emergence or worsening of depression, suicidal thoughts or behavior, and/or any unusual changes in mood or behavior.
Pooled analyses of 199 placebo-controlled clinical trials (mono- and adjunctive therapy) of 11 different AEDs showed that patients randomized to one of the AEDs had approximately twice the risk (adjusted Relative Risk 1.8, 95% CI:1.2, 2.7) of suicidal thinking or behavior compared to patients randomized to placebo. In these trials, which had a median treatment duration of 12 weeks, the estimated incidence rate of suicidal behavior or ideation among 27,863 AED-treated patients was 0.43% compared to 0.24% among 16,029 placebo-treated patients, representing an increase of approximately one case of suicidal thinking or behavior for every 530 patients treated. There were four suicides in drug-treated patients in the trials and none in placebo-treated patients, but the number is too small to allow any conclusion about drug effect on suicide.
The increased risk of suicidal thoughts or behavior with AEDs was observed as early as one week after starting drug treatment with AEDs and persisted for the duration of treatment assessed. Because most trials included in the analysis did not extend beyond 24 weeks, the risk of suicidal thoughts or behavior beyond 24 weeks could not be assessed.
The risk of suicidal thoughts or behavior was generally consistent among drugs in the data analyzed. The finding of increased risk with AEDs of varying mechanisms of action and across a range of indications suggests that the risk applies to all AEDs used for any indication. The risk did not vary substantially by age (5 to 100 years) in the clinical trials analyzed.
Table 1 shows absolute and relative risk by indication for all evaluated AEDs.
Table 1: Risk by Indication for Antiepileptic Drugs in the Pooled Analysis
|
Indication |
Placebo Patients with Events Per 1000 Patients |
Drug Patients with Events Per 1000 Patients |
Relative Risk: Incidence of Events in Drug Patients/Incidence in Placebo Patients |
Risk Difference: Additional Drug Patients with Events per 1000 Patients |
|
Epilepsy |
1.0 |
3.4 |
3.5 |
2.4 |
|
Psychiatric |
5.7 |
8.5 |
1.5 |
2.9 |
|
Other |
1.0 |
1.8 |
1.9 |
0.9 |
|
Total |
2.4 |
4.3 |
1.8 |
1.9 |
The relative risk for suicidal thoughts or behavior was higher in clinical trials for epilepsy than in clinical trials for psychiatric or other conditions, but the absolute risk differences were similar for the epilepsy and psychiatric indications.
Anyone considering prescribing clonazepam or any other AED must balance the risk of suicidal thoughts or behavior with the risk of untreated illness. Epilepsy and many other illnesses for which AEDs are prescribed are themselves associated with morbidity and mortality and with an increased risk of suicidal thoughts and behavior. Should suicidal thoughts and behavior emerge during treatment, the prescriber needs to consider whether the emergence of these symptoms in any given patient may be related to the illness being treated.
Patients, their caregivers, and families should be informed that AEDs increase the risk of suicidal thoughts and behavior and should be advised of the need to be alert for the emergence or worsening of the signs and symptoms of depression, any unusual changes in mood or behavior, or the emergence of suicidal thoughts, behavior, or thoughts about self-harm. Behaviors of concern should be reported immediately to healthcare providers.
PRECAUTIONS
General
Worsening of Seizures
When used in patients in whom several different types of seizure disorders coexist, clonazepam may increase the incidence or precipitate the onset of generalized tonic-clonic seizures (grand mal). This may require the addition of appropriate anticonvulsants or an increase in their dosages. The concomitant use of valproic acid and clonazepam may produce absence status.
Loss of Effect
In some studies, up to 30% of patients who initially responded have shown a loss of anticonvulsant activity, often within 3 months of administration. In some cases, dosage adjustment may reestablish efficacy.
Laboratory Testing During Long-Term Therapy
Periodic blood counts and liver function tests are advisable during long-term therapy with clonazepam.
Psychiatric and Paradoxical Reactions
Paradoxical reactions, such as agitation, irritability, aggression, anxiety, anger, nightmares, hallucinations, and psychoses are known to occur when using benzodiazepines
(see
ADVERSE REACTIONS: Psychiatric).
Should this occur, the use of the drug should be discontinued gradually
(see
WARNINGS: Dependence and Withdrawal Reactions and
DRUG ABUSE AND DEPENDENCE: Dependence).
Paradoxical reactions are more likely to occur in children and in the elderly.
Caution in Renally Impaired Patients
Metabolites of clonazepam are excreted by the kidneys; to avoid their excess accumulation, caution should be exercised in the administration of the drug to patients with impaired renal function.
Hypersalivation
Clonazepam may produce an increase in salivation. This should be considered before giving the drug to patients who have difficulty handling secretions.
Respiratory Depression
Clonazepam may cause respiratory depression and should be used with caution in patients with compromised respiratory function (e.g., chronic obstructive pulmonary disease, sleep apnea).
Porphyria
Clonazepam may have a porphyrogenic effect and should be used with care in patients with porphyria.
Information for Patients
A clonazepam tablets Medication Guide must be given to the patient each time clonazepam tablets are dispensed, as required by law. Patients should be instructed to take clonazepam only as prescribed. Physicians are advised to discuss the following issues with patients for whom they prescribe clonazepam:
Risks from Concomitant Use with Opioids
Inform patients and caregivers that potentially fatal additive effects may occur if clonazepam is used with opioids and not to use such drugs concomitantly unless supervised by a health care provider
(see
WARNINGS, Risks from Concomitant Use With Opioids and
PRECAUTIONS, Drug Interactions).
Abuse, Misuse, and Addiction
Inform patients that the use of clonazepam tablets, even at recommended dosages, exposes users to risks of abuse, misuse, and addiction, which can lead to overdose and death, especially when used in combination with other medications (e.g., opioid analgesics), alcohol, and/or illicit substances. Inform patients about the signs and symptoms of benzodiazepine abuse, misuse, and addiction; to seek medical help if they develop these signs and/or symptoms; and on the proper disposal of unused drug
(see
WARNINGS: Abuse, Misuse, and Addiction and
DRUG ABUSE AND DEPENDENCE).
Withdrawal Reactions
Inform patients that the continued use of clonazepam tablets may lead to clinically significant physical dependence and that abrupt discontinuation or rapid dosage reduction of clonazepam tablets may precipitate acute withdrawal reactions, which can be life-threatening. Inform patients that in some cases, patients taking benzodiazepines have developed a protracted withdrawal syndrome with withdrawal symptoms lasting weeks to more than 12 months. Instruct patients that discontinuation or dosage reduction of clonazepam tablets may require a slow taper
(see
WARNINGS: Dependence and Withdrawal Reactions and
DRUG ABUSE AND DEPENDENCE).
Interference With Cognitive and Motor Performance
Because benzodiazepines have the potential to impair judgment, thinking or motor skills, patients should be cautioned about operating hazardous machinery, including automobiles, until they are reasonably certain that clonazepam therapy does not affect them adversely.
Suicidal Thinking and Behavior
Patients, their caregivers, and families should be counseled that AEDs, including clonazepam, may increase the risk of suicidal thoughts and behavior and should be advised of the need to be alert for the emergence or worsening of symptoms of depression, any unusual changes in mood or behavior, or the emergence of suicidal thoughts, behavior, or thoughts about self-harm. Behaviors of concern should be reported immediately to healthcare providers.
Pregnancy
Patients should be advised to notify their physician if they become pregnant or intend to become pregnant during therapy with clonazepam
(see
PRECAUTIONS: Pregnancy).
Patients should be encouraged to enroll in the North American Antiepileptic Drug (NAAED) Pregnancy Registry if they become pregnant. This registry is collecting information about the safety of antiepileptic drugs during pregnancy. To enroll, patients can call the toll free number 1-888-233-2334
(see
PRECAUTIONS: Pregnancy).
Nursing
Patients should be advised to notify their physician if they are breastfeeding or intend to breastfeed during therapy.
Concomitant Medication
Patients should be advised to inform their physicians if they are taking, or plan to take, any prescription or over-the-counter drugs, since there is a potential for interactions.
Alcohol
Patients should be advised to avoid alcohol while taking clonazepam.
Drug Interaction
Effect of Concomitant Use of Benzodiazepines and Opioids
The concomitant use of benzodiazepines and opioids increases the risk of respiratory depression because of actions at different receptor sites in the CNS that control respiration. Benzodiazepines interact at GABA
A sites, and opioids interact primarily at mu receptors. When benzodiazepines and opioids are combined, the potential for benzodiazepines to significantly worsen opioid-related respiratory depression exists. Limit dosage and duration of concomitant use of benzodiazepines and opioids, and follow patients closely for respiratory depression and sedation.
Effect of Clonazepam on the Pharmacokinetics of Other Drugs
Clonazepam does not appear to alter the pharmacokinetics of carbamazepine or phenobarbital. Clonazepam has the potential to influence concentrations of phenytoin. Monitoring of phenytoin concentration is recommended when clonazepam is coadministrated with phenytoin. The effect of clonazepam on the metabolism of other drugs has not been investigated.
Effect of Other Drugs on the Pharmacokinetics of Clonazepam
Literature reports suggest that ranitidine, an agent that decreases stomach acidity, does not greatly alter clonazepam pharmacokinetics.
In a study in which the 2 mg clonazepam orally disintegrating tablet was administered with and without propantheline (an anticholinergic agent with multiple effects on the GI tract) to healthy volunteers, the AUC of clonazepam was 10% lower and the C max of clonazepam was 20% lower when the orally disintegrating tablet was given with propantheline compared to when it was given alone.
The selective serotonin reuptake inhibitors sertraline (weak CYP3A4 inducer) and fluoxetine (CYP2D6 inhibitor), and the anti-epileptic drug felbamate (CYP2C19 inhibitor and CYP3A4 inducer) do not affect the pharmacokinetics of clonazepam. Cytochrome P-450 inducers, such as phenytoin, carbamazepine, lamotrigine, and phenobarbital induce clonazepam metabolism, causing an approximately 38% decrease in plasma clonazepam levels. Although clinical studies have not been performed, based on the involvement of the cytochrome P-450 3A family in clonazepam metabolism, inhibitors of this enzyme system, notably oral antifungal agents (e.g., fluconazole), should be used cautiously in patients receiving clonazepam because they may impair the metabolism of clonazepam leading to exaggerated concentrations and effects.
Pharmacodynamic Interactions
The CNS-depressant action of the benzodiazepine class of drugs may be potentiated by alcohol, narcotics, barbiturates, nonbarbiturate hypnotics, antianxiety agents, the phenothiazines, thioxanthene and butyrophenone classes of antipsychotic agents, monoamine oxidase inhibitors and the tricyclic antidepressants, and by other anticonvulsant drugs.
Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis
Carcinogenicity studies have not been conducted with clonazepam.
Mutagenesis
The data currently available are not sufficient to determine the genotoxic potential of clonazepam.
Impairment of Fertility
In a two-generation fertility study in which clonazepam was given orally to rats at 10 and 100 mg/kg/day, there was a decrease in the number of pregnancies and in the number of offspring surviving until weaning. The lowest dose tested is approximately 5 and 24 times the maximum recommended human dose (MRHD) of 20 mg/day for seizure disorders and 4 mg/day for panic disorder, respectively, on a body surface area (mg/m
2) basis.
Pregnancy
There are no adequate and well-controlled studies of clonazepam in pregnant women. Available human data on the risk of teratogenicity are inconclusive. There is insufficient evidence in humans to assess the effect of benzodiazepine exposure during pregnancy on neurodevelopment. Administration of benzodiazepines immediately prior to or during childbirth can result in a syndrome of hypothermia, hypotonia, respiratory depression, and difficulty feeding. In addition, infants born to mothers who have taken benzodiazepines during the later stages of pregnancy can develop dependence, and subsequently withdrawal, during the postnatal period.
In three studies in which clonazepam was administered orally to pregnant rabbits at doses of 0.2, 1, 5, or 10 mg/kg/day during the period of organogenesis, a similar pattern of malformations (cleft palate, open eyelid, fused sternebrae and limb defects) was observed at all doses, in a low, non-dose-related incidence. The lowest dose tested is less than the maximum recommended human dose (MRHD) of 20 mg/day for seizure disorders and similar to the MRHD of 4 mg/day for panic disorder, on a mg/m 2 basis. Reductions in maternal weight gain occurred at doses of 5 mg/kg/day or greater and reduction in embryofetal growth occurred in one study at a dose of 10 mg/kg/day.
No adverse maternal or embryofetal effects were observed in mice or rats following oral administration of clonazepam during organogenesis of doses up to 15 or 40 mg/kg/day, respectively (4 and 20 times the MRHD of 20 mg/day for seizure disorders and 20 and 100 times the MRHD of 4 mg/day for panic disorder, respectively, on a mg/m 2 basis).
Data for other benzodiazepines suggest the possibility of adverse developmental effects (long-term effects on neurobehavioral and immunological function) in animals following prenatal exposure to benzodiazepines.
To provide information regarding the effects of in utero exposure to clonazepam, physicians are advised to recommend that pregnant patients taking clonazepam enroll in the NAAED Pregnancy Registry. This can be done by calling the toll free number 1-888-233-2334, and must be done by patients themselves. Information on this registry can also be found at the website http://www.aedpregnancyregistry.org/.
Labor and Delivery
The effect of clonazepam on labor and delivery in humans has not been specifically studied; however, perinatal complications have been reported in children born to mothers who have been receiving benzodiazepines late in pregnancy, including findings suggestive of either excess benzodiazepine exposure or of withdrawal phenomena (see PRECAUTIONS: Pregnancy).
Nursing Mothers
The effects of clonazepam on the breastfed infant and on milk production are unknown. The developmental and health benefits of breastfeeding should be considered along with the mother's clinical need for clonazepam and any potential adverse effects on the breastfed infant from clonazepam or from the underlying maternal condition.
Pediatric Use
Because of the possibility that adverse effects on physical or mental development could become apparent only after many years, a benefit-risk consideration of the long-term use of clonazepam is important in pediatric patients being treated for seizure disorder (see INDICATIONS AND USAGEand DOSAGE AND ADMINISTRATION).
Safety and effectiveness in pediatric patients with panic disorder below the age of 18 have not been established.
Geriatric Use
Clinical studies of clonazepam did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy.
Because clonazepam undergoes hepatic metabolism, it is possible that liver disease will impair clonazepam elimination. Metabolites of clonazepam are excreted by the kidneys; to avoid their excess accumulation, caution should be exercised in the administration of the drug to patients with impaired renal function. Because elderly patients are more likely to have decreased hepatic and/or renal function, care should be taken in dose selection, and it may be useful to assess hepatic and/or renal function at the time of dose selection.
Sedating drugs may cause confusion and over-sedation in the elderly; elderly patients generally should be started on low doses of clonazepam and observed closely
ADVERSE REACTIONS
The adverse experiences for clonazepam are provided separately for patients with seizure disorders and with panic disorder.
Seizure Disorders
The most frequently occurring side effects of clonazepam are referable to CNS depression. Experience in treatment of seizures has shown that drowsiness has occurred in approximately 50% of patients and ataxia in approximately 30%. In some cases, these may diminish with time; behavior problems have been noted in approximately 25% of patients. Others, listed by system, including those identified during postapproval use of clonazepam are:
Cardiovascular: Palpitations
Dermatologic: Hair loss, hirsutism, skin rash, ankle and facial edema
Gastrointestinal: Anorexia, coated tongue, constipation, diarrhea, dry mouth, encopresis, gastritis, increased appetite, nausea, sore gums
Genitourinary: Dysuria, enuresis, nocturia, urinary retention
Hematopoietic: Anemia, leukopenia, thrombocytopenia, eosinophilia
Hepatic: Hepatomegaly, transient elevations of serum transaminases and alkaline phosphatase
Musculoskeletal: Muscle weakness, pains
Miscellaneous: Dehydration, general deterioration, fever, lymphadenopathy, weight loss or gain
Neurologic: Abnormal eye movements, aphonia, choreiform movements, coma, diplopia, dysarthria, dysdiadochokinesis, “glassy-eyed” appearance, headache, hemiparesis, hypotonia, nystagmus, respiratory depression, slurred speech, tremor, vertigo
Psychiatric: Confusion, depression, amnesia, hysteria, increased libido, insomnia, psychosis (the behavior effects are more likely to occur in patients with a history of psychiatric disturbances).
The following paradoxical reactions have been observed: irritability, aggression, agitation, nervousness, hostility, anxiety, sleep disturbances, nightmares, abnormal dreams, hallucinations.
Respiratory: Chest congestion, rhinorrhea, shortness of breath, hypersecretion in upper respiratory passages
Panic Disorder
Adverse events during exposure to clonazepam were obtained by spontaneous report and recorded by clinical investigators using terminology of their own choosing. Consequently, it is not possible to provide a meaningful estimate of the proportion of individuals experiencing adverse events without first grouping similar types of events into a smaller number of standardized event categories. In the tables and tabulations that follow, CIGY dictionary terminology has been used to classify reported adverse events, except in certain cases in which redundant terms were collapsed into more meaningful terms, as noted below.
The stated frequencies of adverse events represent the proportion of individuals who experienced, at least once, a treatment-emergent adverse event of the type listed. An event was considered treatment-emergent if it occurred for the first time or worsened while receiving therapy following baseline evaluation.
Adverse Findings Observed in Short-Term, Placebo-Controlled Trials
Adverse Events Associated with Discontinuation of Treatment
Overall, the incidence of discontinuation due to adverse events was 17% in clonazepam compared to 9% for placebo in the combined data of two 6-to 9-week trials. The most common events (≥ 1%) associated with discontinuation and a dropout rate twice or greater for clonazepam than that of placebo included the following:
|
Adverse Event |
Clonazepam (N = 574) |
Placebo (N = 294) |
|
Somnolence |
7% |
1% |
|
Depression |
4% |
1% |
|
Dizziness |
1% |
< 1% |
|
Nervousness |
1% |
0% |
|
Ataxia |
1% |
0% |
|
Intellectual Ability Reduced |
1% |
0% |
Adverse Events Occurring at an Incidence of 1% or More among Clonazepam-Treated Patients
Table 3 enumerates the incidence, rounded to the nearest percent, of treatment-emergent adverse events that occurred during acute therapy of panic disorder from a pool of two 6-to 9-week trials. Events reported in 1% or more of patients treated with clonazepam (doses ranging from 0.5 to 4 mg/day) and for which the incidence was greater than that in placebo-treated patients are included.
The prescriber should be aware that the figures in Table 3 cannot be used to predict the incidence of side effects in the course of usual medical practice where patient characteristics and other factors differ from those that prevailed in the clinical trials. Similarly, the cited frequencies cannot be compared with figures obtained from other clinical investigations involving different treatments, uses and investigators. The cited figures, however, do provide the prescribing physician with some basis for estimating the relative contribution of drug and nondrug factors to the side effect incidence in the population studied.
|
||||||
|
Clonazepam Maximum Daily Dose | ||||||
|
Adverse Event by Body System |
< 1 mg n = 96 % |
1 to < 2 mg n = 129 % |
2 to < 3 mg n = 113 % |
≥ 3 mg n = 235 % |
All Clonazepam Groups N = 574 % |
Placebo N = 294 % |
|
Central & Peripheral Nervous System | ||||||
|
Somnolence † |
26 |
35 |
50 |
36 |
37 |
10 |
|
Dizziness |
5 |
5 |
12 |
8 |
8 |
4 |
|
Coordination Abnormal † |
1 |
2 |
7 |
9 |
6 |
0 |
|
Ataxia † |
2 |
1 |
8 |
8 |
5 |
0 |
|
Dysarthria † |
0 |
0 |
4 |
3 |
2 |
0 |
|
Psychiatric | ||||||
|
Depression |
7 |
6 |
8 |
8 |
7 |
1 |
|
Memory Disturbance |
2 |
5 |
2 |
5 |
4 |
2 |
|
Nervousness |
1 |
4 |
3 |
4 |
3 |
2 |
|
Intellectual Ability Reduced |
0 |
2 |
4 |
3 |
2 |
0 |
|
Emotional Lability |
0 |
1 |
2 |
2 |
1 |
1 |
|
Libido Decreased |
0 |
1 |
3 |
1 |
1 |
0 |
|
Confusion |
0 |
2 |
2 |
1 |
1 |
0 |
|
Respiratory System | ||||||
|
Upper Respiratory Tract Infection † |
10 |
10 |
7 |
6 |
8 |
4 |
|
Sinusitis |
4 |
2 |
8 |
4 |
4 |
3 |
|
Rhinitis |
3 |
2 |
4 |
2 |
2 |
1 |
|
Coughing |
2 |
2 |
4 |
0 |
2 |
0 |
|
Pharyngitis |
1 |
1 |
3 |
2 |
2 |
1 |
|
Bronchitis |
1 |
0 |
2 |
2 |
1 |
1 |
|
Gastrointestinal System | ||||||
|
Constipation † |
0 |
1 |
5 |
3 |
2 |
2 |
|
Appetite Decreased |
1 |
1 |
0 |
3 |
1 |
1 |
|
Abdominal Pain † |
2 |
2 |
2 |
0 |
1 |
1 |
|
Body as a Whole | ||||||
|
Fatigue |
9 |
6 |
7 |
7 |
7 |
4 |
|
Allergic Reaction |
3 |
1 |
4 |
2 |
2 |
1 |
|
Musculoskeletal | ||||||
|
Myalgia |
2 |
1 |
4 |
0 |
1 |
1 |
|
Resistance Mechanism Disorders | ||||||
|
Influenza |
3 |
2 |
5 |
5 |
4 |
3 |
|
Urinary System | ||||||
|
Micturition Frequency |
1 |
2 |
2 |
1 |
1 |
0 |
|
Urinary Tract Infection † |
0 |
0 |
2 |
2 |
1 |
0 |
|
Vision Disorders | ||||||
|
Blurred Vision |
1 |
2 |
3 |
0 |
1 |
1 |
|
Reproductive Disorders‡ | ||||||
|
Female | ||||||
|
Dysmenorrhea |
0 |
6 |
5 |
2 |
3 |
2 |
|
Colpitis |
4 |
0 |
2 |
1 |
1 |
1 |
|
Male | ||||||
|
Ejaculation Delayed |
0 |
0 |
2 |
2 |
1 |
0 |
|
Impotence |
3 |
0 |
2 |
1 |
1 |
0 |
Commonly Observed Adverse Events
|
||
|
Adverse Event |
Clonazepam
|
Placebo
|
|
Somnolence |
37% |
10% |
|
Depression |
7% |
1% |
|
Coordination Abnormal |
6% |
0% |
|
Ataxia |
5% |
0% |
Treatment-Emergent Depressive Symptoms
In the pool of two short-term placebo-controlled trials, adverse events classified under the preferred term “depression” were reported in 7% of clonazepam-treated patients compared to 1% of placebo-treated patients, without any clear pattern of dose relatedness. In these same trials, adverse events classified under the preferred term “depression” were reported as leading to discontinuation in 4% of clonazepam-treated patients compared to 1% of placebo-treated patients. While these findings are noteworthy, Hamilton Depression Rating Scale (HAM-D) data collected in these trials revealed a larger decline in HAM-D scores in the clonazepam group than the placebo group suggesting that clonazepam-treated patients were not experiencing a worsening or emergence of clinical depression.
Other Adverse Events Observed During the Premarketing Evaluation of Clonazepam in Panic Disorder
Following is a list of modified CIGY terms that reflect treatment-emergent adverse events reported by patients treated with clonazepam at multiple doses during clinical trials. All reported events are included except those already listed in Table 3 or elsewhere in labeling, those events for which a drug cause was remote, those event terms which were so general as to be uninformative, and events reported only once and which did not have a substantial probability of being acutely life-threatening. It is important to emphasize that, although the events occurred during treatment with clonazepam, they were not necessarily caused by it.
Events are further categorized by body system and listed in order of decreasing frequency. These adverse events were reported infrequently, which is defined as occurring in 1/100 to 1/1000 patients.
Body as a Whole: weight increase, accident, weight decrease, wound, edema, fever, shivering, abrasions, ankle edema, edema foot, edema periorbital, injury, malaise, pain, cellulitis, inflammation localized
Cardiovascular Disorders: chest pain, hypotension postural
Central and Peripheral Nervous System Disorders: migraine, paresthesia, drunkenness, feeling of enuresis, paresis, tremor, burning skin, falling, head fullness, hoarseness, hyperactivity, hypoesthesia, tongue thick, twitching
Gastrointestinal System Disorders: abdominal discomfort, gastrointestinal inflammation, stomach upset, toothache, flatulence, pyrosis, saliva increased, tooth disorder, bowel movements frequent, pain pelvic, dyspepsia, hemorrhoids
Hearing and Vestibular Disorders: vertigo, otitis, earache, motion sickness
Heart Rate and Rhythm Disorders: palpitation
Metabolic and Nutritional Disorders: thirst, gout
Musculoskeletal System Disorders: back pain, fracture traumatic, sprains and strains, pain leg, pain nape, cramps muscle, cramps leg, pain ankle, pain shoulder, tendinitis, arthralgia, hypertonia, lumbago, pain feet, pain jaw, pain knee, swelling knee
Platelet, Bleeding and Clotting Disorders: bleeding dermal
Psychiatric Disorders: insomnia, organic disinhibition, anxiety, depersonalization, dreaming excessive, libido loss, appetite increased, libido increased, reactions decreased, aggression, apathy, disturbance in attention, excitement, anger, hunger abnormal, illusion, nightmares, sleep disorder, suicide ideation, yawning
Reproductive Disorders, Female: breast pain, menstrual irregularity
Reproductive Disorders, Male: ejaculation decreased
Resistance Mechanism Disorders: infection mycotic, infection viral, infection streptococcal, herpes simplex infection, infectious mononucleosis, moniliasis
Respiratory System Disorders: sneezing excessive, asthmatic attack, dyspnea, nosebleed, pneumonia, pleurisy
Skin and Appendages Disorders: acne flare, alopecia, xeroderma, dermatitis contact, flushing, pruritus, pustular reaction, skin burns, skin disorder
Special Senses Other, Disorders: taste loss
Urinary System Disorders: dysuria, cystitis, polyuria, urinary incontinence, bladder dysfunction, urinary retention, urinary tract bleeding, urine discoloration
Vascular (Extracardiac) Disorders: thrombophlebitis leg
Vision Disorders: eye irritation, visual disturbance, diplopia, eye twitching, styes, visual field defect, xerophthalmia
To report SUSPECTED ADVERSE REACTIONS, contact Teva at 1-888-838-2872 of FDA-1088 or http://www.fda.gov/medwatch.
DRUG ABUSE AND DEPENDENCE
Abuse
Clonazepam tablets is a benzodiazepine and a CNS depressant with a potential for abuse and addiction. Abuse is the intentional, non-therapeutic use of a drug, even once, for its desirable psychological or physiological effects. Misuse is the intentional use, for therapeutic purposes, of a drug by an individual in a way other than prescribed by a health care provider or for whom it was not prescribed. Drug addiction is a cluster of behavioral, cognitive, and physiological phenomena that may include a strong desire to take the drug, difficulties in controlling drug use (e.g., continuing drug use despite harmful consequences, giving a higher priority to drug use than other activities and obligations), and possible tolerance or physical dependence. Even taking benzodiazepines as prescribed may put patients at risk for abuse and misuse of their medication. Abuse and misuse of benzodiazepines may lead to addiction.
Abuse and misuse of benzodiazepines often (but not always) involve the use of doses greater than the maximum recommended dosage and commonly involve concomitant use of other medications, alcohol, and/or illicit substances, which is associated with an increased frequency of serious adverse outcomes, including respiratory depression, overdose, or death. Benzodiazepines are often sought by individuals who abuse drugs and other substances, and by individuals with addictive disorders (see WARNINGS: Abuse, Misuse, and Addiction).
The following adverse reactions have occurred with benzodiazepine abuse and/or misuse: abdominal pain, amnesia, anorexia, anxiety, aggression, ataxia, blurred vision, confusion, depression, disinhibition, disorientation, dizziness, euphoria, impaired concentration and memory, indigestion, irritability, muscle pain, slurred speech, tremors, and vertigo.
The following severe adverse reactions have occurred with benzodiazepine abuse and/or misuse: delirium, paranoia, suicidal ideation and behavior, seizures, coma, breathing difficulty, and death. Death is more often associated with polysubstance use (especially benzodiazepines with other CNS depressants such as opioids and alcohol).
Dependence
Physical Dependence
Clonazepam tablets may produce physical dependence from continued therapy. Physical dependence is a state that develops as a result of physiological adaptation in response to repeated drug use, manifested by withdrawal signs and symptoms after abrupt discontinuation or a significant dose reduction of a drug. Abrupt discontinuation or rapid dosage reduction of benzodiazepines or administration of flumazenil, a benzodiazepine antagonist, may precipitate acute withdrawal reactions, including seizures, which can be life-threatening. Patients at an increased risk of withdrawal adverse reactions after benzodiazepine discontinuation or rapid dosage reduction include those who take higher dosages (i.e., higher and/or more frequent doses) and those who have had longer durations of use
(see
WARNINGS: Dependence and Withdrawal Reactions).
To reduce the risk of withdrawal reactions, use a gradual taper to discontinue clonazepam tablets or reduce the dosage (see DOSAGE AND ADMINISTRATION: Discontinuation or Dosage Reduction of clonazepam tablets and WARNINGS: Dependence and Withdrawal Reactions).
Acute Withdrawal Signs and Symptoms
Acute withdrawal signs and symptoms associated with benzodiazepines have included abnormal involuntary movements, anxiety, blurred vision, depersonalization, depression, derealization, dizziness, fatigue, gastrointestinal adverse reactions (e.g., nausea, vomiting, diarrhea, weight loss, decreased appetite), headache, hyperacusis, hypertension, irritability, insomnia, memory impairment, muscle pain and stiffness, panic attacks, photophobia, restlessness, tachycardia, and tremor. More severe acute withdrawal signs and symptoms, including life-threatening reactions, have included catatonia, convulsions, delirium tremens, depression, hallucinations, mania, psychosis, seizures, and suicidality.
Protracted Withdrawal Syndrome
Protracted withdrawal syndrome associated with benzodiazepines is characterized by anxiety, cognitive impairment, depression, insomnia, formication, motor symptoms (e.g., weakness, tremor, muscle twitches), paresthesia, and tinnitus that persists beyond 4 to 6 weeks after initial benzodiazepine withdrawal. Protracted withdrawal symptoms may last weeks to more than 12 months. As a result, there may be difficulty in differentiating withdrawal symptoms from potential re-emergence or continuation of symptoms for which the benzodiazepine was being used.
Tolerance
Tolerance to clonazepam tablets may develop from continued therapy. Tolerance is a physiological state characterized by a reduced response to a drug after repeated administration (i.e., a higher dose of a drug is required to produce the same effect that was once obtained at a lower dose). Tolerance to the therapeutic effect of clonazepam tablets may develop; however, little tolerance develops to the amnestic reactions and other cognitive impairments caused by benzodiazepines. Following the short-term treatment of patients with panic disorder in Studies 1 and 2
(see
CLINICAL PHARMACOLOGY: Clinical Trials),
patients were gradually withdrawn during a 7 week downward-titration (discontinuance) period. Overall, the discontinuance period was associated with good tolerability and a very modest clinical deterioration, without evidence of a significant rebound phenomenon. However, there are not sufficient data from adequate and well-controlled long-term clonazepam studies in patients with panic disorder to accurately estimate the risks of withdrawal symptoms and dependence that may be associated with such use.
OVERDOSAGE
Human Experience
Symptoms of clonazepam overdosage, like those produced by other CNS depressants, include somnolence, confusion, coma, and diminished reflexes.
Overdose Management
Treatment includes monitoring of respiration, pulse and blood pressure, general supportive measures and immediate gastric lavage. Intravenous fluids should be administered and an adequate airway maintained. Hypotension may be combated by the use of levarterenol or metaraminol. Dialysis is of no known value.
Flumazenil, a specific benzodiazepine-receptor antagonist, is indicated for the complete or partial reversal of the sedative effects of benzodiazepines and may be used in situations when an overdose with a benzodiazepine is known or suspected. Prior to the administration of flumazenil, necessary measures should be instituted to secure airway, ventilation and intravenous access. Flumazenil is intended as an adjunct to, not as a substitute for, proper management of benzodiazepine overdose. Patients treated with flumazenil should be monitored for resedation, respiratory depression and other residual benzodiazepine effects for an appropriate period after treatment. The prescriber should be aware of a risk of seizure in association with flumazenil treatment, particularly in long-term benzodiazepine users and in cyclic antidepressant overdose. The complete flumazenil package insert, including CONTRAINDICATIONS, WARNINGS and PRECAUTIONS, should be consulted prior to use.
Flumazenil is not indicated in patients with epilepsy who have been treated with benzodiazepines. Antagonism of the benzodiazepine effect in such patients may provoke seizures.
Serious sequelae are rare unless other drugs or alcohol have been taken concomitantly.
DOSAGE AND ADMINISTRATION
Clonazepam tablets should be administered with water by swallowing the tablet whole.
Seizure Disorders
The use of multiple anticonvulsants may result in an increase of CNS depressant adverse effects. This should be considered before adding clonazepam to an existing anticonvulsant regimen.
Adults
The initial dose for adults with seizure disorders should not exceed 1.5 mg/day divided into three doses. Dosage may be increased in increments of 0.5 to 1 mg every 3 days until seizures are adequately controlled or until side effects preclude any further increase. Maintenance dosage must be individualized for each patient depending upon response. Maximum recommended daily dose is 20 mg.
Pediatric Patients
Clonazepam is administered orally. In order to minimize drowsiness, the initial dose for infants and children (up to 10 years of age or 30 kg of body weight) should be between 0.01 and 0.03 mg/kg/day but not to exceed 0.05 mg/kg/day given in two or three divided doses. Dosage should be increased by no more than 0.25 to 0.5 mg every third day until a daily maintenance dose of 0.1 to 0.2 mg/kg of body weight has been reached, unless seizures are controlled or side effects preclude further increase. Whenever possible, the daily dose should be divided into three equal doses. If doses are not equally divided, the largest dose should be given before retiring.
Geriatric Patients
There is no clinical trial experience with clonazepam in seizure disorder patients 65 years of age and older. In general, elderly patients should be started on low doses of clonazepam and observed closely (see PRECAUTIONS, Geriatric Use).
Panic Disorder
Adults
The initial dose for adults with panic disorder is 0.25 mg twice daily. An increase to the target dose for most patients of 1 mg/day may be made after 3 days. The recommended dose of 1 mg/day is based on the results from a fixed dose study in which the optimal effect was seen at 1 mg/day. Higher doses of 2, 3 and 4 mg/day in that study were less effective than the 1 mg/day dose and were associated with more adverse effects. Nevertheless, it is possible that some individual patients may benefit from doses of up to a maximum dose of 4 mg/day, and in those instances, the dose may be increased in increments of 0.125 to 0.25 mg twice daily every 3 days until panic disorder is controlled or until side effects make further increases undesired. To reduce the inconvenience of somnolence, administration of one dose at bedtime may be desirable.
Treatment should be discontinued gradually, with a decrease of 0.125 mg twice daily every 3 days, until the drug is completely withdrawn.
There is no body of evidence available to answer the question of how long the patient treated with clonazepam should remain on it. Therefore, the physician who elects to use clonazepam for extended periods should periodically reevaluate the long-term usefulness of the drug for the individual patient.
Pediatric Patients
There is no clinical trial experience with clonazepam in panic disorder patients under 18 years of age.
Geriatric Patients
There is no clinical trial experience with clonazepam in panic disorder patients 65 years of age and older. In general, elderly patients should be started on low doses of clonazepam and observed closely (see PRECAUTIONS, Geriatric Use).
Discontinuation or Dosage Reduction of Clonazepam tablets
To reduce the risk of withdrawal reactions, increased seizure frequency, and status epilepticus, use a gradual taper to discontinue clonazepam tablets or reduce the dosage. If a patient develops withdrawal reactions, consider pausing the taper or increasing the dosage to the previous tapered dosage level. Subsequently decrease the dosage more slowly (see WARNINGS: Dependence and Withdrawal Reactions and DRUG ABUSE AND DEPENDENCE: Dependence).
HOW SUPPLIED
Clonazepam tablets 0.5 mg are available as yellow, round, flat beveled, tablets. Scored and debossed with "832" above the score on one side, debossed with "TEVA" on the other side. Packaged in: Unit dose packages of 100 (10 x 10) NDC 60687-544-01.
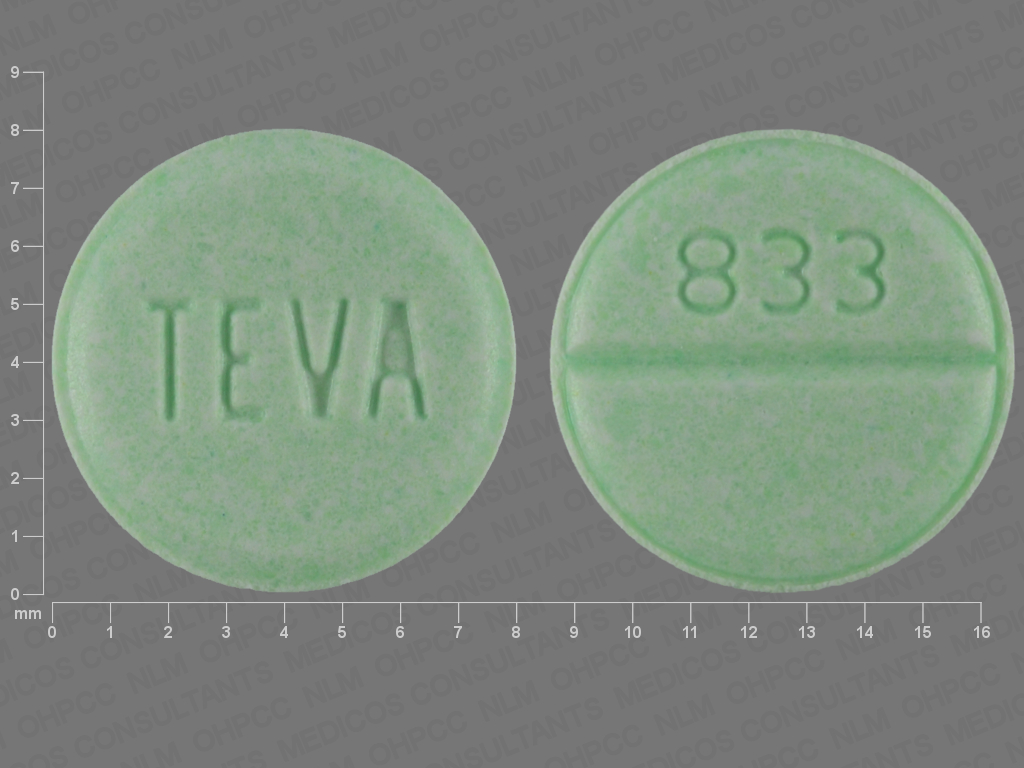
Clonazepam tablets 1 mg are available as mottled green, round, flat beveled tablets. Debossed with "833" on one side and debossed with "TEVA" on the other side. Packaged in: Unit dose packages of 100 (10 x 10) NDC 60687-555-01.
Store at 20° to 25°C (68° to 77°F) [See USP Controlled Room Temperature].
FOR YOUR PROTECTION: Do not use if blister is torn or broken.
Dispense with Medication Guide
To order more Medication Guides call American Health Packaging at 1-800-707-4621.
PACKAGING INFORMATION
American Health Packaging unit dose blisters (see
How Supplied section) contain drug product from Teva Pharmaceuticals as follows:
(0.5 mg / 100 UD) NDC 60687-544-01 packaged from NDC 0093-0832
(1 mg / 100 UD) NDC 60687-555-01 packaged from NDC 0093-3212
Distributed by:
American Health Packaging
Columbus, OH 43217
8454401/0322
MEDICATION GUIDE
8454401/0322
Dispense with Medication Guide
To order more Medication Guides call American Health Packaging at 1-800-707-4621.
Clonazepam (klo-NAY-zeh-pam) Tablets USP, for oral use, CIV
What is the most important information I should know about clonazepam tablets?
- Clonazepam tablets are a benzodiazepine medicine. Taking benzodiazepines with opioid medicines, alcohol, or other central nervous system (CNS) depressants (including street drugs) can cause severe drowsiness, breathing problems (respiratory depression), coma, and death.
Get emergency help right away if any of the following happens:
- shallow or slowed breathing
- breathing stops (which may lead to the heart stopping)
- excessive sleepiness (sedation)
Do not drive or operate heavy machinery until you know how taking clonazepam tablets and opioids affects you.
- Risk of abuse, misuse, and addiction. There is a risk of abuse, misuse, and addiction with benzodiazepines, including clonazepam tablets, which can lead to overdose and serious side effects including coma and death.
- Serious side effects including coma and death have happened in people who have abused or misused benzodiazepines, including clonazepam tablets. These serious side effects may also include delirium, paranoia, suicidal thoughts or actions, seizures, and difficulty breathing. Call your healthcare provider or go to the nearest hospital emergency room right away if you get any of these serious side effects.
- You can develop an addiction even if you take clonazepam tablets as prescribed by your healthcare provider.
- Take clonazepam tablets exactly as your healthcare provider prescribed.
- Do not share your clonazepam tablets with other people.
- Keep clonazepam tablets in a safe place and away from children.
- Physical dependence and withdrawal reactions. Clonazepam tablets can cause physical dependence and withdrawal reactions.
- Do not suddenly stop taking clonazepam tablets. Stopping clonazepam tablets suddenly can cause serious and life-threatening side effects, including, unusual movements, responses, or expressions, seizures, sudden and severe mental or nervous system changes, depression, seeing or hearing things that others do not see or hear, an extreme increase in activity or talking, losing touch with reality, and suicidal thoughts or actions. Call your healthcare provider or go to the nearest hospital emergency room right away if you get any of these symptoms.
- Some people who suddenly stop benzodiazepines have symptoms that can last for several weeks to more than 12 months, including, anxiety, trouble remembering, learning, or concentrating, depression, problems sleeping, feeling like insects are crawling under your skin, weakness, shaking, muscle twitching, burning or prickling feeling in your hands, arms, legs or feet, and ringing in your ears.
- Physical dependence is not the same as drug addiction. Your healthcare provider can tell you more about the differences between physical dependence and drug addiction.
- Do not take more clonazepam tablets than prescribed or take clonazepam tablets for longer than prescribed.
- Clonazepam tablets can make you sleepy or dizzy and can slow your thinking and motor skills. This may get better over time.
- Do not drive, operate heavy machinery, or do other dangerous activities until you know how clonazepam tablets affect you.
- Clonazepam tablets may cause problems with your coordination, especially when you are walking or picking things up.
- Do not drink alcohol or take other drugs that may make you sleepy or dizzy while taking clonazepam tablets until you talk to your healthcare provider. When taken with alcohol or drugs that cause sleepiness or dizziness, clonazepam tablets may make your sleepiness or dizziness worse.
-
Like other antiepileptic medicines, clonazepam tablets may cause suicidal thoughts or actions in a very small number of people, about 1 in 500.
Call your healthcare provider right away if you have any of these symptoms, especially if they are new, worse, or worry you: - thoughts about suicide or dying
- attempt to commit suicide
- new or worse depression
- new or worse anxiety
- feeling agitated or restless
- panic attacks
- trouble sleeping (insomnia)
- new or worse irritability
- acting aggressive, being angry, or violent
- acting on dangerous impulses
- an extreme increase in activity and talking (mania)
- other unusual changes in behavior or mood
How can I watch for early symptoms of suicidal thoughts and actions?
- Pay attention to any changes, especially sudden changes, in mood, behaviors, thoughts, or feelings.
- Keep all follow-up visits with your healthcare provider as scheduled.
Call your healthcare provider between visits as needed, especially if you are worried about symptoms.
Suicidal thoughts or actions can be caused by things other than medicines. If you have suicidal thoughts or actions, your healthcare provider may check for other causes.
- Do not stop clonazepam tablets without first talking to a healthcare provider.
- Stopping clonazepam tablets suddenly can cause serious problems. Stopping clonazepam tablets suddenly can cause seizures that will not stop (status epilepticus).
What are clonazepam tablets?
- Clonazepam tablets is a prescription medicine used alone or with other medicines to treat:
- certain types of seizure disorders (epilepsy) in adults and children
- panic disorder with or without fear of open spaces (agoraphobia) in adults
- Clonazepam tablets are a federally controlled substance (C-IV) because it contains clonazepam that can be abused or lead to dependence. Keep clonazepam tablets in a safe place to prevent misuse and abuse. Selling or giving away clonazepam tablets may harm others, and is against the law. Tell your healthcare provider if you have ever abused or been dependent on alcohol, prescription medicines or street drugs.
It is not known if clonazepam tablets is safe or effective in treating panic disorder in children younger than 18 years old.
Who should not take clonazepam tablets?
Do not take clonazepam tablets if you:
- are allergic to benzodiazepines
- have significant liver disease
- have an eye disease called acute narrow angle glaucoma
Ask your healthcare provider if you are not sure if you have any of the problems listed above.
Before you take clonazepam tablets, tell your healthcare provider if you:
- have liver or kidney problems
- have lung problems (respiratory disease)
- have or have had depression, mood problems, or suicidal thoughts or behavior
- have any other medical problems
- are pregnant or plan to become pregnant. It is not known if clonazepam tablets can harm your unborn baby. Tell your healthcare provider right away if you become pregnant while taking clonazepam tablets. You and your healthcare provider will decide if you should take clonazepam tablets while you are pregnant.
- Studies in pregnant animals have shown harmful effects of benzodiazepine medications (including the active ingredient in clonazepam tablets) on the developing fetus.
- Children born to mothers receiving benzodiazepine medications (including clonazepam tablets) late in pregnancy may be at some risk of experiencing breathing problems, feeding problems, hypothermia, and withdrawal symptoms.
- If you become pregnant while taking clonazepam tablets, talk to your healthcare provider about registering with the North American Antiepileptic Drug Pregnancy Registry. You can register by calling 1-888-233-2334. The purpose of this registry is to collect information about the safety of antiepileptic drugs during pregnancy.
- are breastfeeding or plan to breastfeed. Clonazepam can pass into breast milk. You and your healthcare provider should decide how you will feed your baby while you take clonazepam tablets.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements.
Taking clonazepam tablets with certain other medicines can cause side effects or affect how well clonazepam tablets or the other medicines work. Do not start or stop other medicines without talking to your healthcare provider.
How should I take clonazepam tablets?
- Take clonazepam tablets exactly as your healthcare provider tells you. If you take clonazepam tablets for seizures, your healthcare provider may change the dose until you are taking the right amount of medicine to control your symptoms.
- Clonazepam is available as a tablet.
- Do not stop taking clonazepam tablets without first talking to your healthcare provider. Stopping clonazepam tablets suddenly can cause serious problems.
- Clonazepam tablets should be taken with water and swallowed whole.
- If you take too many clonazepam tablets, call your healthcare provider or local Poison Control Center right away.
What should I avoid while taking clonazepam tablets?
- Clonazepam tablets can slow your thinking and motor skills. Do not drive, operate heavy machinery, or do other dangerous activities until you know how clonazepam tablets affect you.
- Do not drink alcohol or take other medicines that may make you sleepy or dizzy while taking clonazepam tablets until you talk to your healthcare provider. When taken with alcohol or medicines that cause sleepiness or dizziness, clonazepam tablets may make your sleepiness or dizziness worse.
What are the possible side effects of clonazepam tablets?
See “What is the most important information I should know about clonazepam tablets?”
Clonazepam tablets can also make your seizures happen more often or make them worse. Call your healthcare provider right away if your seizures get worse while taking clonazepam tablets.
The most common side effects of clonazepam tablets include:
- drowsiness
- problems with walking and coordination
- dizziness
- depression
- fatigue
- problems with memory
These are not all the possible side effects of clonazepam tablets. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088. You may also report side effects to Teva at 1-888-838-2872.
How should I store clonazepam tablets?
- Store clonazepam tablets between 68° to 77°F (20° to 25°C)
- Keep clonazepam tablets and all medicines out of the reach of children
General Information about the safe and effect use of clonazepam tablets
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use clonazepam tablets for a condition for which they were not prescribed. Do not give clonazepam tablets to other people, even if they have the same symptoms that you have. They may harm them.
You can ask your pharmacist or healthcare provider for information about clonazepam tablets that is written for health professionals.
What are the ingredients in clonazepam tablets?
Active ingredient: clonazepam
Inactive ingredients:
- 0.5 mg tablets contain corn starch, lactose monohydrate, magnesium stearate, microcrystalline cellulose, povidone, and Yellow D&C No. 10 Aluminum Lake
- 1 mg tablets contain corn starch, FD&C Blue No. 1 Aluminum Lake, lactose monohydrate, magnesium stearate, microcrystalline cellulose, povidone, and Yellow D&C No. 10 Aluminum Lake
- 2 mg tablets contain corn starch, lactose monohydrate, magnesium stearate, microcrystalline cellulose, and povidone
For more information about the drug product, call Teva Pharmaceuticals at 1-888-838-2872.
For more information about the packaging or labeling, call American Health Packaging at 1-800-707-4621. .
This Medication Guide has been approved by the U.S. Food and Drug Administration.
Distributed by:
American Health Packaging
Columbus, OH 43217
8454401/0322
Package/Label Display Panel – Carton – 0.5 mg

NDC 60687- 544-01
Clonazepam CIV
Tablets USP
0.5 mg
100 Tablets (10 x 10) Rx Only
PHARMACIST: Dispense with the accompanying Medication
Guide to each patient.
Each Tablet Contains:
Clonazepam, USP ....................................................................... 0.5 mg
Usual Dosage: See package insert for full prescribing information.
Store at 20° to 25°C (68° to 77°F); excursions permitted between
15° to 30°C (59° to 86°F) [see USP Controlled Room Temperature].
Keep this and all drugs out of reach of children.
FOR YOUR PROTECTION: Do not use if blister is torn or broken.
The drug product contained in this package is from
NDC # 0093-0832, Teva Pharmaceuticals USA, Inc.
Distributed by:
American Health Packaging
Columbus, Ohio 43217
754401
0454401/0422
Package/Label Display Panel – Carton – 1 mg

NDC 60687- 555-01
Clonazepam CIV
Tablets USP
1 mg
100 Tablets (10 x 10) Rx Only
PHARMACIST: Dispense with the accompanying Medication
Guide to each patient.
Each Tablet Contains:
Clonazepam, USP ....................................................................... 1 mg
Usual Dosage: See package insert for full prescribing information.
Store at 20° to 25°C (68° to 77°F); excursions permitted between
15° to 30°C (59° to 86°F) [see USP Controlled Room Temperature].
Keep this and all drugs out of reach of children.
FOR YOUR PROTECTION: Do not use if blister is torn or broken.
The drug product contained in this package is from
NDC # 0093-3212, Teva Pharmaceuticals USA, Inc.
Distributed by:
American Health Packaging
Columbus, Ohio 43217
755501
0455501/0322
INGREDIENTS AND APPEARANCE
| CLONAZEPAM
clonazepam tablet |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
 |
||||||||||||||||||||
|
||||||||||||||||||||
| CLONAZEPAM
clonazepam tablet |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
 |
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - American Health Packaging (929561009) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| American Health Packaging | 929561009 | repack(60687-544, 60687-555) | |