Search by Drug Name or NDC
NDC 63481-0529-10 Cortisporin TC 3; 10; 3.3; .5 mg/mL; mg/mL; mg/mL; mg/mL Details
Cortisporin TC 3; 10; 3.3; .5 mg/mL; mg/mL; mg/mL; mg/mL
Cortisporin TC is a AURICULAR (OTIC) SUSPENSION in the HUMAN PRESCRIPTION DRUG category. It is labeled and distributed by Endo Pharmaceuticals, Inc.. The primary component is COLISTIN SULFATE; HYDROCORTISONE ACETATE; NEOMYCIN SULFATE; THONZONIUM BROMIDE.
Product Information
| NDC | 63481-0529 |
|---|---|
| Product ID | 63481-529_7d79882e-12eb-4b51-88f8-6d0089fa5122 |
| Associated GPIs | 87991004201820 |
| GCN Sequence Number | 048618 |
| GCN Sequence Number Description | neomyc/colist/hydrocort/thonzn DROPS SUSP 3.3-3-10/1 OTIC (EAR) |
| HIC3 | Q8W |
| HIC3 Description | EAR PREPARATIONS,ANTIBIOTICS |
| GCN | 14106 |
| HICL Sequence Number | 018381 |
| HICL Sequence Number Description | NEOMYCIN SULF/COLISTIN SUL/HYDROCORTISONE AC/THONZONIUM BROM |
| Brand/Generic | Brand |
| Proprietary Name | Cortisporin TC |
| Proprietary Name Suffix | n/a |
| Non-Proprietary Name | colistin sulfate, neomycin sulfate, thonzonium bromide and hydrocortisone acetate |
| Product Type | HUMAN PRESCRIPTION DRUG |
| Dosage Form | SUSPENSION |
| Route | AURICULAR (OTIC) |
| Active Ingredient Strength | 3; 10; 3.3; .5 |
| Active Ingredient Units | mg/mL; mg/mL; mg/mL; mg/mL |
| Substance Name | COLISTIN SULFATE; HYDROCORTISONE ACETATE; NEOMYCIN SULFATE; THONZONIUM BROMIDE |
| Labeler Name | Endo Pharmaceuticals, Inc. |
| Pharmaceutical Class | Aminoglycoside Antibacterial [EPC], Aminoglycosides [CS], Corticosteroid Hormone Receptor Agonists [MoA], Corticosteroid [EPC] |
| DEA Schedule | n/a |
| Marketing Category | NDA |
| Application Number | NDA050356 |
| Listing Certified Through | 2024-12-31 |
Package
Package Images


NDC 63481-0529-10 (63481052910)
| NDC Package Code | 63481-529-10 |
|---|---|
| Billing NDC | 63481052910 |
| Package | 10 mL in 1 BOTTLE, DROPPER (63481-529-10) |
| Marketing Start Date | 2019-06-03 |
| NDC Exclude Flag | N |
| Pricing Information | |
| Price Per Unit | 22.1 |
| Pricing Unit | ML |
| Effective Date | 2022-10-19 |
| NDC Description | CORTISPORIN-TC EAR SUSPENSION |
| Pharmacy Type Indicator | C/I |
| OTC | N |
| Explanation Code | 4, 5 |
| Classification for Rate Setting | B |
| As of Date | 2022-11-23 |
Standard Product Labeling (SPL)/Prescribing Information SPL 9c6fc1c7-3350-42ae-b999-aca48e704bb1 Details
DESCRIPTION
Cortisporin®TC Otic with Neomycin and Hydrocortisone (colistin sulfate—neomycin sulfate—thonzonium bromide—hydrocortisone acetate otic suspension) is a sterile antibacterial and anti-inflammatory aqueous suspension containing in each mL: Colistin base activity, 3 mg (as the sulfate); Neomycin base activity, 3.3 mg (as the sulfate); Hydrocortisone acetate, 10 mg (1%); Thonzonium bromide, 0.5 mg (0.05%); Polysorbate 80, acetic acid, and sodium acetate in a buffered aqueous vehicle. Thimerosal (mercury derivative), 0.002%, is added as a preservative. It is a nonviscous liquid, buffered at pH 5, for instillation into the canal of the external ear or direct application to the affected aural skin.
The structural formulas of colistin sulfate (mixture of Colistin A & B), neomycin sulfate (mixture of neomycin A, B & C), hydrocortisone acetate ((11β)-21-(acetyloxy)-11,17-dihydroxypregn) methyl]-2 pyrimidinylamino] ethyl]-N,N-dimethyl-1-hexadecanaminium, bromide) are represented below:

Thonzonium Bromide

Colistin sulfate

Neomycin A

Neomycin B Sulfate
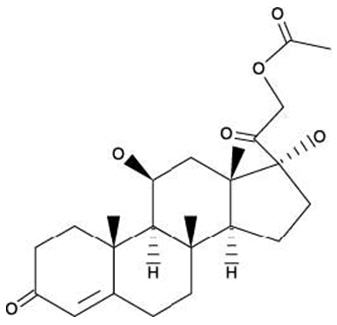
Hydrocortisone Acetate

Neomycin C Sulfate
CLINICAL PHARMACOLOGY
Colistin sulfate is a polypeptide antibiotic which penetrates into and disrupts the bacterial cell membrane. Neomycin sulfate is an aminoglycoside antibiotic which inhibits protein synthesis, disrupting the normal cycle of ribosomal function. Hydrocortisone acetate is a corticosteroid hormone which is thought to act by regulating the rate of protein synthesis; it controls inflammation, edema, pruritus and other dermal reactions. Cortiscosteroids suppress the inflammatory response to a variety of agents and they may delay healing. Since corticoids may inhibit the body's defense mechanism against infection, a concomitant antimicrobial drug may be used when this inhibition is considered to be clinically significant in a particular case.
The relative potency of corticosteroids depends on the molecular structure, concentration, and release from the vehicle.
Thonzonium bromide is a surface-active agent that promotes tissue contact by dispersion and penetration of the cellular debris and exudate.
Microbiology
Together, colistin sulfate and neomycin sulfate have bactericidal activity against most strains of the following microorganisms, both in vitro and in clinical infections as described in the INDICATIONS AND USAGE section.
Aerobic gram-positive microorganisms:
Staphylococcus aureus.
Aerobic gram-negative microorganisms:
Enterobacter aerogenes
Escherichia coli
Klebsiella pneumoniae
Pseudomonas aeruginosa.
Susceptibility Tests:
It is not recommended that colistin sulfate or neomycin sulfate be routinely tested and reported by clinical microbiology laboratories.
INDICATIONS AND USAGE
Cortisporin®TC Otic is indicated for the treatment of superficial bacterial infections of the external auditory canal, caused by organisms susceptible to the action of the antibiotics; and for the treatment of infections of mastoidectomy and fenestration cavities, caused by organisms susceptible to the antibiotics.
CONTRAINDICATIONS
WARNINGS
Neomycin can induce permanent sensorineural hearing loss due to cochlear damage, mainly destruction of hair cells in the organ of Corti. The risk is greater with prolonged use. Therapy should be limited to 10 consecutive days. (See PRECAUTIONS-General.) Patients being treated with eardrops containing neomycin should be under close clinical observation. Cortisporin®TC Otic should be used cautiously in any patient with a perforated tympanic membrane.
Neomycin sulfate may cause cutaneous sensitization. A precise incidence of hypersensitivity reactions (primarily skin rash) due to topical neomycin is not known. Discontinue promptly if sensitivity or irritation occurs.
When using neomycin-containing products to control secondary infection in the chronic dermatoses, such as chronic otitis externa or stasis dermatitis, it should be borne in mind that the skin in these conditions is more liable than is normal skin to become sensitized to many substances, including neomycin. The manifestation of sensitization to neomycin is ususally a low-grade reddening with swelling, dry scaling, and itching; it may be manifest simply as a failure to heal. Periodic examination for such signs is advisable, and the patient should be told to discontinue the product if they are observed. These symptoms regress quickly on withdrawing the medication. Neomycin containing applications should be avoided for the patient thereafter.
PRECAUTIONS
General
As with any other antibiotic preparation, prolonged treatment may result in overgrowth of nonsusceptible organisms and fungi. If the infection is not improved after one week, cultures should be repeated to verify the identity of the organism and to determine whether therapy should be changed.
Treatment should not be continued for longer than ten days.
Allergic cross-reactions may occur which could prevent the use of any or all of the aminoglycoside antibiotics for the treatment of future infections.
Information for Patients
Avoid contaminating the dropper with material from the ear, fingers, or other source. This caution is necessary if the sterility of the drops is to be preserved.
If sensitization or irritation occurs, discontinue use immediately and contact your physician.
Do not use in the eyes.
If you prefer to warm the medication before using it, do not heat the suspension above body temperature in order to avoid loss of potency.
SHAKE WELL BEFORE USING.
Laboratory Tests
Systemic effects of excessive levels of hydrocortisone may include a reduction in the number of circulating eosinophils and a decrease in urinary excretion of 17-hydroxycorticosteroids.
Carcinogenesis, Mutagenesis, Impairment of Fertility
Long-term animal carcinogenicity studies have not been performed with colistin or neomycin, or Cortisporin®TC Otic. An increased incidence of chromosome aberrations in human lymphocytes has been reported following in vitro exposure to colistin or neomycin.
Fertility studies have not been performed with neomycin, but reports from the scientific literature suggest that it may decrease spermatogenesis in rats. No adverse effects on fertility were observed in male or female rats given intramuscular doses of colistimethate sodium, the methanesulfonate salt of colistin, up to 20 mg/kg (equivalent to 9.3 mg/kg of colistin base). This is approximately 30 times the clinical daily dose based on body surface area, assuming 100% absorption from the ear; however, significant systemic levels of colistin or neomycin would not be anticipated in humans when Cortisporin®TC Otic is used as directed.
Long term studies in rodents showed no evidence of carcinogenicity attributable to oral administration of corticosteroids. Mutagenicity studies with hydrocortisone were negative. Studies have not been performed to evaluate the effect on fertility of topical corticosteroids.
Pregnancy
Teratogenic Effects
Pregnancy Category C
There are no adequate and well controlled studies of Cortisporin®TC Otic in pregnant women. It is not known whether Cortisporin®TC Otic can cause fetal harm when administered to a pregnant woman.
Colistimethate sodium, the methanesulfonate salt of colistin, was not teratogenic in rats or rabbits given intramuscular doses up to 20 mg/kg (equivalent to 9.3 mg/kg of colisitin base, approximately 30 times (rats) or 55 times (rabbits) the clinical daily dose based on body suface area and assuming 100% absorption from the ear). Increased resorptions were observed in rabbits at 20 mg/kg, but not 10 mg/kg (equivalent to 4.15 mg/kg of colistin base). Decreased pup survival at weaning was observed in rats at 20 mg/kg, a maternally toxic dose of colistin, but not 10 mg/kg. Colistin has not been shown to have any adverse effects on the developing embryo or fetus at doses relevant to the amount that will be delivered ototopically at the recommended clinical doses.
Although aminoglycosides can cause congenital deafness in humans if administered during pregnancy, significant systemic levels of neomycin would not be anticipated when Cortisporin®TC Otic is used as directed.
Corticosteroids are generally teratogenic in laboratory animals when administered systemically at relatively low dosage levels. The more potent corticosteroids have been shown to be teratogenic after dermal application in laboratory animals.
Cortisporin®TC Otic should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
Nursing Mothers
Hydrocortisone and colistin sulfate appear in human milk following oral administration of the drugs. Since systemic absorption of these drugs may occur when they are used topically, caution should be exercised when Cortisporin®TC Otic Suspension is used by a nursing woman.
Pediatric Use
See DOSAGE AND ADMINISTRATION.
The safety and effectiveness of Cortisporin®TC Otic in infants below one year of age have not been established. The efficacy of Cortisporin®TC Otic in pediatric patients one year or older in the treatment of superficial bacterial infections of the external auditory canal and for the treatment of infections of mastoidectomy and fenestration cavities has been demonstrated in a controlled clinical trial.
ADVERSE REACTIONS
Neomycin occasionally causes skin sensitization.
Ototoxicity (see WARNINGS section) and nephrotoxicity have also been reported.
Adverse reactions have occurred with topical use of antibiotic combinations. Exact incidence figures are not available since no denominator of treated patients is available. The reaction occurring most often is allergic sensitization. In one clinical study, using a 20% neomycin patch, neomycin-induced allergic skin reactions occurred in two of 2,175 (0.09%) individuals in the general populaton. In another study the incidence was found to be approximately 1%.
The following local adverse events have been reported with topical corticosteroids, especially under occlusive dressings: burning, itching, irritation, dryness, folliculitis, hypertrichosis, acneiform eruptions, hypopigmentation, perioral dermatitis, allergic contact dermatitis, maceration of the skin, secondary infection, skin atrophy, striae, and miliaria.
For medical advice about adverse reactions contact your medical professional. To report SUSPECTED ADVERSE REACTIONS, contact Endo Pharmaceuticals Inc. at 1-800-462-3636 or MEDWATCH at 1-800-FDA-1088 or http://www.fda.gov/medwatch/.
DOSAGE AND ADMINISTRATION
Therapy with this product should be limited to 10 days. (See WARNINGS.)
The external auditory canal should be thoroughly cleansed and dried with a sterile cotton applicator.
When using the calibrated dropper:
For adults, 5 drops of the suspension should be instilled into the affected ear 3 or 4 times daily. For pediatric patients, 4 drops are suggested because of the smaller capacity of the ear canal.
The patient should lie with the affected ear upward and then the drops should be instilled. This position should be maintained for 5 minutes to facilitate penetration of the drops into the ear canal. Repeat, if necessary, for the opposite ear.
If preferred, a cotton wick may be inserted into the canal and then the cotton may be saturated with the suspension. This wick should be kept moist by adding further solution every 4 hours. The wick should be replaced at least once every 24 hours.
HOW SUPPLIED
Cortisporin®TC is supplied as:
NDC 63481-529-10 10 mL bottle with dropper
Each mL contains: Colistin sulfate equivalent to 3 mg of colistin base activity, Neomycin sulfate equivalent to 3.3 mg neomycin base activity, Hydrocortisone acetate 10 mg (1%), Thonzonium bromide 0.5 mg (0.05%), and Polysorbate 80 in an aqueous vehicle buffered with acetic acid and sodium acetate. Thimerosal (mercury derivative) 0.002% is added as a preservative.
A sterilized dropper-cap assembly for use on the bottle of suspension is included in the package.
Shake well before using.
SPL UNCLASSIFIED SECTION
INGREDIENTS AND APPEARANCE
| CORTISPORIN TC
colistin sulfate, neomycin sulfate, thonzonium bromide and hydrocortisone acetate suspension |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Labeler - Endo Pharmaceuticals, Inc. (178074951) |