Search by Drug Name or NDC
NDC 63304-0096-30 Erlotinib 100 mg/1 Details
Erlotinib 100 mg/1
Erlotinib is a ORAL TABLET, FILM COATED in the HUMAN PRESCRIPTION DRUG category. It is labeled and distributed by Sun Pharmaceutical Industries, Inc.. The primary component is ERLOTINIB.
MedlinePlus Drug Summary
Erlotinib is used to treat certain types of non-small cell lung cancer that has spread to nearby tissues or to other parts of the body in patients who have already been treated with at least one other chemotherapy medication and have not gotten better. Erlotinib is also used in combination with another medication (gemcitabine [Gemzar]) to treat pancreatic cancer that has spread to nearby tissues or to other parts of the body and cannot be treated with surgery. Erlotinib is in a class of medications called kinase inhibitors. It works by blocking the action of an abnormal protein that signals cancer cells to multiply. This helps slow or stop the spread of cancer cells.
Related Packages: 63304-0096-30Last Updated: 12/01/2022
MedLinePlus Full Drug Details: Erlotinib
Product Information
| NDC | 63304-0096 |
|---|---|
| Product ID | 63304-096_c160713b-c06f-49b4-80ea-fc1afb030b81 |
| Associated GPIs | 21360025100330 |
| GCN Sequence Number | 058375 |
| GCN Sequence Number Description | erlotinib HCl TABLET 100 MG ORAL |
| HIC3 | V1Q |
| HIC3 Description | ANTINEOPLASTIC SYSTEMIC ENZYME INHIBITORS |
| GCN | 23794 |
| HICL Sequence Number | 026745 |
| HICL Sequence Number Description | ERLOTINIB HCL |
| Brand/Generic | Generic |
| Proprietary Name | Erlotinib |
| Proprietary Name Suffix | n/a |
| Non-Proprietary Name | Erlotinib |
| Product Type | HUMAN PRESCRIPTION DRUG |
| Dosage Form | TABLET, FILM COATED |
| Route | ORAL |
| Active Ingredient Strength | 100 |
| Active Ingredient Units | mg/1 |
| Substance Name | ERLOTINIB |
| Labeler Name | Sun Pharmaceutical Industries, Inc. |
| Pharmaceutical Class | Kinase Inhibitor [EPC], Protein Kinase Inhibitors [MoA] |
| DEA Schedule | n/a |
| Marketing Category | ANDA |
| Application Number | ANDA210300 |
| Listing Certified Through | 2024-12-31 |
Package
Package Images


NDC 63304-0096-30 (63304009630)
| NDC Package Code | 63304-096-30 |
|---|---|
| Billing NDC | 63304009630 |
| Package | 30 TABLET, FILM COATED in 1 BOTTLE (63304-096-30) |
| Marketing Start Date | 2019-11-05 |
| NDC Exclude Flag | N |
| Pricing Information | N/A |
Standard Product Labeling (SPL)/Prescribing Information SPL e36fa076-e23b-437f-bbd2-0433ce333175 Details
HIGHLIGHTS OF PRESCRIBING INFORMATION
ERLOTINIB tablets, for oral use
Initial U.S. Approval: 2004
RECENT MAJOR CHANGES
Indications and Usage, Non-Small Cell Lung Cancer (NSCLC) (1.1) 10/2016
Dosage and Administration (2.1) 06/2016
Dosage and Administration, Dose Modifications (2.4) 05/2016
Warnings and Precautions, Cerebrovascular Accident (5.6) 10/2016
Warnings and Precautions, Embryo-fetal Toxicity (5.10) 10/2016
INDICATIONS AND USAGE
Erlotinib tablets are kinase inhibitors indicated for: (1)
- •
- The treatment of patients with metastatic non-small cell lung cancer (NSCLC) whose tumors have epidermal growth factor receptor (EGFR) exon 19 deletions or exon 21 (L858R) substitution mutations as detected by an FDA-approved test receiving first-line, maintenance, or second or greater line treatment after progression following at least one prior chemotherapy regimen. (1.1)
- •
- First-line treatment of patients with locally advanced, unresectable or metastatic pancreatic cancer, in combination with gemcitabine. (1.2)
Limitations of Use: (1)
- •
- Safety and efficacy of erlotinib tablets have not been established in patients with NSCLC whose tumors have other EGFR mutations. (1.1)
- •
- Erlotinib tablets are not recommended for use in combination with platinum-based chemotherapy. (1.1)
DOSAGE AND ADMINISTRATION
- •
- NSCLC: 150 mg orally, on an empty stomach, once daily. (2.2)
- •
- Pancreatic cancer: 100 mg orally, on an empty stomach, once daily. (2.3)
DOSAGE FORMS AND STRENGTHS
Tablets: 25 mg, 100 mg, and 150 mg (3) (3)
CONTRAINDICATIONS
None. (4) (4)
WARNINGS AND PRECAUTIONS
- •
- Interstitial lung disease (ILD): Occurs in 1.1% of patients. Withhold erlotinib for acute onset of new or progressive unexplained pulmonary symptoms, such as dyspnea, cough and fever. Discontinue erlotinib if ILD is diagnosed. (5.1)
- •
- Renal failure: Monitor renal function and electrolytes, particularly in patients at risk of dehydration. Withhold erlotinib for severe renal toxicity. (5.2)
- •
- Hepatotoxicity: Occurs with or without hepatic impairment, including hepatic failure and hepatorenal syndrome: Monitor periodic liver testing. Withhold or discontinue erlotinib for severe or worsening liver tests. (5.3)
- •
- Gastrointestinal perforations: Discontinue erlotinib. (5.4)
- •
- Bullous and exfoliative skin disorders: Discontinue erlotinib. (5.5)
- •
- Cerebrovascular accident (CVA): The risk of CVA is increased in patients with pancreatic cancer. (5.6)
- •
- Microangiopathic hemolytic anemia (MAHA): The risk of MAHA is increased in patients with pancreatic cancer. (5.7)
- •
- Ocular disorders: Discontinue erlotinib for corneal perforation, ulceration or persistent severe keratitis. (5.8)
- •
- Hemorrhage in patients taking warfarin: Regularly monitor INR in patients taking warfarin or other coumarin-derivative anticoagulants. (5.9)
- •
- Embryo-fetal toxicity: Can cause fetal harm. Advise females of reproductive potential of the potential risk to the fetus and to use effective contraception. (5.10, 8.1, 8.3)
ADVERSE REACTIONS
The most common adverse reactions (≥ 20%) with erlotinib from a pooled analysis in patients with NSCLC across all approved lines of therapy, with and without EGFR mutations, and in patients with pancreatic cancer were rash, diarrhea, anorexia, fatigue, dyspnea, cough, nausea, and vomiting. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Sun Pharmaceutical Industries, Inc. at 1-800-406-7984 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS
- •
- CYP3A4 inhibitors or a combined CYP3A4 and CYP1A2 inhibitor increase erlotinib plasma concentrations. Avoid concomitant use. If not possible, reduce erlotinib dose. (2.4, 7)
- •
- CYP3A4 inducers decrease erlotinib plasma concentrations. Avoid concomitant use. If not possible, increase erlotinib dose. (2.4, 7)
- •
- Cigarette smoking and CYP1A2 inducers decrease erlotinib plasma concentrations. Avoid concomitant use. If not possible, increase erlotinib dose. (2.4, 7)
- •
- Drugs that increase gastric pH decrease erlotinib plasma concentrations. For proton pump inhibitors avoid concomitant use if possible. For H-2 receptor antagonists, take erlotinib 10 hours after H-2 receptor antagonist dosing. For use with antacids, separate dosing by several hours. (2.4, 7)
USE IN SPECIFIC POPULATIONS
Lactation: Do not breastfeed (8.2) (8)
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 11/2019
Table Of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
1.1 Non-Small Cell Lung Cancer (NSCLC)
1.2 Pancreatic Cancer
2 DOSAGE AND ADMINISTRATION
2.1 Selection of Patients with Metastatic NSCLC
2.2 Recommended Dose – NSCLC
2.3 Recommended Dose – Pancreatic Cancer
2.4 Dose Modifications
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Interstitial Lung Disease (ILD)
5.2 Renal Failure
5.3 Hepatotoxicity with or without Hepatic Impairment
5.4 Gastrointestinal Perforation
5.5 Bullous and Exfoliative Skin Disorders
5.6 Cerebrovascular Accident
5.7 Microangiopathic Hemolytic Anemia with Thrombocytopenia
5.8 Ocular Disorders
5.9 Hemorrhage in Patients Taking Warfarin
5.10 Embryo-fetal Toxicity
6 ADVERSE REACTIONS
6.1 Clinical Trial Experience
6.2 Post-Marketing Experience
7 DRUG INTERACTIONS
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.3 Females and Males of Reproductive Potential
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Hepatic Impairment
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
14.1 Non-Small Cell Lung Cancer (NSCLC) – First-Line Treatment of Patients with EGFR Mutations
14.2 NSCLC - Lack of Efficacy of Erlotinib in Maintenance Treatment of Patients without EGFR Mutations
14.3 NSCLC – Maintenance Treatment or Second/Third Line Treatment
14.4 NSCLC – Lack of Efficacy of Erlotinib Administered Concurrently with Chemotherapy
14.5 Pancreatic Cancer - Erlotinib Administered Concurrently with Gemcitabine
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- *
- Sections or subsections omitted from the full prescribing information are not listed.
1 INDICATIONS AND USAGE
1.1 Non-Small Cell Lung Cancer (NSCLC)
Erlotinib tablets are indicated for:
- •
- The treatment of patients with metastatic non-small cell lung cancer (NSCLC) whose tumors have epidermal growth factor receptor (EGFR) exon 19 deletions or exon 21 (L858R) substitution mutations as detected by an FDA-approved test receiving first-line, maintenance, or second or greater line treatment after progression following at least one prior chemotherapy regimen [see Clinical Studies (14.1, 14.3)].
Limitations of use:
- •
- Safety and efficacy of erlotinib tablets have not been established in patients with NSCLC whose tumors have other EGFR mutations [see Clinical Studies (14.1, 14.2)].
- •
- Erlotinib tablets are not recommended for use in combination with platinum-based chemotherapy [see ClinicalStudies (14.4)].
2 DOSAGE AND ADMINISTRATION
2.1 Selection of Patients with Metastatic NSCLC
Select patients for the treatment of metastatic NSCLC with erlotinib tablets based on the presence of EGFR exon 19 deletions or exon 21 (L858R) substitution mutations in tumor or plasma specimens [See Clinical Studies (14.1, 14.2)]. If these mutations are not detected in a plasma specimen, test tumor tissue if available. Information on FDA-approved tests for the detection of EGFR mutations in NSCLC is available at: http://www.fda.gov/CompanionDiagnostics.
2.2 Recommended Dose – NSCLC
The recommended daily dose of erlotinib tablets for NSCLC is 150 mg taken on an empty stomach, i.e., at least one hour before or two hours after the ingestion of food. Treatment should continue until disease progression or unacceptable toxicity occurs.
2.3 Recommended Dose – Pancreatic Cancer
The recommended daily dose of erlotinib tablets for pancreatic cancer is 100 mg taken once daily in combination with gemcitabine. Take erlotinib tablets on an empty stomach, i.e., at least one hour before or two hours after the ingestion of food. Treatment should continue until disease progression or unacceptable toxicity occurs [see Clinical Studies (14.5)].
2.4 Dose Modifications
|
||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
- † For additional information see Warnings and Precautions (5).
- * Reduce erlotinib tablets by 50 mg decrements when restarting therapy following withholding treatment for a dose-limiting toxicity that has resolved to baseline or grade ≤ 1.
- ‡ For additional information see Drug Interactions (7).
- § For additional information see Clinical Pharmacology (12.3).
3 DOSAGE FORMS AND STRENGTHS
25 mg tablets: white to off-white, round, biconvex film-coated tablets debossed with “RL” on one side and “11” on other side.
100 mg tablets: white to off-white, round, biconvex film-coated tablets debossed with “RL” on one side and “12”on other side.
150 mg tablets: white to off-white, round, biconvex film-coated tablets debossed with “RL” on one side and “13” on other side.
5 WARNINGS AND PRECAUTIONS
5.1 Interstitial Lung Disease (ILD)
Cases of serious ILD, including fatal cases, can occur with erlotinib treatment. The overall incidence of ILD in approximately 32,000 erlotinib-treated patients in uncontrolled studies and studies with concurrent chemotherapy was approximately 1.1%. In patients with ILD, the onset of symptoms was between 5 days to more than 9 months (median 39 days) after initiating erlotinib therapy.
Withhold erlotinib for acute onset of new or progressive unexplained pulmonary symptoms such as dyspnea, cough, and fever pending diagnostic evaluation. If ILD is confirmed, permanently discontinue erlotinib [see Dosage and Administration (2.4)].
5.2 Renal Failure
Hepatorenal syndrome, severe acute renal failure including fatal cases, and renal insufficiency can occur with erlotinib treatment. Renal failure may arise from exacerbation of underlying baseline hepatic impairment or severe dehydration. The pooled incidence of severe renal impairment in the 3 monotherapy lung cancer studies was 0.5% in the erlotinib arms and 0.8% in the control arms. The incidence of renal impairment in the pancreatic cancer study was 1.4% in the erlotinib plus gemcitabine arm and 0.4% in the control arm. Withhold erlotinib in patients developing severe renal impairment until renal toxicity is resolved. Perform periodic monitoring of renal function and serum electrolytes during erlotinib treatment [see Adverse Reactions (6.1) and Dosage and Administration (2.4)].
5.3 Hepatotoxicity with or without Hepatic Impairment
Hepatic failure and hepatorenal syndrome, including fatal cases, can occur with erlotinib treatment in patients with normal hepatic function; the risk of hepatic toxicity is increased in patients with baseline hepatic impairment. In clinical studies where patients with moderate to severe hepatic impairment were excluded, the pooled incidence of hepatic failure in the 3 monotherapy lung cancer studies was 0.4% in the erlotinib arms and 0% in the control arms. The incidence of hepatic failure in the pancreatic cancer study was 0.4% in the erlotinib plus gemcitabine arm and 0.4% in the control arm. In a pharmacokinetic study in 15 patients with moderate hepatic impairment (Child-Pugh B) associated with significant liver tumor burden, 10 of these 15 patients died within 30 days of the last erlotinib dose. One patient died from hepatorenal syndrome, 1 patient died from rapidly progressing liver failure and the remaining 8 patients died from progressive disease. Six out of the 10 patients who died had baseline total bilirubin > 3 times ULN.
Perform periodic liver testing (transaminases, bilirubin, and alkaline phosphatase) during treatment with erlotinib. Increased frequency of monitoring of liver function is required for patients with pre-existing hepatic impairment or biliary obstruction. Withhold erlotinib in patients without pre-existing hepatic impairment for total bilirubin levels greater than 3 times the upper limit of normal or transaminases greater than 5 times the upper limit of normal. Withhold erlotinib in patients with pre-existing hepatic impairment or biliary obstruction for doubling of bilirubin or tripling of transaminases values over baseline. Discontinue erlotinib in patients whose abnormal liver tests meeting the above criteria do not improve significantly or resolve within three weeks [see Dosage and Administration (2.4) and Clinical Pharmacology (12.3)].
5.4 Gastrointestinal Perforation
Gastrointestinal perforation, including fatal cases, can occur with erlotinib treatment. Patients receiving concomitant anti-angiogenic agents, corticosteroids, NSAIDs, or taxane-based chemotherapy, or who have prior history of peptic ulceration or diverticular disease may be at increased risk of perforation [see Adverse Reactions (6.1, 6.2)]. The pooled incidence of gastrointestinal perforation in the 3 monotherapy lung cancer studies was 0.2% in the erlotinib arms and 0.1% in the control arms. The incidence of gastrointestinal perforation in the pancreatic cancer study was 0.4% in the erlotinib plus gemcitabine arm and 0% in the control arm. Permanently discontinue erlotinib in patients who develop gastrointestinal perforation [see Dosage and Administration (2.4)].
5.5 Bullous and Exfoliative Skin Disorders
Bullous, blistering and exfoliative skin conditions, including cases suggestive of Stevens-Johnson syndrome/toxic epidermal necrolysis, which in some cases were fatal, can occur with erlotinib treatment [see Adverse Reactions (6.1, 6.2)]. The pooled incidence of bullous and exfoliative skin disorders in the 3 monotherapy lung cancer studies was 1.2% in the erlotinib arms and 0% in the control arms. The incidence of bullous and exfoliative skin disorders in the pancreatic cancer study was 0.4% in the erlotinib plus gemcitabine arm and 0% in the control arm. Discontinue erlotinib treatment if the patient develops severe bullous, blistering or exfoliating conditions [see Dosage and Administration (2.4)].
5.6 Cerebrovascular Accident
In the pancreatic carcinoma trial, seven patients in the erlotinib/gemcitabine group developed cerebrovascular accidents (incidence: 2.5%). One of these was hemorrhagic and was the only fatal event. In comparison, in the placebo/gemcitabine group there were no cerebrovascular accidents. The pooled incidence of cerebrovascular accident in the 3 monotherapy lung cancer studies was 0.6% in the erlotinib arms and not higher than that observed in the control arms.
5.7 Microangiopathic Hemolytic Anemia with Thrombocytopenia
The pooled incidence of microangiopathic hemolytic anemia with thrombocytopenia in the 3 monotherapy lung cancer studies was 0% in the erlotinib arms and 0.1% in the control arms. The incidence of microangiopathic hemolytic anemia with thrombocytopenia in the pancreatic cancer study was 1.4% in the erlotinib plus gemcitabine arm and 0% in the control arm.
5.8 Ocular Disorders
Decreased tear production, abnormal eyelash growth, keratoconjunctivitis sicca or keratitis can occur with erlotinib treatment and can lead to corneal perforation or ulceration [see Adverse Reactions (6.1) and (6.2)]. The pooled incidence of ocular disorders in the 3 monotherapy lung cancer studies was 17.8% in the erlotinib arms and 4% in the control arms. The incidence of ocular disorders in the pancreatic cancer study was 12.8% in the erlotinib plus gemcitabine arm and 11.4% in the control arm. Interrupt or discontinue erlotinib therapy if patients present with acute or worsening ocular disorders such as eye pain [see Dosage and Administration (2.4)].
5.9 Hemorrhage in Patients Taking Warfarin
Severe and fatal hemorrhage associated with International Normalized Ratio (INR) elevations can occur when erlotinib and warfarin are administered concurrently. Regularly monitor prothrombin time and INR during erlotinib treatment in patients taking warfarin or other coumarin-derivative anticoagulants [see Adverse Reactions (6.1) and Drug Interactions (7)].
5.10 Embryo-fetal Toxicity
Based on animal data and its mechanism of action, erlotinib can cause fetal harm when administered to a pregnant woman. When given during organogenesis, erlotinib administration resulted in embryo-fetal lethality and abortion in rabbits at exposures approximately 3 times the exposure at the recommended human daily dose of 150 mg. Advise pregnant women of the potential risk to a fetus.
Advise females of reproductive potential to use effective contraception during therapy and for one month after the last dose of erlotinib [see Use in Specific Populations (8.1) and (8.3), Clinical Pharmacology (12.1)].
6 ADVERSE REACTIONS
The following serious adverse reactions, which may include fatalities, are discussed in greater detail in other sections of the labeling:
- •
- Interstitial Lung Disease (ILD) [see Warnings and Precautions (5.1)]
- •
- Renal Failure [see Warnings and Precautions (5.2)]
- •
- Hepatotoxicity with or without Hepatic Impairment [see Warnings and Precautions (5.3)]
- •
- Gastrointestinal Perforation [see Warnings and Precautions (5.4)]
- •
- Bullous and Exfoliative Skin Disorders [see Warnings and Precautions (5.5)]
- •
- Cerebrovascular Accident [see Warnings and Precautions (5.6)]
- •
- Microangiopathic Hemolytic Anemia with Thrombocytopenia [see Warnings and Precautions (5.7)]
- •
- Ocular Disorders [see Warnings and Precautions (5.8)]
- •
- Hemorrhage in Patients Taking Warfarin [see Warnings and Precautions (5.9)]
6.1 Clinical Trial Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Safety evaluation of erlotinib is based on more than 1200 cancer patients who received erlotinib as monotherapy, more than 300 patients who received erlotinib 100 or 150 mg plus gemcitabine, and 1228 patients who received erlotinib concurrently with other chemotherapies. The most common adverse reactions with erlotinib are rash and diarrhea usually with onset during the first month of treatment. The incidences of rash and diarrhea from clinical studies of erlotinib for the treatment of NSCLC and pancreatic cancer were 70% for rash and 42% for diarrhea.
Non-Small Cell Lung Cancer
First-Line Treatment of Patients with EGFR Mutations
The most frequent (≥ 30%) adverse reactions in erlotinib-treated patients were diarrhea, asthenia, rash, cough, dyspnea, and decreased appetite. In erlotinib-treated patients the median time to onset of rash was 15 days and the median time to onset of diarrhea was 32 days.
The most frequent Grade 3 to 4 adverse reactions in erlotinib-treated patients were rash and diarrhea.
Dose interruptions or reductions due to adverse reactions occurred in 37% of erlotinib-treated patients, and 14.3% of erlotinib-treated patients discontinued therapy due to adverse reactions. In erlotinib-treated patients, the most frequently reported adverse reactions leading to dose modification were rash (13%), diarrhea (10%), and asthenia (3.6%).
Common adverse reactions in Study 1, occurring in at least 10% of patients who received erlotinib or chemotherapy and an increase in ≥ 5% in the erlotinib-treated group, are graded by National Cancer Institute Common Toxicity Criteria for Adverse Events version 3 (NCI-CTCAE v3) Grade in Table 1. The median duration of erlotinib treatment was 9.6 months in Study 1.
Table 1: Adverse Reactions with an Incidence Rate ≥ 10% and an Increase of ≥ 5% in the Erlotinib-Treated Group (Study 1)
|
Erlotinib N = 84 |
Chemotherapy† N = 83 |
|||
|
Adverse Reaction |
All Grades % |
Grades 3 to 4 % |
All Grades % |
Grades 3 to 4 % |
|
Rash‡ |
85 |
14 |
5 |
0 |
|
Diarrhea |
62 |
5 |
21 |
1 |
|
Cough |
48 |
1 |
40 |
0 |
|
Dyspnea |
45 |
8 |
30 |
4 |
|
Dry skin |
21 |
1 |
2 |
0 |
|
Back pain |
19 |
2 |
5 |
0 |
|
Chest pain |
18 |
1 |
12 |
0 |
|
Conjunctivitis |
18 |
0 |
0 |
0 |
|
Mucosal inflammation |
18 |
1 |
6 |
0 |
|
Pruritus |
16 |
0 |
1 |
0 |
|
Paronychia |
14 |
0 |
0 |
0 |
|
Arthralgia |
13 |
1 |
6 |
1 |
|
Musculoskeletal pain |
11 |
1 |
1 |
0 |
† Platinum-based chemotherapy (cisplatin or carboplatin with gemcitabine or docetaxel).
‡ Rash as a composite term includes rash, acne, folliculitis, erythema, acneiform dermatitis, dermatitis, palmar-plantar erythrodysesthesia syndrome, exfoliative rash, erythematous rash, rash pruritic, skin toxicity, eczema, follicular rash, skin ulcer.
Hepatic Toxicity: One erlotinib-treated patient experienced fatal hepatic failure and four additional patients experienced grade 3 to 4 liver test abnormalities in Study 1 [see Warnings and Precautions (5.3)].
Maintenance Treatment
Adverse reactions, regardless of causality, that occurred in at least 3% of patients treated with single-agent erlotinib at 150 mg and at least 3% more often than in the placebo group in the randomized maintenance trial (Study 3) are summarized by NCI-CTCAE v3Grade in Table 2.
The most common adverse reactions in patients receiving single-agent erlotinib 150 mg were rash and diarrhea. Grade 3 to 4 rash and diarrhea occurred in 9% and 2%, respectively, in erlotinib-treated patients. Rash and diarrhea resulted in study discontinuation in 1% and 0.5% of erlotinib-treated patients, respectively. Dose reduction or interruption for rash and diarrhea was needed in 5% and 3% of patients, respectively. In erlotinib-treated patients the median time to onset of rash was 10 days, and the median time to onset of diarrhea was 15 days.
Table 2: NSCLC Maintenance Study: Adverse Reactions Occurring with an Incidence Rate ≥ 10% and an Increase of ≥ 5% in the Single-Agent Erlotinib Group compared to the Placebo Group (Study 3)
|
Adverse Reaction |
ERLOTINIB N = 433 |
PLACEBO N = 445 |
||||
|
Any Grade |
Grade 3 |
Grade 4 |
Any Grade |
Grade 3 |
Grade 4 |
|
|
% |
% |
% |
% |
% |
% |
|
|
Rash † |
60 |
9 |
0 |
9 |
0 |
0 |
|
Diarrhea |
20 |
2 |
0 |
4 |
0 |
0 |
† Rash as a composite term includes: rash, acne, acneiform dermatitis, skin fissures, erythema, papular rash, rash generalized, pruritic rash, skin exfoliation, urticaria, dermatitis, eczema, exfoliative rash, exfoliative dermatitis, furuncle, macular rash, pustular rash, skin hyperpigmentation, skin reaction, skin ulcer.
Liver test abnormalities including ALT elevations were observed at Grade 2 or greater severity in 3% of erlotinib-treated patients and 1% of placebo-treated patients. Grade 2 and above bilirubin elevations were observed in 5% of erlotinib-treated patients and in < 1% in the placebo group [see Dosage and Administration (2.4) and Warnings and Precautions (5.3)].
Second/Third Line Treatment
Adverse reactions, regardless of causality, that occurred in at least 10% of patients treated with single-agent erlotinib at 150 mg and at least 5% more often than in the placebo group in the randomized trial of patients with NSCLC are summarized by NCI-CTC v2 Grade in Table 3.
The most common adverse reactions in this patient population were rash and diarrhea. Grade 3 to 4 rash and diarrhea occurred in 9% and 6%, respectively, in erlotinib-treated patients. Rash and diarrhea each resulted in study discontinuation in 1% of erlotinib-treated patients. Six percent and 1% of patients needed dose reduction for rash and diarrhea, respectively. The median time to onset of rash was 8 days, and the median time to onset of diarrhea was 12 days.
Table 3: NSCLC 2nd/3rd Line Study: Adverse Reactions Occurring with an Incidence Rate ≥ 10% and an Increase of ≥ 5% in the Single-Agent Erlotinib Group Compared to the Placebo Group (Study 4)
|
Adverse Reaction |
Erlotinib 150 mg N = 485 |
Placebo N = 242 |
||||
|
Any Grade |
Grade 3 |
Grade 4 |
Any Grade |
Grade 3 |
Grade 4 |
|
|
% |
% |
% |
% |
% |
% |
|
|
Rash † |
75 |
8 |
< 1 |
17 |
0 |
0 |
|
Diarrhea |
54 |
6 |
< 1 |
18 |
< 1 |
0 |
|
Anorexia |
52 |
8 |
1 |
38 |
5 |
< 1 |
|
Fatigue |
52 |
14 |
4 |
45 |
16 |
4 |
|
Dyspnea |
41 |
17 |
11 |
35 |
15 |
11 |
|
Nausea |
33 |
3 |
0 |
24 |
2 |
0 |
|
Infection |
24 |
4 |
0 |
15 |
2 |
0 |
|
Stomatitis |
17 |
< 1 |
0 |
3 |
0 |
0 |
|
Pruritus |
13 |
< 1 |
0 |
5 |
0 |
0 |
|
Dry skin |
12 |
0 |
0 |
4 |
0 |
0 |
|
Conjunctivitis |
12 |
< 1 |
0 |
2 |
< 1 |
0 |
|
Keratoconjunctivitis sicca |
12 |
0 |
0 |
3 |
0 |
0 |
† Rash as a composite term includes: rash, palmar-plantar erythrodysesthesia syndrome, acne, skin disorder, pigmentation disorder, erythema, skin ulcer, exfoliative dermatitis, papular rash, skin desquamation.
Liver function test abnormalities [including elevated alanine aminotransferase (ALT), aspartate aminotransferase (AST) and bilirubin] were observed in patients receiving single-agent erlotinib 150 mg. These elevations were mainly transient or associated with liver metastases. Grade 2 [> 2.5 to 5 times upper limit of normal (ULN)] ALT elevations occurred in 4% and < 1% of erlotinib and placebo treated patients, respectively. Grade 3 (> 5 to 20 times ULN) elevations were not observed in erlotinib-treated patients. Erlotinib dosing should be interrupted or discontinued if changes in liver function are severe [see Dosage and Administration (2.4)].
Pancreatic Cancer - Erlotinib Administered Concurrently with Gemcitabine
This was a randomized, double–blind, placebo-controlled study of erlotinib (150 mg or 100 mg daily) or placebo plus gemcitabine (1000 mg/m2 by intravenous infusion) in patients with locally advanced, unresectable or metastatic pancreatic cancer (Study 5). The safety population comprised 282 patients in the erlotinib group (259 in the 100 mg cohort and 23 in the 150 mg cohort) and 280 patients in the placebo group (256 in the 100 mg cohort and 24 in the 150 mg cohort).
Adverse reactions that occurred in at least 10% of patients treated with erlotinib 100 mg plus gemcitabine in the randomized trial of patients with pancreatic cancer (Study 5) were graded according to NCI-CTC v2 in Table 4.
The most common adverse reactions in pancreatic cancer patients receiving erlotinib 100 mg plus gemcitabine were fatigue, rash, nausea, anorexia and diarrhea. In the erlotinib plus gemcitabine arm, Grade 3 to 4 rash and diarrhea were each reported in 5% of patients. The median time to onset of rash and diarrhea was 10 days and 15 days, respectively. Rash and diarrhea each resulted in dose reductions in 2% of patients, and resulted in study discontinuation in up to 1% of patients receiving erlotinib plus gemcitabine. Severe adverse reactions (≥ Grade 3 NCI-CTC) in the erlotinib plus gemcitabine group with incidences < 5% included syncope, arrhythmias, ileus, pancreatitis, hemolytic anemia including microangiopathic hemolytic anemia with thrombocytopenia, myocardial infarction/ischemia, cerebrovascular accidents including cerebral hemorrhage, and renal insufficiency [see Warnings and Precautions (5)].
The 150 mg cohort was associated with a higher rate of certain class-specific adverse reactions including rash and required more frequent dose reduction or interruption.
Table 4: Adverse Reactions Occurring with an Incidence Rate ≥ 10% and an Increase of ≥ 5% in Erlotinib-Treated Pancreatic Cancer Patients: 100 mg Cohort (Study 5)
|
Adverse Reaction |
Erlotinib + Gemcitabine 1000 mg/m2 IV N = 259 |
Placebo + Gemcitabine 1000 mg/m2 IV N = 256 |
||||
|
Any Grade |
Grade 3 |
Grade 4 |
Any Grade |
Grade 3 |
Grade 4 |
|
|
% |
% |
% |
% |
% |
% |
|
|
Rash† |
70 |
5 |
0 |
30 |
1 |
0 |
|
Diarrhea |
48 |
5 |
< 1 |
36 |
2 |
0 |
|
Decreased weight |
39 |
2 |
0 |
29 |
< 1 |
0 |
|
Infection* |
39 |
13 |
3 |
30 |
9 |
2 |
|
Pyrexia |
36 |
3 |
0 |
30 |
4 |
0 |
|
Stomatitis |
22 |
< 1 |
0 |
12 |
0 |
0 |
|
Depression |
19 |
2 |
0 |
14 |
< 1 |
0 |
|
Cough |
16 |
0 |
0 |
11 |
0 |
0 |
|
Headache |
15 |
< 1 |
0 |
10 |
0 |
0 |
* Infections as a composite term include infections with unspecified pathogens as well as bacterial (including chlamydial, rickettsial, mycobacterial and mycoplasmal), parasitic (including helminthic, ectoparasitic and protozoal), viral and fungal infectious disorders.
† Rash as a composite term includes: rash, palmar-plantar erythrodysesthesia syndrome, pigmentation disorder, acneiform dermatitis, folliculitis, photosensitivity reaction, Stevens-Johnson syndrome, urticaria, erythematous rash, skin disorder, skin ulcer.
Ten patients (4%) in the erlotinib/gemcitabine group and three patients (1%) in the placebo/gemcitabine group developed deep venous thrombosis. The overall incidence of grade 3 or 4 thrombotic events, including deep venous thrombosis was 11% for erlotinib plus gemcitabine and 9% for placebo plus gemcitabine.
The incidences of liver test abnormalities (≥ Grade 2) in Study 5 are provided in Table 5 [see Dosage and Administration (2.4) and Warnings and Precautions (5.3)].
Table 5: Liver Test Abnormalities in Pancreatic Cancer Patients: 100 mg Cohort (Study 5)
|
Erlotinib + Gemcitabine 1000 mg/m2 IV N = 259 |
Placebo + Gemcitabine 1000 mg/m2 IV N = 256 |
|||||
|
Grade 2 |
Grade 3 |
Grade 4 |
Grade 2 |
Grade 3 |
Grade 4 |
|
|
Bilirubin |
17% |
10% |
< 1% |
11% |
10% |
3% |
|
ALT |
31% |
13% |
< 1% |
22% |
9% |
0% |
|
AST |
24% |
10% |
< 1% |
19% |
9% |
0% |
NSCLC and Pancreatic Indications: Selected Low Frequency Adverse Reactions
Gastrointestinal Disorders
Cases of gastrointestinal bleeding (including fatalities) have been reported, some associated with concomitant warfarin or NSAID administration [see Warnings and Precautions (5.9) and Drug Interactions (7)]. These adverse reactions were reported as peptic ulcer bleeding (gastritis, gastroduodenal ulcers), hematemesis, hematochezia, melena and hemorrhage from possible colitis.
6.2 Post-Marketing Experience
The following adverse reactions have been identified during post approval use of erlotinib. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Musculoskeletal and Connective Tissue Disorders: myopathy, including rhabdomyolysis, in combination with statin therapy
Eye Disorders: ocular inflammation including uveitis
7 DRUG INTERACTIONS
CYP3A4 Inhibitors
Co-administration of erlotinib with a strong CYP3A4 inhibitor or a combined CYP3A4 and CYP1A2 inhibitor increased erlotinib exposure. Erlotinib is metabolized primarily by CYP3A4 and to a lesser extent by CYP1A2. Increased erlotinib exposure may increase the risk of exposure-related toxicity [see Clinical Pharmacology (12.3)].
Avoid co-administering erlotinib with strong CYP3A4 inhibitors (e.g., boceprevir, clarithromycin, conivaptan, indinavir, itraconazole, ketoconazole, lopinavir/ritonavir, nefazodone, nelfinavir, posaconazole, ritonavir, saquinavir, telithromycin, voriconazole, grapefruit or grapefruit juice) or a combined CYP3A4 and CYP1A2 inhibitor (e.g., ciprofloxacin). Reduce the erlotinib dosage when co-administering with a strong CYP3A4 inhibitor or a combined CYP3A4 and CYP1A2 inhibitor if co-administration is unavoidable [see Dosage and Administration (2.4)].
CYP3A4 Inducers
Pre-treatment with a CYP3A4 inducer prior to erlotinib decreased erlotinib exposure [see Clinical Pharmacology (12.3)]. Increase the erlotinib dosage if co-administration with CYP3A4 inducers (e.g., carbamazepine, phenytoin, rifampin, rifabutin, rifapentine, phenobarbital and St. John's wort) is unavoidable [see Dosage and Administration (2.4)].
CYP1A2 Inducers and Cigarette Smoking
Cigarette smoking decreased erlotinib exposure. Avoid smoking tobacco (CYP1A2 inducer) and avoid concomitant use of erlotinib with moderate CYP1A2 inducers (e.g., teriflunomide, rifampin, or phenytoin). Increase the erlotinib dosage in patients that smoke tobacco or when co-administration with moderate CYP1A2 inducers is unavoidable [see Dosage and Administration (2.4) and Clinical Pharmacology (12.3)].
Drugs that Increase Gastric pH
Co-administration of erlotinib with proton pump inhibitors (e.g., omeprazole) and H-2 receptor antagonists (e.g., ranitidine) decreased erlotinib exposure [see Clinical Pharmacology (12.3)]. For proton pump inhibitors, avoid concomitant use if possible. For H‑2 receptor antagonists and antacids, modify the dosing schedule [see Dosage and Administration (2.4)]. Increasing the dose of erlotinib when co-administered with gastric PH elevating agents is not likely to compensate for the loss of exposure.
Anticoagulants
Interaction with coumarin-derived anticoagulants, including warfarin, leading to increased International Normalized Ratio (INR) and bleeding adverse reactions, which in some cases were fatal, have been reported in patients receiving erlotinib. Regularly monitor prothrombin time or INR in patients taking coumarin-derived anticoagulants. Dose modifications of erlotinib are not recommended [see Warnings and Precautions (5.9) and Adverse Reactions (6.1)].
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
Based on animal data and its mechanism of action, erlotinib can cause fetal harm when administered to a pregnant woman. Limited available data on use of erlotinib in pregnant women are not sufficient to inform a risk of major birth defects or miscarriage. When given during organogenesis, erlotinib administration resulted in embryo-fetal lethality and abortion in rabbits at exposures approximately 3 times the exposure at the recommended human daily dose of 150 mg. Advise pregnant women of the potential risk to a fetus.
In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2 to 4% and 15 to 20%, respectively.
Data
Animal Data
Erlotinib has been shown to cause maternal toxicity resulting in embryo-fetal lethality and abortion in rabbits when given during the period of organogenesis at doses that result in plasma drug concentrations approximately 3 times those achieved at the recommended dose in humans (AUCs at 150 mg daily dose). During the same period, there was no increase in the incidence of embryo-fetal lethality or abortion in rabbits or rats at doses resulting in exposures approximately equal to those in humans at the recommended daily dose. In an independent fertility study female rats treated with 30 mg/m2/day or 60 mg/m2/day (0.3 or 0.7 times the recommended daily dose, on a mg/m2 basis) of erlotinib had an increase in early resorptions that resulted in a decrease in the number of live fetuses.
No teratogenic effects were observed in rabbits or rats dosed with erlotinib during organogenesis at doses up to 600 mg/m2/day in the rabbit (3 times the plasma drug concentration seen in humans at 150 mg/day) and up to 60 mg/m2/day in the rat (0.7 times the recommended dose of 150 mg/day on a mg/m2 basis).
8.2 Lactation
Risk Summary
There are no data on the presence of erlotinib in human milk, or the effects of erlotinib on the breastfed infant or on milk production. Because of the potential for serious adverse reactions in breastfed infants from erlotinib, including interstitial lung disease, hepatotoxicity, bullous and exfoliative skin disorders, microangiopathic hemolytic anemia with thrombocytopenia, ocular disorders, and diarrhea. Advise a lactating woman not to breastfeed during treatment with erlotinib and for 2 weeks after the final dose.
8.3 Females and Males of Reproductive Potential
Contraception
Females
Erlotinib can cause fetal harm when administered to a pregnant woman [see Use in Specific Populations (8.1)]. Advise females of reproductive potential to use effective contraception during treatment with erlotinib and for one month after the last dose of erlotinib.
8.4 Pediatric Use
The safety and effectiveness of erlotinib in pediatric patients have not been established.
In an open-label, multicenter trial, 25 pediatric patients (median age 14 years, range 3 to 20 years) with recurrent or refractory ependymoma were randomized (1:1) to erlotinib or etoposide. Thirteen patients received erlotinib at a dose of 85 mg/m2/day orally until disease progression, death, patient request, investigator decision to discontinue study drug, or intolerable toxicity. Four patients randomized to etoposide also received erlotinib following disease progression. The trial was terminated prematurely for lack of efficacy; there were no objective responses observed in these 17 erlotinib-treated patients.
No new adverse events were identified in the pediatric population.
Based on the population pharmacokinetics analysis conducted in 105 pediatric patients (2 to 21 years old) with cancer, the geometric mean estimates of CL/F/BSA (apparent clearance normalized to body surface area) were comparable across the three age groups: 2 to 6 years (n = 29), 7 to 16 years (n = 59), and 17 to 21 years (n = 17).
8.5 Geriatric Use
Of the 1297 subjects in clinical studies of erlotinib for the treatment of NSCLC and pancreatic cancer 40% were 65 and older while 10% were 75 and older. No overall differences in safety or efficacy were observed between subjects 65 years and older and those younger than 65.
8.6 Hepatic Impairment
Hepatic failure and hepatorenal syndrome, including fatal cases, can occur with erlotinib treatment in patients with normal hepatic function; the risk of hepatic toxicity is increased in patients with baseline hepatic impairment [see Warnings and Precautions (5.3), Adverse Reactions (6.1, 6.2), and Dosage and Administration]. Monitor patients with hepatic impairment (total bilirubin greater than upper limit of normal (ULN) or Child-Pugh A, B and C) during therapy with erlotinib. Treatment with erlotinib should be used with increased monitoring in patients with total bilirubin greater than 3 times ULN [see Warnings and Precautions (5.3), Adverse Reactions (6.1, 6.2), and Dosage and Administration (2.4)].
10 OVERDOSAGE
11 DESCRIPTION
Erlotinib, a kinase inhibitor, is a quinazolinamine with the chemical name N-(3-ethynylphenyl)-6,7-bis(2-methoxyethoxy)‑4-quinazolinamine hydrochloride. Erlotinib tablets contain erlotinib as the hydrochloride salt that has the following structural formula:
Erlotinib hydrochloride is off-white to pale yellow powder and has the molecular formula C22H24ClN3O4 and a molecular weight of 429.90. The molecule has a pKa of about 4.44 at 25°C. Erlotinib hydrochloride is slightly soluble in methanol; practically insoluble in acetonitrile and in acetone.
Erlotinib hydrochloride is insoluble over the pH range of 1.2 to 8.0
Erlotinib tablets for oral administration are available in three dosage strengths containing erlotinib hydrochloride (27.3 mg, 109.3 mg and 163.9 mg) equivalent to 25 mg, 100 mg and 150 mg erlotinib and the following inactive ingredients: hypromellose, lactose monohydrate, magnesium stearate, microcrystalline cellulose, polyethylene glycol, sodium lauryl sulfate, sodium starch glycolate, talc and titanium dioxide.
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Epidermal growth factor receptor (EGFR) is expressed on the cell surface of both normal and cancer cells. In some tumor cells signaling through this receptor plays a role in tumor cell survival and proliferation irrespective of EGFR mutation status. Erlotinib reversibly inhibits the kinase activity of EGFR, preventing autophosphorylation of tyrosine residues associated with the receptor and thereby inhibiting further downstream signaling. Erlotinib binding affinity for EGFR exon 19 deletion or exon 21 (L858R) mutations is higher than its affinity for the wild type receptor. Erlotinib inhibition of other tyrosine kinase receptors has not been fully characterized.
12.3 Pharmacokinetics
Absorption
Erlotinib is about 60% absorbed after oral administration. Peak plasma levels occur 4 hours after dosing.
Effect of Food
Food increased the bioavailability of erlotinib to approximately 100%.
Distribution:
Erlotinib is 93% protein bound to plasma albumin and alpha-1 acid glycoprotein (AAG).
Erlotinib has an apparent volume of distribution of 232 liters.
Elimination
Erlotinib is eliminated with a median half-life of 36.2 hours in patients receiving the single-agent erlotinib 2nd/3rd line regimen. Time to reach steady state plasma concentration would therefore be 7 to 8 days.
Metabolism
Erlotinib is metabolized primarily by CYP3A4 and to a lesser extent by CYP1A2, and the extrahepatic isoform CYP1A1, in vitro.
Excretion
Following a 100 mg oral dose, 91% of the dose was recovered: 83% in feces (1% of the dose as intact parent) and 8% in urine (0.3% of the dose as intact parent).
Specific Populations
Neither age, body weight, nor gender had a clinically significant effect on the systemic exposure of erlotinib in NSCLC patients receiving single-agent erlotinib for 2nd/3rd line treatment or for maintenance treatment, and in pancreatic cancer patients who received erlotinib plus gemcitabine. The pharmacokinetics of erlotinib in patients with compromised renal function is unknown.
Patients with Hepatic Impairment
In vitro and in vivo evidence suggest that erlotinib is cleared primarily by the liver. However, erlotinib exposure was similar in patients with moderately impaired hepatic function (Child-Pugh B) compared with patients with adequate hepatic function including patients with primary liver cancer or hepatic metastases.
Patients That Smoke Tobacco Cigarettes
In a single-dose pharmacokinetics trial in healthy volunteers, cigarette smoking (moderate CYP1A2 inducer) increased erlotinib clearance and decreased erlotinib AUC0-inf by 64% (95% CI, 46 to 76%) in current smokers compared with former/never smokers. In a NSCLC trial, current smokers achieved erlotinib steady-state trough plasma concentrations which were approximately 2-fold less than the former smokers or patients who had never smoked. This effect was accompanied by a 24% increase in apparent erlotinib plasma clearance. In another study which was conducted in NSCLC patients who were current smokers, pharmacokinetic analyses at steady-state indicated a dose-proportional increase in erlotinib exposure when the erlotinib dose was increased from 150 mg to 300 mg. [see Dosage and Administration (2.4), Drug Interactions (7) and Patient Counseling Information (17)].
Drug Interaction Studies
Co-administration of gemcitabine had no effect on erlotinib plasma clearance.
CYP3A4 Inhibitors
Co-administration with a strong CYP3A4 inhibitor, ketoconazole, increased erlotinib AUC by 67%. Co-administration with a combined CYP3A4 and CYP1A2 inhibitor, ciprofloxacin, increased erlotinib exposure [AUC] by 39%, and increased erlotinib maximum concentration [Cmax] by 17%. [see Dose Modifications (2.4), Drug Interactions (7)].
CYP3A4 Inducers
Pre-treatment with the CYP3A4 inducer rifampicin, for 7 to 11 days prior to erlotinib, decreased erlotinib AUC by 58% to 80% [see Dose Modifications (2.4), Drug Interactions (7)].
CYP1A2 Inducers or Smoking Tobacco
See Specific Populations Section [see Dose Modifications (2.4), Drug Interactions (7)].
Drugs that Increase Gastric pH
Erlotinib solubility is pH dependent and decreases as pH increases. When a proton pump inhibitor (omeprazole) was co-administered with erlotinib the erlotinib exposure [AUC] was decreased by 46% and the erlotinib maximum concentration [Cmax] was decreased by 61%. When erlotinib was administered 2 hours following a 300 mg dose of an H-2 receptor antagonist (ranitidine), the erlotinib AUC was reduced by 33% and the erlotinib Cmax was reduced by 54%. When erlotinib was administered with ranitidine 150 mg twice daily (at least 10 h after the previous ranitidine evening dose and 2 h before the ranitidine morning dose), the erlotinib AUC was decreased by 15% and the erlotinib Cmax was decreased by 17% [see Dose Modifications (2.4), Drug Interactions (7)].
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Two-year carcinogenicity studies were conducted in mice and rats with erlotinib at oral doses of up to 60 mg/kg/day in mice, 5 mg/kg/day in female rats, and 10 mg/kg/day in male rats. The studies were negative for carcinogenic findings. Exposure in mice at the highest dose tested was approximately 10 times the exposure in humans at the erlotinib dose of 150 mg/day. The highest dose evaluated in male rats resulted in exposures that were twice those in humans and exposures at the highest tested dose in female rats were slightly lower than those in humans.
Erlotinib did not cause genetic damage in a series of in vitro assays (bacterial mutation, human lymphocyte chromosome aberration and mammalian cell mutation) and in the in vivo mouse bone marrow micronucleus test.
Erlotinib did not impair fertility in either male or female rats.
14 CLINICAL STUDIES
14.1 Non-Small Cell Lung Cancer (NSCLC) – First-Line Treatment of Patients with EGFR Mutations
Study 1
The safety and efficacy of erlotinib as monotherapy for the first-line treatment of patients with metastatic NSCLC containing EGFR exon 19 deletions or exon 21 (L858R) substitution mutations was demonstrated in Study 1, a randomized, open-label, clinical trial conducted in Europe. One hundred seventy-four (174) White patients were randomized 1:1 to receive erlotinib 150 mg once daily until disease progression (n = 86) or four cycles of a standard platinum-based doublet chemotherapy (n = 88); standard chemotherapy regimens were cisplatin plus gemcitabine, cisplatin plus docetaxel, carboplatin plus gemcitabine, and carboplatin plus docetaxel. The main efficacy outcome measure was progression-free survival (PFS) as assessed by the investigator. Randomization was stratified by EGFR mutation (exon 19 deletion or exon 21 (L858R) substitution) and Eastern Cooperative Oncology Group Performance Status (ECOG PS) (0 vs. 1 vs. 2). EGFR mutation status for screening and enrollment of patients was determined by a clinical trials assay (CTA). Tumor samples from 134 patients (69 patients from the erlotinib arm and 65 patients from the chemotherapy arm) were tested retrospectively by the FDA-approved companion diagnostic, cobas® EGFR Mutation Test.
Baseline demographics of the overall study population were: female (72%), White (99%), age ≥ 65 years (51%), ECOG PS 1 (53%), with ECOG PS 0 (33%), and ECOG PS 2 (14%), current smoker (11%), past-smoker (20%), and never smoker (69%). The disease characteristics were 93% Stage IV and 7% Stage IIIb with pleural effusion as classified by the American Joint Commission on Cancer (AJCC, 6th edition), 93% adenocarcinoma, 66% exon 19 mutation deletions and 34% exon 21 (L858R) point mutation by CTA.
A statistically significant improvement in investigator-determined PFS (based on RECIST 1 or clinical progression) was demonstrated for patients randomized to erlotinib compared to those randomized to chemotherapy (see Table 6 and Figure 1). Similar results for PFS (based on RECIST 1) were observed for the subgroup evaluated by an independent-review committee (approximately 75% of patients evaluated in Study 1) and in the subgroup of 134 patients (77% of Study 1 population) with EGFR mutations confirmed by the cobas® EGFR Mutation Test.
A protocol-specified analysis of overall survival (OS) conducted at the time of the final analysis of PFS showed no statistically significant difference between the erlotinib and chemotherapy arms. At the time of the data cut-off, 84% of patients in the chemotherapy arm had received at least one subsequent treatment, of whom 97% received an EGFR-tyrosine kinase inhibitor. In the erlotinib arm, 66% of patients had received at least one subsequent treatment.
Table 6: Efficacy Results (Study 1)
|
Efficacy Parameter |
Erlotinib (N = 86) |
Chemotherapy (N = 88) |
|
Progression-Free Survival |
||
|
71 (83%) |
63 (72%) |
|
10.4 (8.7, 12.9) |
5.2 (4.6, 6) |
|
0.34 (0.23, 0.49) |
|
|
< 0.001 |
|
|
Overall Survival |
||
|
55 (64%) |
54 (61%) |
|
22.9 (17, 26.8) |
19.5 (17.3, 28.4) |
|
0.93 (0.64, 1.35) |
|
|
Objective Response |
||
|
|
16% (9%, 25.3%) |
(1) Unstratified Cox regression model.
Figure 1: Kaplan-Meier Curves of Investigator-Assessed PFS in Study 1
In exploratory subgroup analyses based on EGFR mutation subtype, the hazard ratio (HR) for PFS was 0.27 (95% CI 0.17 to 0.43) in patients with exon 19 deletions and 0.52 (95% CI 0.29 to 0.95) in patients with exon 21 (L858R) substitution. The HR for OS was 0.94 (95% CI 0.57 to 1.54) in the exon 19 deletion subgroup and 0.99 (95% CI 0.56 to 1.76) in the exon 21 (L858R) substitution subgroup.
14.2 NSCLC - Lack of Efficacy of Erlotinib in Maintenance Treatment of Patients without EGFR Mutations
Lack of efficacy of erlotinib for the maintenance treatment of patients with NSCLC without EGFR activating mutations was demonstrated in Study 2. Study 2 was a multicenter, placebo-controlled, randomized trial of 643 patients with advanced NSCLC without an EGFR exon 19 deletion or exon 21 L858R mutation who had not experienced disease progression after four cycles of platinum-based chemotherapy. Patients were randomized 1:1 to receive erlotinib 150 mg or placebo orally once daily (322 erlotinib, 321 placebo) until disease progression or unacceptable toxicity. Following progression on initial therapy, patients were eligible to enter an open-label phase. Baseline characteristics were as follows: median age 61 years (35% age ≥ 65 years), 75% male, 77% White, 21% Asian, 28% ECOG PS 0, 72% ECOG PS 1, 16% never smokers, 58% current smokers, 57% adenocarcinoma, 35% squamous cell carcinoma, 22% stage IIIB disease not amenable to combined modality treatment, and 78% stage IV disease. Fifty percent of patients randomized to erlotinib entered the open-label phase and received chemotherapy, while 77% of patients randomized to placebo entered the open-label phase and received erlotinib.
The main efficacy outcome was overall survival (OS). Median OS was 9.7 months in the erlotinib arm and 9.5 months in the placebo arm; the hazard ratio for OS was 1.02 (95% CI 0.85, 1.22). Median PFS was 3 months in the erlotinib arm and 2.8 months in the placebo arm; the hazard ratio for PFS was 0.94 (95% CI 0.80, 1.11).
14.3 NSCLC – Maintenance Treatment or Second/Third Line Treatment
Two randomized, double-blind, placebo-controlled trials, Studies 3 and 4, examined the efficacy and safety of erlotinib administered to patients with metastatic NSCLC as maintenance therapy after initial treatment with chemotherapy (Study 3) or with disease progression following initial treatment with chemotherapy (Study 4). Determination of EGFR mutation status was not required for enrollment.
Study 3
The efficacy and safety of erlotinib as maintenance treatment of NSCLC were demonstrated in Study 3, a randomized, double-blind, placebo-controlled trial conducted in 26 countries, in 889 patients with metastatic NSCLC whose disease did not progress during first-line platinum-based chemotherapy. Patients were randomized 1:1 to receive erlotinib 150 mg or placebo orally once daily (438 erlotinib, 451 placebo) until disease progression or unacceptable toxicity. The primary objective of the study was to determine if the administration of erlotinib after standard platinum-based chemotherapy in the treatment of NSCLC resulted in improved progression-free survival (PFS) when compared with placebo, in all patients or in patients with EGFR immunohistochemistry (IHC) positive tumors.
Baseline demographics of the overall study population were as follows: male (74%), age < 65 years (66%), ECOG PS 1 (69%), ECOG PS 0 (31%), white (84%), Asian (15%), current smoker (55%), past-smoker (27%), and never smoker (17%). Disease characteristics were as follows: Stage IV (75%), Stage IIIb with effusion (25%) as classified by AJCC (6th edition) with histologic subtypes of adenocarcinoma including bronchioalveolar (45%), squamous (40%) and large cell (5%); and EGFR IHC positive (70%), negative (14%), indeterminate (4%), and missing (12%).
Table 7: Efficacy Results (Study 3): (ITT Population)1
|
Efficacy Parameter |
Erlotinib (N = 438) |
Placebo (N = 451) |
|
Progression-Free Survival (PFS) based on investigator assessment |
||
|
349 (80%) |
400 (89%) |
|
2.8 (2.8, 3.1) |
2.6 (1.9, 2.7) |
|
0.71 (0.62, 0.82) |
|
|
p < 0.0001 |
|
|
Overall Survival (OS) |
||
|
298 (68%) |
350 (78%) |
|
12 (10.6, 13.9) |
11 (9.9, 12.1) |
|
0.81 (0.70, 0.95) |
|
|
0.0088 |
|
- (1) Patients with PD prior to randomization were excluded from PFS and TTP analysis.
- (2) Univariate Cox regression model.
- (3) Unstratified log-rank test.
Figure 2 depicts the Kaplan-Meier Curves for Overall Survival (ITT Population).
- Figure 2: Kaplan-Meier Curves for Overall Survival of Patients by Treatment Group in Study 3
Study 4
The efficacy and safety of single-agent erlotinib was assessed in Study 4, a randomized, double blind, placebo-controlled trial in 731 patients with locally advanced or metastatic NSCLC after failure of at least one chemotherapy regimen. Patients were randomized 2:1 to receive erlotinib 150 mg or placebo (488 erlotinib, 243 placebo) orally once daily until disease progression or unacceptable toxicity. Efficacy outcome measures included overall survival, response rate, and progression-free survival (PFS). Duration of response was also examined. The primary endpoint was survival. The study was conducted in 17 countries.
Baseline demographics of the overall study population were as follows: male (65%), White (78%), Asian (12%), Black (4%), age < 65 years (62%), ECOG PS 1 (53%), ECOG PS 0 (13%), ECOG PS 2 (25%), ECOG PS 3 (9%), current or ex-smoker (75%), never smoker (20%), and exposure to prior platinum therapy (93%). Tumor characteristics were as follows: adenocarcinoma (50%), squamous (30%), undifferentiated large cell (9%), and mixed non-small cell (2%).
The results of the study are shown in Table 8.
Table 8: Efficacy Results (Study 4)
|
Efficacy Parameter |
Erlotinib (N = 488) |
Placebo (N = 243) |
|
Overall Survival (OS) |
||
|
378 (77%) |
209 (86%) |
|
6.7 (5.5, 7.8) |
4.7 (4.1, 6.3) |
|
0.73 (0.61, 0.86) |
|
|
p < 0.001 |
|
|
Progression-Free Survival (PFS) |
||
|
402 (82%) |
211 (87%) |
|
2.3 (1.9, 3.3) |
1.8 (1.8, 1.9) |
|
0.59 (0.50, 0.70) |
|
|
Objective Response |
||
|
8.9% (6.4, 12) |
0.9% (0.1, 3.4) |
- (1) Cox regression model with the following covariates: ECOG performance status, number of prior regimens, prior platinum, best response to prior chemotherapy.
- (2) Two-sided log-rank test stratified by ECOG performance status, number of prior regimens, prior platinum, best response to prior chemotherapy.
Figure 3 depicts the Kaplan-Meier curves for overall survival.
- Figure 3: Kaplan-Meier Curves for Overall Survival of Patients by Treatment Group in Study 4
14.4 NSCLC – Lack of Efficacy of Erlotinib Administered Concurrently with Chemotherapy
Results from two, multicenter, placebo-controlled, randomized, trials in over 1000 patients conducted in first-line patients with locally advanced or metastatic NSCLC showed no clinical benefit with the concurrent administration of erlotinib with platinum-based chemotherapy [carboplatin and paclitaxel (erlotinib, N = 526) or gemcitabine and cisplatin (erlotinib, N = 580)].
14.5 Pancreatic Cancer - Erlotinib Administered Concurrently with Gemcitabine
The efficacy and safety of erlotinib in combination with gemcitabine as a first-line treatment was assessed in Study 5, a randomized, double-blind, placebo-controlled trial in 569 patients with locally advanced, unresectable or metastatic pancreatic cancer. Patients were randomized 1:1 to receive erlotinib (100 mg or 150 mg) or placebo once daily on a continuous schedule plus gemcitabine by intravenous infusion (1000 mg/m2, Cycle 1 - Days 1, 8, 15, 22, 29, 36 and 43 of an 8-week cycle; Cycle 2 and subsequent cycles - Days 1, 8 and 15 of a 4-week cycle [the approved dose and schedule for pancreatic cancer, see the gemcitabine package insert]). Erlotinib or placebo was taken orally once daily until disease progression or unacceptable toxicity. The primary endpoint was survival. Secondary endpoints included response rate, and progression-free survival (PFS). Duration of response was also examined. The study was conducted in 18 countries. A total of 285 patients were randomized to receive gemcitabine plus erlotinib (261 patients in the 100 mg cohort and 24 patients in the 150 mg cohort) and 284 patients were randomized to receive gemcitabine plus placebo (260 patients in the 100 mg cohort and 24 patients in the 150 mg cohort). Too few patients were treated in the 150 mg cohort to draw conclusions.
In the 100 mg cohort, baseline demographics of the overall study population were as follows: male (52%), white (88%), Asian (7%), black (2%), age < 65 years (53%), ECOG PS 1 (51%), ECOG PS 0 (32%), and ECOG PS 2 (17%). There was a slightly larger proportion of females in the erlotinib arm (51%) compared with the placebo arm (44%). The median time from initial diagnosis to randomization was approximately 1 month. The majority of the patients (76%) had distant metastases at baseline and 24% had locally advanced disease.
The results of the study are shown in Table 9.
Table 9: Efficacy Results: Erlotinib 100 mg Cohort (Study 5)
|
Efficacy Parameter |
Erlotinib + Gemcitabine (N = 261) |
Placebo + Gemcitabine (N = 260) |
|
Overall Survival (OS) |
||
|
250 |
254 |
|
6.5 (6, 7.4) |
6 (5.1, 6.7) |
|
0.81 (0.68, 0.97) |
|
|
0.028 |
|
|
Progression-Free Survival (PFS) |
||
|
225 |
232 |
|
3.8 (3.6, 4.9) |
3.6 (3.3, 3.8) |
|
0.76 (0.64, 0.92) |
|
|
Objective Response |
||
|
8.6% (5.4, 12.9) |
7.9% (4.8, 12) |
- (1) Cox regression model with the following covariates: ECOG performance status and extent of disease.
- (2) Two-sided log-rank test stratified by ECOG performance status and extent of disease.
Survival was evaluated in the intent-to-treat population. Figure 4 depicts the Kaplan-Meier curves for overall survival in the 100 mg cohort. The primary survival and PFS analyses were two-sided log-rank tests stratified by ECOG performance status and extent of disease.
Figure 4: Kaplan-Meier Curves for Overall Survival: 100 mg Cohort in Study 5
16 HOW SUPPLIED/STORAGE AND HANDLING
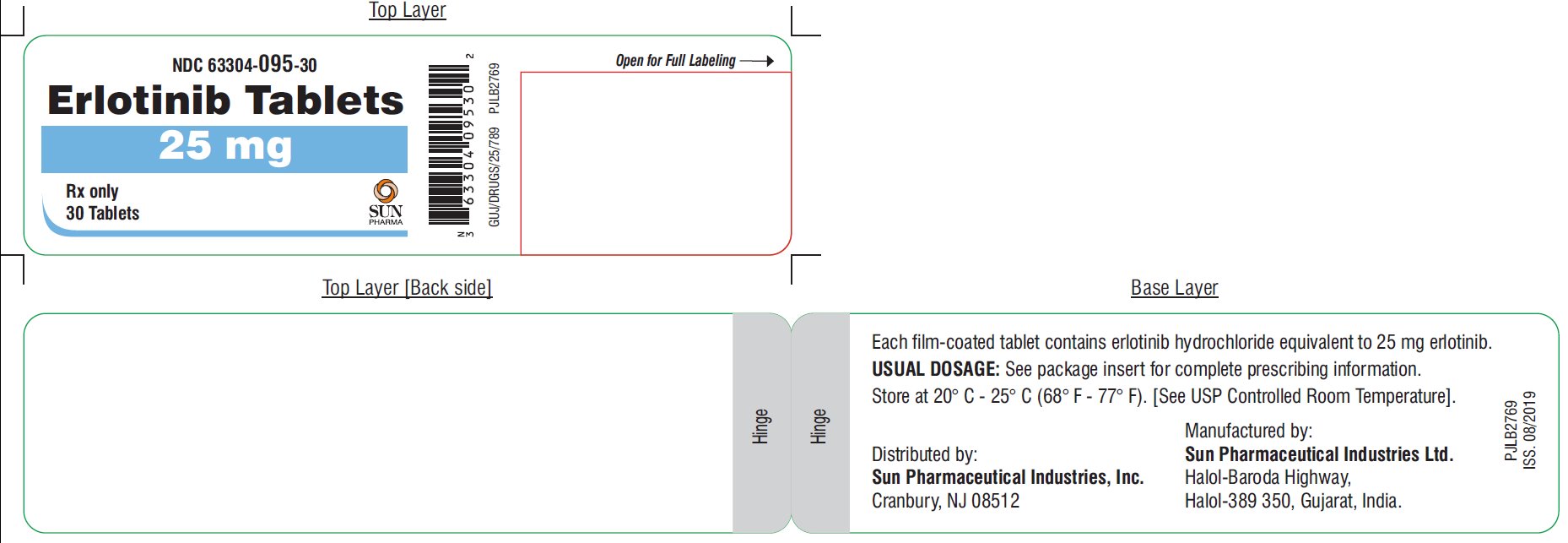
25 mg Tablets: white to off-white, round, biconvex film-coated tablets debossed with “RL” on one side and “11”on other side; supplied in bottle of 30 and blister pack of 3 x 10 tablets:
- NDC 63304-095-30 Bottles of 30
- NDC 63304-095-31 Blister Pack of 3X10
100 mg Tablets: white to off-white, round, biconvex film-coated tablets debossed with “RL” on one side and “12”on other side; supplied in bottle of 30 and blister pack of 3 x 10 tablets:
- NDC 63304-096-30 Bottles of 30
- NDC 63304-096-31 Blister Pack of 3X10
150 mg Tablets: white to off-white, film-coated tablets debossed with “RL” on one side and “13”on other side; supplied in bottle of 30 and blister pack of 3 x 10 tablets:
- NDC 63304-135-30 Bottles of 30
- NDC 63304-135-31 Blister Pack of 3X10
Store at 20° C - 25° C (68° F - 77° F). [See USP Controlled Room Temperature].
17 PATIENT COUNSELING INFORMATION
Skin rash, bullous and exfoliative skin disorders
- •
- Advise patients that skin reactions can occur or worsen on sun-exposed areas while taking erlotinib tablets, and proactive intervention may include alcohol-free emollient cream and use of sunscreen or avoidance of sun exposure. Advise patients that hyperpigmentation or dry skin, with or without digital skin fissures, have been reported and in the majority of cases were associated with rash [see Adverse Reactions (6.1)].
- •
- Advise patients that erlotinib tablets can increase the risk of bullous and exfoliative skin disorders and to seek immediately medical attention for severe skin reactions [see Warnings and Precautions (5.5)].
Diarrhea
Advise patients that diarrhea can usually be managed with loperamide and to contact their healthcare provider for severe or persistent diarrhea [see Adverse Reactions (6.1)].
Interstitial lung disease
Advise patients of the risk of severe or fatal ILD, including pneumonitis. Advise patients to contact their healthcare provider immediately to report new of worsening unexplained shortness of breath or coughing [see Dosage and Administration (2.4) and Warnings and Precautions (5.1)].
Renal failure
Advise patients of the risk of developing renal failure. Inform patients of the need for the healthcare provider to monitor kidney function and electrolytes [see Warnings and Precautions (5.2)].
Hepatotoxicity
Advise patients to immediately report signs or symptoms of hepatotoxicity [see Warnings and Precautions (5.3)].
Gastrointestinal perforations
Advise patients that erlotinib tablets can increase the risk of gastrointestinal perforation or fistula and to seek immediate medical attention for severe abdominal pain [see Dosage and Administration (2.4) and Warnings and Precautions (5.4)].
Cerebrovascular accident
Advise patients of the risk of cerebrovascular accident and see immediate medical attention [see Dosage and Administration (2.4) and Warnings and Precautions (5.6)].
Ocular disorders
Advise patients promptly to contact their healthcare provider if they develop eye signs or symptoms, lacrimation, light sensitivity, blurred vision, eye pain, red eye, or changes in vision [see Dosage and Administration (2.4) and Warnings and Precautions (5.8)].
Hemorrhage in patients taking warfarin
Advise patients who are receiving warfarin of the need to monitor INR or other coumarin-derivative anticoagulants [see Warnings and Precautions (5.9) and Drug Interactions (7)].
Hair and nail disorders
Advise patients that hair and nail disorders, including hirsutism and brittle and loose nails, have been reported [see Adverse Reactions (6.1)].
Embryo-fetal toxicity
- •
- Advise pregnant women and females of reproductive potential of the potential risk to a fetus. Advise females of reproductive potential to inform their healthcare provider of a known or suspected pregnancy [see Warnings and Precautions (5.10), Use in Specific Populations (8.1)].
- •
- Advise females of reproductive potential to use effective contraception during treatment with erlotinib tablets, and for 1 month after the last dose [see Use in Specific Populations (8.3)].
Lactation
- •
- Advise women not to breastfeed during treatment with erlotinib tablets and for 2 weeks after the final dose [see Use in Specific Populations (8.2)].
Smoking
- •
- Advise patients to contact their health care provider for any changes in smoking status and that the dose of erlotinib tablets may need to be adjusted if they smoke [see Drug Interactions (7) and Clinical Pharmacology (12.3)]
- •
- Advise patients to stop smoking [see Clinical Pharmacology (12.3)].
Distributed by:
Sun Pharmaceutical Industries, Inc.
Cranbury, NJ 08512
Manufactured by:
Sun Pharmaceutical Industries Ltd.
Halol-Baroda Highway,
Halol-389 350, Gujarat, India.
All trademarks are the property of their respective owners.
- August 2019 FDA-02
PACKAGE/LABEL PRINCIPAL DISPLAY PANEL
INGREDIENTS AND APPEARANCE
| ERLOTINIB
erlotinib tablet, film coated |
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
| ERLOTINIB
erlotinib tablet, film coated |
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
| ERLOTINIB
erlotinib tablet, film coated |
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
| Labeler - Sun Pharmaceutical Industries, Inc. (146974886) |
| Registrant - Sun Pharmaceutical Industries, Inc. (146974886) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Sun Pharmaceutical Industries Limited | 725959238 | MANUFACTURE(63304-095, 63304-096, 63304-135) | |




