Search by Drug Name or NDC
NDC 60687-0661-01 Carbidopa and Levodopa 25; 100 mg/1; mg/1 Details
Carbidopa and Levodopa 25; 100 mg/1; mg/1
Carbidopa and Levodopa is a ORAL TABLET in the HUMAN PRESCRIPTION DRUG category. It is labeled and distributed by American Health Packaging. The primary component is CARBIDOPA; LEVODOPA.
MedlinePlus Drug Summary
The combination of levodopa and carbidopa is used to treat the symptoms of Parkinson's disease and Parkinson's-like symptoms that may develop after encephalitis (swelling of the brain) or injury to the nervous system caused by carbon monoxide poisoning or manganese poisoning. Parkinson's symptoms, including tremors (shaking), stiffness, and slowness of movement, are caused by a lack of dopamine, a natural substance usually found in the brain. Levodopa is in a class of medications called central nervous system agents. It works by being converted to dopamine in the brain. Carbidopa is in a class of medications called decarboxylase inhibitors. It works by preventing levodopa from being broken down before it reaches the brain. This allows for a lower dose of levodopa, which causes less nausea and vomiting.
Related Packages: 60687-0661-01Last Updated: 12/01/2022
MedLinePlus Full Drug Details: Levodopa and Carbidopa
Product Information
| NDC | 60687-0661 |
|---|---|
| Product ID | 60687-661_de57abb7-42d1-9ccd-e053-2995a90a24ef |
| Associated GPIs | 73209902100320 |
| GCN Sequence Number | 002538 |
| GCN Sequence Number Description | carbidopa/levodopa TABLET 25MG-100MG ORAL |
| HIC3 | H6A |
| HIC3 Description | ANTIPARKINSONISM DRUGS,OTHER |
| GCN | 62741 |
| HICL Sequence Number | 013894 |
| HICL Sequence Number Description | CARBIDOPA/LEVODOPA |
| Brand/Generic | Generic |
| Proprietary Name | Carbidopa and Levodopa |
| Proprietary Name Suffix | n/a |
| Non-Proprietary Name | Carbidopa and Levodopa |
| Product Type | HUMAN PRESCRIPTION DRUG |
| Dosage Form | TABLET |
| Route | ORAL |
| Active Ingredient Strength | 25; 100 |
| Active Ingredient Units | mg/1; mg/1 |
| Substance Name | CARBIDOPA; LEVODOPA |
| Labeler Name | American Health Packaging |
| Pharmaceutical Class | Amino Acids, Aromatic [CS], Aromatic Amino Acid [EPC] |
| DEA Schedule | n/a |
| Marketing Category | ANDA |
| Application Number | ANDA074260 |
| Listing Certified Through | 2024-12-31 |
Package
Package Images


NDC 60687-0661-01 (60687066101)
| NDC Package Code | 60687-661-01 |
|---|---|
| Billing NDC | 60687066101 |
| Package | 100 BLISTER PACK in 1 CARTON (60687-661-01) / 1 TABLET in 1 BLISTER PACK (60687-661-11) |
| Marketing Start Date | 2022-04-01 |
| NDC Exclude Flag | N |
| Pricing Information | |
| Price Per Unit | 0.08968 |
| Pricing Unit | EA |
| Effective Date | 2024-02-21 |
| NDC Description | CARBIDOPA-LEVODOPA 25-100 TAB |
| Pharmacy Type Indicator | C/I |
| OTC | N |
| Explanation Code | 1 |
| Classification for Rate Setting | G |
| As of Date | 2024-02-21 |
Standard Product Labeling (SPL)/Prescribing Information SPL a62ae74d-6677-459c-b338-00e6851a1e00 Details
DESCRIPTION
Carbidopa and levodopa tablets, USP are a combination product for the treatment of Parkinson's disease and syndrome.
Carbidopa, USP an inhibitor of aromatic amino acid decarboxylation, is a white, crystalline compound, slightly soluble in water, with a molecular weight of 244.24. It is designated chemically as (-)-L-α-hydrazino-α-methyl-β-(3,4-dihydroxybenzene) propanoic acid monohydrate. Its molecular formula is C 10H 14N 2O 4•H 2O, and its structural formula is:

Tablet content is expressed in terms of anhydrous carbidopa which has a molecular weight of 226.23.
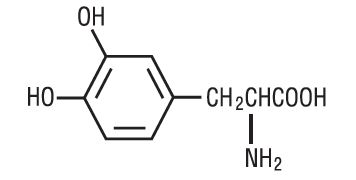
Levodopa, USP an aromatic amino acid, is a white, crystalline compound, slightly soluble in water, with a molecular weight of 197.19. It is designated chemically as (-)-L-α-amino-β-(3,4-dihydroxybenzene) propanoic acid. Its molecular formula is C 9H 11NO 4, and its structural formula is:
Carbidopa and Levodopa Tablets, USP for oral administration, are supplied in three strengths:
10 mg/100 mg, containing 10 mg of carbidopa, USP and 100 mg of levodopa, USP.
25 mg/100 mg, containing 25 mg of carbidopa, USP and 100 mg of levodopa, USP.
25 mg/250 mg, containing 25 mg of carbidopa, USP and 250 mg of levodopa, USP.
In addition, each tablet contains the following inactive ingredients: corn starch, magnesium stearate, microcrystalline cellulose, pregelatinized starch (maize). In addition, the 25 mg/100 mg strength contain D&C yellow #10 aluminum lake and FD&C yellow #6 aluminum lake (sunset yellow lake). The 10 mg/100 mg and 25 mg/250 mg strengths contain FD&C blue #2 aluminum lake.
CLINICAL PHARMACOLOGY
Mechanism of Action
Parkinson's disease is a progressive, neurodegenerative disorder of the extrapyramidal nervous system affecting the mobility and control of the skeletal muscular system. Its characteristic features include resting tremor, rigidity, and bradykinetic movements. Symptomatic treatments, such as levodopa therapies, may permit the patient better mobility.
Current evidence indicates that symptoms of Parkinson's disease are related to depletion of dopamine in the corpus striatum. Administration of dopamine is ineffective in the treatment of Parkinson's disease apparently because it does not cross the blood-brain barrier. However, levodopa, the metabolic precursor of dopamine, does cross the blood-brain barrier, and presumably is converted to dopamine in the brain. This is thought to be the mechanism whereby levodopa relieves symptoms of Parkinson's disease.
Pharmacodynamics
When levodopa is administered orally, it is rapidly decarboxylated to dopamine in extracerebral tissues so that only a small portion of a given dose is transported unchanged to the central nervous system. For this reason, large doses of levodopa are required for adequate therapeutic effect, and these may often be accompanied by nausea and other adverse reactions, some of which are attributable to dopamine formed in extracerebral tissues.
Since levodopa competes with certain amino acids for transport across the gut wall, the absorption of levodopa may be impaired in some patients on a high protein diet.
Carbidopa inhibits decarboxylation of peripheral levodopa. It does not cross the blood-brain barrier and does not affect the metabolism of levodopa within the central nervous system.
The incidence of levodopa-induced nausea and vomiting is less with the combination product than with levodopa. In many patients, this reduction in nausea and vomiting will permit more rapid dosage titration.
Since its decarboxylase inhibiting activity is limited to extracerebral tissues, administration of carbidopa with levodopa makes more levodopa available for transport to the brain.
Pharmacokinetics
Carbidopa reduces the amount of levodopa required to produce a given response by about 75% and, when administered with levodopa, increases both plasma levels and the plasma half-life of levodopa, and decreases plasma and urinary dopamine and homovanillic acid.
The plasma half-life of levodopa is about 50 minutes, without carbidopa. When carbidopa and levodopa are administered together, the half-life of levodopa is increased to about 1.5 hours. At steady state, the bioavailability of carbidopa from carbidopa and levodopa tablets is approximately 99% relative to the concomitant administration of carbidopa and levodopa.
In clinical pharmacologic studies, simultaneous administration of carbidopa and levodopa produced greater urinary excretion of levodopa in proportion to the excretion of dopamine than administration of the two drugs at separate times.
Pyridoxine hydrochloride (vitamin B 6), in oral doses of 10 mg to 25 mg, may reverse the effects of levodopa by increasing the rate of aromatic amino acid decarboxylation. Carbidopa inhibits this action of pyridoxine; therefore, carbidopa and levodopa can be given to patients receiving supplemental pyridoxine (vitamin B 6).
Special Populations
Geriatric: A study in eight young healthy subjects (21 to 22 yr) and eight elderly healthy subjects (69 to 76 yr) showed that the absolute bioavailability of levodopa was similar between young and elderly subjects following oral administration of levodopa and carbidopa. However, the systemic exposure (AUC) of levodopa was increased by 55% in elderly subjects compared to young subjects. Based on another study in forty patients with Parkinson’s disease, there was a correlation between age of patients and the increase of AUC of levodopa following administration of levodopa and an inhibitor of peripheral dopa decarboxylase. AUC of levodopa was increased by 28% in elderly patients (greater than or equal to 65 yr) compared to young patients (less than 65 yr). Additionally, mean value of C
max for levodopa was increased by 24% in elderly patients (greater than or equal to 65 yr) compared to young patients (less than 65 yr) (see
PRECAUTIONS, Geriatric Use).
The AUC of carbidopa was increased in elderly subjects (n=10, 65 to 76 yr) by 29% compared to young subjects (n=24, 23 to 64 yr) following IV administration of 50 mg levodopa with carbidopa (50 mg). This increase is not considered a clinically significant impact.
INDICATIONS AND USAGE
Carbidopa and levodopa tablets are indicated in the treatment of Parkinson's disease, post-encephalitic parkinsonism, and symptomatic parkinsonism that may follow carbon monoxide intoxication or manganese intoxication.
Carbidopa allows patients treated for Parkinson's disease to use much lower doses of levodopa. Some patients who responded poorly to levodopa have improved on carbidopa and levodopa. This is most likely due to decreased peripheral decarboxylation of levodopa caused by administration of carbidopa rather than by a primary effect of carbidopa on the nervous system. Carbidopa has not been shown to enhance the intrinsic efficacy of levodopa.
Carbidopa may also reduce nausea and vomiting and permit more rapid titration of levodopa.
CONTRAINDICATIONS
Nonselective monoamine oxidase (MAO) inhibitors are contraindicated for use with carbidopa and levodopa. These inhibitors must be discontinued at least two weeks prior to initiating therapy with this combination product. Carbidopa and levodopa may be administered concomitantly with the manufacturer's recommended dose of an MAO inhibitor with selectivity for MAO type B (e.g., selegiline HCl) (see PRECAUTIONS, Drug Interactions).
Carbidopa and levodopa is contraindicated in patients with known hypersensitivity to any component of this drug, and in patients with narrow-angle glaucoma.
WARNINGS
When carbidopa and levodopa tablets are to be given to patients who are being treated with levodopa, levodopa must be discontinued at least twelve hours before therapy with the combination product is started. In order to reduce adverse reactions, it is necessary to individualize therapy. See DOSAGE AND ADMINISTRATION section before initiating therapy.
The addition of carbidopa with levodopa in the form of this combination product reduces the peripheral effects (nausea, vomiting) due to decarboxylation of levodopa; however, carbidopa does not decrease the adverse reactions due to the central effects of levodopa. Because carbidopa permits more levodopa to reach the brain and more dopamine to be formed, certain adverse central nervous system (CNS) effects, e.g., dyskinesias (involuntary movements), may occur at lower dosages and sooner with carbidopa and levodopa than with levodopa alone.
All patients should be observed carefully for the development of depression with concomitant suicidal tendencies.
Carbidopa and levodopa should be administered cautiously to patients with severe cardiovascular or pulmonary disease, bronchial asthma, renal, hepatic or endocrine disease.
As with levodopa, care should be exercised in administering the combination product, to patients with a history of myocardial infarction who have residual atrial, nodal, or ventricular arrhythmias. In such patients, cardiac function should be monitored with particular care during the period of initial dosage adjustment, in a facility with provisions for intensive cardiac care.
As with levodopa, treatment with the combination product may increase the possibility of upper gastrointestinal hemorrhage in patients with a history of peptic ulcer.
Falling Asleep During Activities of Daily Living and Somnolence
Patients taking carbidopa and levodopa alone or with other dopaminergic drugs have reported suddenly falling asleep without prior warning of sleepiness while engaged in activities of daily living (includes operation of motor vehicles). Road traffic accidents attributed to sudden sleep onset have been reported. Although many patients reported somnolence while on dopaminergic medications, there have been reports of road traffic accidents attributed to sudden onset of sleep in which the patient did not perceive any warning signs, such as excessive drowsiness, and believed that they were alert immediately prior to the event. Sudden onset of sleep has been reported to occur as long as one year after the initiation of treatment.
Falling asleep while engaged in activities of daily living usually occurs in patients experiencing preexisting somnolence, although some patients may not give such a history. For this reason, prescribers should reassess patients for drowsiness or sleepiness especially since some of the events occur well after the start of treatment. Prescribers should be aware that patients may not acknowledge drowsiness or sleepiness until directly questioned about drowsiness or sleepiness during specific activities. Patients should be advised to exercise caution while driving or operating machines during treatment with carbidopa and levodopa. Patients who have already experienced somnolence or an episode of sudden sleep onset should not participate in these activities during treatment with carbidopa and levodopa.
Before initiating treatment with carbidopa and levodopa, advise patients about the potential to develop drowsiness and ask specifically about factors that may increase the risk for somnolence with carbidopa and levodopa such as the use of concomitant sedating medications and the presence of sleep disorders. Consider discontinuing carbidopa and levodopa in patients who report significant daytime sleepiness or episodes of falling asleep during activities that require active participation (e.g., conversations, eating, etc.). If treatment with carbidopa and levodopa continues, patients should be advised not to drive and to avoid other potentially dangerous activities that might result in harm if the patients become somnolent. There is insufficient information to establish that dose reduction will eliminate episodes of falling asleep while engaged in activities of daily living.
Hyperpyrexia and Confusion
Sporadic cases of a symptom complex resembling neuroleptic malignant syndrome (NMS) have been reported in association with dose reductions or withdrawal of certain antiparkinsonian agents such as levodopa, carbidopa and levodopa, or carbidopa and levodopa extended release. Therefore, patients should be observed carefully when the dosage of levodopa is reduced abruptly or discontinued, especially if the patient is receiving neuroleptics.
NMS is an uncommon but life-threatening syndrome characterized by fever or hyperthermia. Neurological findings, including muscle rigidity, involuntary movements, altered consciousness, mental status changes; other disturbances, such as autonomic dysfunction, tachycardia, tachypnea, sweating, hyper- or hypotension; laboratory findings, such as creatine phosphokinase elevation, leukocytosis, myoglobinuria, and increased serum myoglobin have been reported.
The early diagnosis of this condition is important for the appropriate management of these patients. Considering NMS as a possible diagnosis and ruling out other acute illnesses (e.g., pneumonia, systemic infection, etc.) is essential. This may be especially complex if the clinical presentation includes both serious medical illness and untreated or inadequately treated extrapyramidal signs and symptoms (EPS). Other important considerations in the differential diagnosis include central anticholinergic toxicity, heat stroke, drug fever, and primary central nervous system (CNS) pathology.
The management of NMS should include: 1) intensive symptomatic treatment and medical monitoring and 2) treatment of any concomitant serious medical problems for which specific treatments are available. Dopamine agonists, such as bromocriptine, and muscle relaxants, such as dantrolene, are often used in the treatment of NMS; however, their effectiveness has not been demonstrated in controlled studies.
PRECAUTIONS
General
As with levodopa, periodic evaluations of hepatic, hematopoietic, cardiovascular, and renal function are recommended during extended therapy.
Patients with chronic wide-angle glaucoma may be treated cautiously with carbidopa and levodopa provided the intraocular pressure is well-controlled and the patient is monitored carefully for changes in intraocular pressure during therapy.
Dyskinesia
Levodopa alone, as well as carbidopa and levodopa, is associated with dyskinesias. The occurrence of dyskinesias may require dosage reduction.
Hallucinations/Psychotic-Like Behavior
Hallucinations and psychotic-like behavior have been reported with dopaminergic medications. In general, hallucinations present shortly after the initiation of therapy and may be responsive to dose reduction in levodopa. Hallucinations may be accompanied by confusion and to a lesser extent sleep disorder (insomnia) and excessive dreaming.
Carbidopa and levodopa may have similar effects on thinking and behavior. This abnormal thinking and behavior may present with one or more symptoms, including paranoid ideation, delusions, hallucinations, confusion, psychotic-like behavior, disorientation, aggressive behavior, agitation, and delirium.
Ordinarily, patients with a major psychotic disorder should not be treated with carbidopa and levodopa, because of the risk of exacerbating psychosis. In addition, certain medications used to treat psychosis may exacerbate the symptoms of Parkinson’s disease and may decrease the effectiveness of carbidopa and levodopa.
Impulse Control/Compulsive Behaviors
Reports of patients taking dopaminergic medications (medications that increase central dopaminergic tone), suggest that patients may experience an intense urge to gamble, increased sexual urges, intense urges to spend money, binge eating, and/or other intense urges, and the inability to control these urges. In some cases, although not all, these urges were reported to have stopped when the dose was reduced or the medication was discontinued. Because patients may not recognize these behaviors as abnormal, it is important for prescribers to specifically ask patients or the caregivers about the development of new or increased gambling urges, sexual urges, uncontrolled spending or other urges while being treated with carbidopa and levodopa. Physicians should consider dose reduction or stopping the medication if a patient develops such urges while taking carbidopa and levodopa (see
Information for Patients).
Melanoma
Epidemiological studies have shown that patients with Parkinson’s disease have a higher risk (2- to approximately 6-fold higher) of developing melanoma than the general population. Whether the increased risk observed was due to Parkinson’s disease or other factors, such as drugs used to treat Parkinson’s disease, is unclear.
For the reasons stated above, patients and providers are advised to monitor for melanomas frequently and on a regular basis when using carbidopa and levodopa for any indication. Ideally, periodic skin examinations should be performed by appropriately qualified individuals (e.g., dermatologists).
Information for Patients
The patient should be informed that this combination product is an immediate-release formulation of carbidopa and levodopa that is designed to begin release of ingredients within 30 minutes. It is important that carbidopa and levodopa be taken at regular intervals according to the schedule outlined by the physician. The patient should be cautioned not to change the prescribed dosage regimen and not to add any additional antiparkinson medications, including other carbidopa and levodopa preparations, without first consulting the physician.
Patients should be advised that sometimes a 'wearing-off' effect may occur at the end of the dosing interval. The physician should be notified if such response poses a problem to lifestyle.
Patients should be advised that occasionally, dark color (red, brown, or black) may appear in saliva, urine, or sweat after ingestion of carbidopa and levodopa. Although the color appears to be clinically insignificant, garments may become discolored.
The patient should be advised that a change in diet to foods that are high in protein may delay the absorption of levodopa and may reduce the amount taken up in the circulation. Excessive acidity also delays stomach emptying, thus delaying the absorption of levodopa. Iron salts (such as in multivitamin tablets) may also reduce the amount of levodopa available to the body. The above factors may reduce the clinical effectiveness of the levodopa or carbidopa and levodopa therapy.
Patients should be alerted to the possibility of sudden onset of sleep during daily activities, in some cases without awareness or warning signs, when they are taking dopaminergic agents, including levodopa. Patients should be advised to exercise caution while driving or operating machinery and that if they have experienced somnolence and/or sudden sleep onset, they must refrain from these activities. (See WARNINGS, Falling Asleep During Activities of Daily Living and Somnolence.)
There have been reports of patients experiencing intense urges to gamble, increased sexual urges, and other intense urges, and the inability to control these urges while taking one or more of the medications that increase central dopaminergic tone and that are generally used for the treatment of Parkinson’s disease, including carbidopa and levodopa. Although it is not proven that the medications caused these events, these urges were reported to have stopped in some cases when the dose was reduced or the medication was stopped. Prescribers should ask patients about the development of new or increased gambling urges, sexual urges or other urges while being treated with carbidopa and levodopa. Patients should inform their physician if they experience new or increased gambling urges, increased sexual urges, or other intense urges while taking carbidopa and levodopa. Physicians should consider dose reduction or stopping the medication if a patient develops such urges while taking carbidopa and levodopa (See PRECAUTIONS, Impulse Control/Compulsive Behaviors).
Laboratory Tests
Abnormalities in laboratory tests may include elevations of liver function tests such as alkaline phosphatase, SGOT (AST), SGPT (ALT), lactic dehydrogenase (LDH), and bilirubin. Abnormalities in blood urea nitrogen (BUN) and positive Coombs test have also been reported. Commonly, levels of blood urea nitrogen, creatinine, and uric acid are lower during administration of this combination product than with levodopa.
Carbidopa and levodopa may cause a false-positive reaction for urinary ketone bodies when a test tape is used for determination of ketonuria. This reaction will not be altered by boiling the urine specimen. False-negative tests may result with the use of glucose-oxidase methods of testing for glucosuria.
Cases of falsely diagnosed pheochromocytoma in patients on carbidopa and levodopa therapy have been reported very rarely. Caution should be exercised when interpreting the plasma and urine levels of catecholamines and their metabolites in patients on levodopa or carbidopa and levodopa therapy.
Drug Interactions
Caution should be exercised when the following drugs are administered concomitantly with carbidopa and levodopa.
Symptomatic postural hypotension occurred when carbidopa and levodopa was added to the treatment of a patient receiving antihypertensive drugs. Therefore, when therapy with carbidopa and levodopa is started, dosage adjustment of the antihypertensive drug may be required.
For patients receiving MAO inhibitors (Type A or B), see CONTRAINDICATIONS. Concomitant therapy with selegiline and carbidopa and levodopa may be associated with severe orthostatic hypotension not attributable to carbidopa and levodopa alone (see CONTRAINDICATIONS).
There have been rare reports of adverse reactions, including hypertension and dyskinesia, resulting from the concomitant use of tricyclic antidepressants and carbidopa and levodopa.
Dopamine D 2 receptor antagonists (e.g., phenothiazines, butyrophenones, risperidone) and isoniazid may reduce the therapeutic effects of levodopa. In addition, the beneficial effects of levodopa in Parkinson's disease have been reported to be reversed by phenytoin and papaverine. Patients taking these drugs with carbidopa and levodopa should be carefully observed for loss of therapeutic response.
Use of carbidopa and levodopa with dopamine-depleting agents (e.g., reserpine and tetrabenazine) or other drugs known to deplete monoamine stores is not recommended.
Carbidopa and levodopa and iron salts or multivitamins containing iron salts should be coadministered with caution. Iron salts can form chelates with levodopa and carbidopa and consequently reduce the bioavailability of carbidopa and levodopa.
Although metoclopramide may increase the bioavailability of levodopa by increasing gastric emptying, metoclopramide may also adversely affect disease control by its dopamine receptor antagonistic properties.
Carcinogenesis, Mutagenesis, Impairment of Fertility
In a two-year bioassay of carbidopa and levodopa, no evidence of carcinogenicity was found in rats receiving doses of approximately two times the maximum daily human dose of carbidopa and four times the maximum daily human dose of levodopa.
In reproduction studies with carbidopa and levodopa, no effects on fertility were found in rats receiving doses of approximately two times the maximum daily human dose of carbidopa and four times the maximum daily human dose of levodopa.
Pregnancy
No teratogenic effects were observed in a study in mice receiving up to 20 times the maximum recommended human dose of carbidopa and levodopa. There was a decrease in the number of live pups delivered by rats receiving approximately two times the maximum recommended human dose of carbidopa and approximately five times the maximum recommended human dose of levodopa during organogenesis. Carbidopa and levodopa caused both visceral and skeletal malformations in rabbits at all doses and ratios of carbidopa/levodopa tested, which ranged from 10 times/5 times the maximum recommended human dose of carbidopa/levodopa to 20 times/10 times the maximum recommended human dose of carbidopa/levodopa.
There are no adequate or well-controlled studies in pregnant women. It has been reported from individual cases that levodopa crosses the human placental barrier, enters the fetus, and is metabolized. Carbidopa concentrations in fetal tissue appeared to be minimal. Use of carbidopa and levodopa in women of childbearing potential requires that the anticipated benefits of the drug be weighed against possible hazards to mother and child.
Nursing Mothers
Levodopa has been detected in human milk. Caution should be exercised when carbidopa and levodopa is administered to a nursing woman.
Pediatric Use
Safety and effectiveness in pediatric patients have not been established. Use of the drug in patients below the age of 18 is not recommended.
Geriatric Use
In the clinical efficacy trials for carbidopa and levodopa, almost half of the patients were older than 65, but few were older than 75. No overall meaningful differences in safety or effectiveness were observed between these subjects and younger subjects, but greater sensitivity of some older individuals to adverse drug reactions such as hallucinations cannot be ruled out. There is no specific dosing recommendation based upon clinical pharmacology data as carbidopa and levodopa is titrated as tolerated for clinical effect.
ADVERSE REACTIONS
The most common adverse reactions reported with carbidopa and levodopa have included dyskinesias, such as choreiform, dystonic, and other involuntary movements, and nausea.
The following other adverse reactions have been reported with carbidopa and levodopa:
Body as a Whole
Chest pain, asthenia.
Cardiovascular
Cardiac irregularities, hypotension, orthostatic effects including orthostatic hypotension, hypertension, syncope, phlebitis, palpitation.
Gastrointestinal
Dark saliva, gastrointestinal bleeding, development of duodenal ulcer, anorexia, vomiting, diarrhea, constipation, dyspepsia, dry mouth, taste alterations.
Hematologic
Agranulocytosis, hemolytic and non-hemolytic anemia, thrombocytopenia, leukopenia.
Hypersensitivity
Angioedema, urticaria, pruritus, Henoch-Schönlein purpura, bullous lesions (including pemphigus-like reactions).
Musculoskeletal
Back pain, shoulder pain, muscle cramps.
Nervous System/Psychiatric
Psychotic episodes including delusions, hallucinations, and paranoid ideation, bradykinetic episodes ("on-off" phenomenon), confusion, agitation, dizziness, somnolence, dream abnormalities including nightmares, insomnia, paresthesia, headache, depression with or without development of suicidal tendencies, dementia, pathological gambling, increased libido including hypersexuality, impulse control symptoms. Convulsions also have occurred; however, a causal relationship with carbidopa and levodopa has not been established.
Respiratory
Dyspnea, upper respiratory infection.
Skin
Rash, increased sweating, alopecia, dark sweat.
Urogenital
Urinary tract infection, urinary frequency, dark urine.
Laboratory Tests
Decreased hemoglobin and hematocrit; abnormalities in alkaline phosphatase, SGOT (AST), SGPT (ALT), LDH, bilirubin, BUN, Coombs test; elevated serum glucose; white blood cells, bacteria, and blood in the urine.
Other adverse reactions that have been reported with levodopa alone and with various carbidopa and levodopa formulations, and may occur with this combination product are:
Body as a Whole
Abdominal pain and distress, fatigue.
Cardiovascular
Myocardial infarction.
Gastrointestinal
Gastrointestinal pain, dysphagia, sialorrhea, flatulence, bruxism, burning sensation of the tongue, heartburn, hiccups.
Metabolic
Edema, weight gain, weight loss.
Musculoskeletal
Leg pain.
Nervous System/Psychiatric
Ataxia, extrapyramidal disorder, falling, anxiety, gait abnormalities, nervousness, decreased mental acuity, memory impairment, disorientation, euphoria, blepharospasm (which may be taken as an early sign of excess dosage; consideration of dosage reduction may be made at this time), trismus, increased tremor, numbness, muscle twitching, activation of latent Horner's syndrome, peripheral neuropathy.
Respiratory
Pharyngeal pain, cough.
Skin
Malignant melanoma, flushing.
Special Senses
Oculogyric crises, diplopia, blurred vision, dilated pupils.
Urogenital
Urinary retention, urinary incontinence, priapism.
Miscellaneous
Bizarre breathing patterns, faintness, hoarseness, malaise, hot flashes, sense of stimulation.
Laboratory Tests
Decreased white blood cell count and serum potassium; increased serum creatinine and uric acid; protein and glucose in urine.
To report SUSPECTED ADVERSE EVENTS, contact Teva at 1-888-838-2872 or FDA at 1-800-FDA-1088 or http://www.fda.gov/medwatch for voluntary reporting of adverse reactions.
OVERDOSAGE
Management of acute overdosage with carbidopa and levodopa is the same as management of acute overdosage with levodopa. Pyridoxine is not effective in reversing the actions of this product.
General supportive measures should be employed, along with immediate gastric lavage. Intravenous fluids should be administered judiciously and an adequate airway maintained. Electrocardiographic monitoring should be instituted and the patient carefully observed for the development of arrhythmias; if required, appropriate antiarrhythmic therapy should be given. The possibility that the patient may have taken other drugs as well as carbidopa and levodopa tablets should be taken into consideration. To date, no experience has been reported with dialysis; hence, its value in overdosage is not known.
Based on studies in which high doses of levodopa and/or carbidopa were administered, a significant proportion of rats and mice given single oral doses of levodopa of approximately 1,500 to 2,000 mg/kg are expected to die. A significant proportion of infant rats of both sexes are expected to die at a dose of 800 mg/kg. A significant proportion of rats are expected to die after treatment with similar doses of carbidopa. The addition of carbidopa in a 1:10 ratio with levodopa increases the dose at which a significant proportion of mice are expected to die to 3,360 mg/kg.
DOSAGE AND ADMINISTRATION
The optimum daily dosage of carbidopa and levodopa must be determined by careful titration in each patient. Carbidopa and levodopa tablets are available in a 1:4 ratio of carbidopa to levodopa (25 mg/100 mg) as well as 1:10 ratio (25 mg/250 mg and 10 mg/100 mg). Tablets of the two ratios may be given separately or combined as needed to provide the optimum dosage.
Studies show that peripheral dopa decarboxylase is saturated by carbidopa at approximately 70 to 100 mg a day. Patients receiving less than this amount of carbidopa are more likely to experience nausea and vomiting.
Usual Initial Dosage
Dosage is best initiated with one tablet of carbidopa and levodopa 25 mg/100 mg three times a day. This dosage schedule provides 75 mg of carbidopa per day. Dosage may be increased by one tablet every day or every other day, as necessary, until a dosage of eight tablets of carbidopa and levodopa 25 mg/100 mg a day is reached.
If carbidopa and levodopa 10 mg/100 mg is used, dosage may be initiated with one tablet three or four times a day. However, this will not provide an adequate amount of carbidopa for many patients. Dosage may be increased by one tablet every day or every other day until a total of eight tablets (2 tablets four times a day) is reached.
How to Transfer Patients from Levodopa
Levodopa must be discontinued at least twelve hours before starting this combination product.
A daily dosage of carbidopa and levodopa should be chosen that will provide approximately 25% of the previous levodopa dosage. Patients who are taking less than 1,500 mg of levodopa a day should be started on one tablet of carbidopa and levodopa 25 mg/100 mg three or four times a day. The suggested starting dosage for most patients taking more than 1,500 mg of levodopa is one tablet of carbidopa and levodopa 25 mg/250 mg three or four times a day.
Maintenance
Therapy should be individualized and adjusted according to the desired therapeutic response. At least 70 to 100 mg of carbidopa per day should be provided. When a greater proportion of carbidopa is required, one 25 mg/100 mg tablet may be substituted for each 10 mg/100 mg tablet. When more levodopa is required, each 25 mg/250 mg tablet should be substituted for a 25 mg/100 mg tablet or a 10 mg/100 mg tablet. If necessary, the dosage of carbidopa and levodopa 25 mg/250 mg may be increased by one-half or one tablet every day or every other day to a maximum of eight tablets a day. Experience with total daily dosages of carbidopa greater than 200 mg is limited.
Because both therapeutic and adverse responses occur more rapidly with this combination product than with levodopa alone, patients should be monitored closely during the dose adjustment period. Specifically, involuntary movements will occur more rapidly with carbidopa and levodopa than with levodopa. The occurrence of involuntary movements may require dosage reduction. Blepharospasm may be a useful early sign of excess dosage in some patients.
Addition of Other Antiparkinsonian Medications
Standard drugs for Parkinson's disease, other than levodopa without a decarboxylase inhibitor, may be used concomitantly while carbidopa and levodopa is being administered, although dosage adjustments may be required.
Interruption of Therapy
Sporadic cases of hyperpyrexia and confusion have been associated with dose reductions and withdrawal of carbidopa and levodopa. Patients should be observed carefully if abrupt reduction or discontinuation of carbidopa and levodopa is required, especially if the patient is receiving neuroleptics (see
WARNINGS).
If general anesthesia is required, carbidopa and levodopa may be continued as long as the patient is permitted to take fluids and medication by mouth. If therapy is interrupted temporarily, the patient should be observed for symptoms resembling NMS, and the usual daily dosage may be administered as soon as the patient is able to take oral medication.
HOW SUPPLIED
Carbidopa and Levodopa Tablets, USP are supplied as follows:
25 mg/100 mg — Each yellow, mottled, round tablet imprinted with ‘TV’ on one side and ‘9702’on the other side contains 25 mg of carbidopa, USP and 100 mg of levodopa, USP and is supplied in:
Unit dose packages of 100 (10 x 10) NDC 60687-661-01
Store at 25ºC (77ºF); excursions permitted to 15º to 30ºC (59º to 86ºF) [see USP Controlled Room Temperature].
Protect from light.
FOR YOUR PROTECTION: Do not use if blister is torn or broken.
PACKAGING INFORMATION
American Health Packaging unit dose blisters (see
How Supplied section) contain drug product from Teva Pharmaceuticals USA, Inc. as follows:
(25 mg/100 mg / 100 UD) NDC 60687-661-01 packaged from NDC 0093-9702
Distributed by:
American Health Packaging
Columbus, OH 43217
8466101/0222
Package/Label Display Panel – Carton – 25 mg/100 mg

NDC 60687- 661-01
Carbidopa and
Levodopa
Tablets, USP
25 mg/100 mg
100 Tablets (10 x 10) Rx Only
Each Tablet Contains:
Carbidopa, USP (anhydrous equivalent) .................................. 25 mg
Levodopa, USP .......................................................................100 mg
Usual Adult Dosage: See package insert for full prescribing
information.
Store at 20° to 25°C (68° to 77°F); excursions permitted between
15° to 30°C (59° to 86°F) [see USP Controlled Room Temperature].
Protect from light.
Keep this and all drugs out of reach of children.
FOR YOUR PROTECTION: Do not use if blister is torn or broken.
The drug product contained in this package is from
NDC # 0093-9702, Teva Pharmaceuticals USA, Inc.
Distributed by:
American Health Packaging
Columbus, Ohio 43217
766101
0466101/0222
Package/Label Display Panel – Blister – 25 mg/100 mg
INGREDIENTS AND APPEARANCE
| CARBIDOPA AND LEVODOPA
carbidopa and levodopa tablet |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
 |
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - American Health Packaging (929561009) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| American Health Packaging | 929561009 | repack(60687-661) | |