Search by Drug Name or NDC
NDC 63629-8826-01 Medroxyprogesterone Acetate 5 mg/1 Details
Medroxyprogesterone Acetate 5 mg/1
Medroxyprogesterone Acetate is a ORAL TABLET in the HUMAN PRESCRIPTION DRUG category. It is labeled and distributed by Bryant Ranch Prepack. The primary component is MEDROXYPROGESTERONE ACETATE.
MedlinePlus Drug Summary
Medroxyprogesterone is used to treat abnormal menstruation (periods) or irregular vaginal bleeding. Medroxyprogesterone is also used to bring on a normal menstrual cycle in women who menstruated normally in the past but have not menstruated for at least 6 months and who are not pregnant or undergoing menopause (change of life). Medroxyprogesterone is also used to prevent overgrowth of the lining of the uterus (womb) and may decrease the risk of cancer of the uterus in patients who are taking estrogen. Medroxyprogesterone is in a class of medications called progestins. It works by stopping the growth of the lining of the uterus and by causing the uterus to produce certain hormones.
Related Packages: 63629-8826-01Last Updated: 12/01/2022
MedLinePlus Full Drug Details: Medroxyprogesterone
Product Information
| NDC | 63629-8826 |
|---|---|
| Product ID | 63629-8826_61bfc46f-234a-47be-bb4a-f14763834473 |
| Associated GPIs | 26000020200310 |
| GCN Sequence Number | 003273 |
| GCN Sequence Number Description | medroxyprogesterone acetate TABLET 5 MG ORAL |
| HIC3 | G2A |
| HIC3 Description | PROGESTATIONAL AGENTS |
| GCN | 11262 |
| HICL Sequence Number | 001442 |
| HICL Sequence Number Description | MEDROXYPROGESTERONE ACETATE |
| Brand/Generic | Generic |
| Proprietary Name | Medroxyprogesterone Acetate |
| Proprietary Name Suffix | n/a |
| Non-Proprietary Name | Medroxyprogesterone Acetate |
| Product Type | HUMAN PRESCRIPTION DRUG |
| Dosage Form | TABLET |
| Route | ORAL |
| Active Ingredient Strength | 5 |
| Active Ingredient Units | mg/1 |
| Substance Name | MEDROXYPROGESTERONE ACETATE |
| Labeler Name | Bryant Ranch Prepack |
| Pharmaceutical Class | Progesterone Congeners [CS], Progestin [EPC] |
| DEA Schedule | n/a |
| Marketing Category | ANDA |
| Application Number | ANDA040159 |
| Listing Certified Through | 2023-12-31 |
Package
Package Images
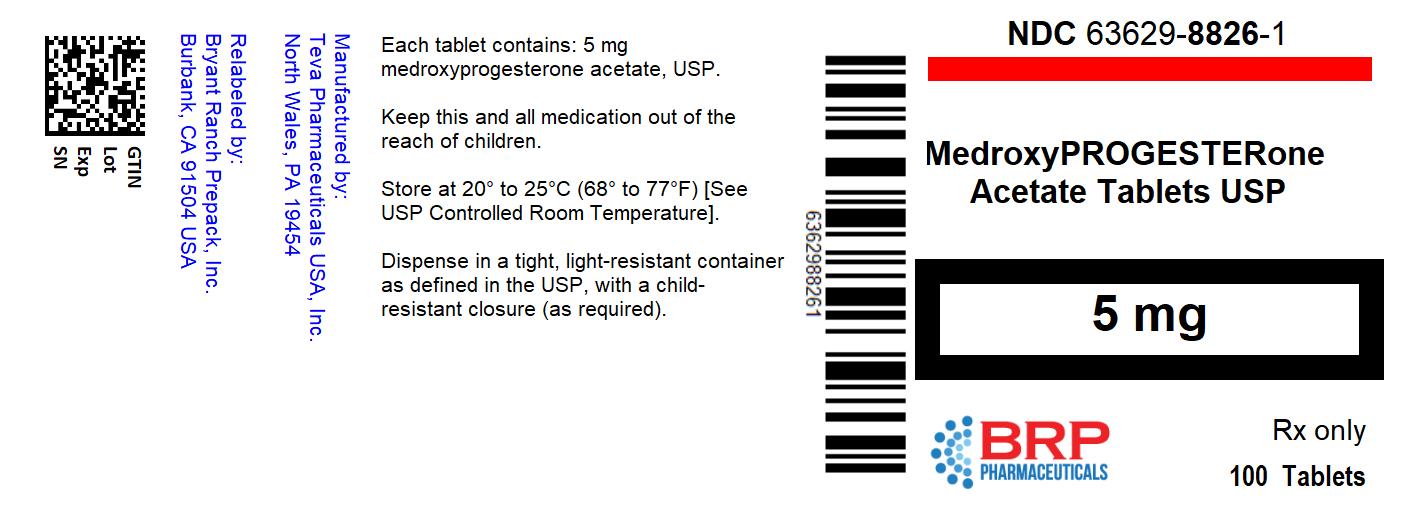
NDC 63629-8826-01 (63629882601)
| NDC Package Code | 63629-8826-1 |
|---|---|
| Billing NDC | 63629882601 |
| Package | 100 TABLET in 1 BOTTLE (63629-8826-1) |
| Marketing Start Date | 2021-09-10 |
| NDC Exclude Flag | N |
| Pricing Information | N/A |
Standard Product Labeling (SPL)/Prescribing Information SPL a88b33ff-842b-4a5b-a27e-9c2b9e85a4d7 Details
SPL UNCLASSIFIED SECTION
0872
0873
0779
Rx only
WARNING: CARDIOVASCULAR DISORDERS, BREAST CANCER AND PROBABLE DEMENTIA FOR ESTROGEN PLUS PROGESTIN THERAPY
Cardiovascular Disorders and Probable Dementia
Estrogen plus progestin therapy should not be used for the prevention of cardiovascular disease or dementia. (See CLINICAL STUDIES and WARNINGS, Cardiovascular Disorders and Probable Dementia.)
The Women’s Health Initiative (WHI) estrogen plus progestin substudy reported an increased risk of deep vein thrombosis (DVT), pulmonary embolism (PE), stroke and myocardial infarction (MI) in postmenopausal women (50 to 79 years of age) during 5.6 years of treatment with daily oral conjugated estrogens (CE) [0.625 mg] combined with medroxyprogesterone acetate (MPA) [2.5 mg], relative to placebo. (See CLINICAL STUDIES and WARNINGS, Cardiovascular Disorders.)
The WHI Memory Study (WHIMS) estrogen plus progestin ancillary study reported an increased risk of developing probable dementia in postmenopausal women 65 years of age or older during 4 years of treatment with daily CE (0.625 mg) combined with MPA (2.5 mg), relative to placebo. It is unknown whether this finding applies to younger postmenopausal women. (See CLINICAL STUDIES and WARNINGS,Probable Dementia and PRECAUTIONS,Geriatric Use.)
Breast Cancer
The WHI estrogen plus progestin substudy demonstrated an increased risk of invasive breast cancer. (See CLINICAL STUDIES and WARNINGS, Malignant Neoplasm,Breast Cancer.)
In the absence of comparable data, these risks should be assumed to be similar for other doses of CE and MPA, and other combinations and dosage forms of estrogens and progestins.
Progestins with estrogens should be prescribed at the lowest effective doses and for the shortest duration consistent with treatment goals and risks for the individual woman.
DESCRIPTION
Medroxyprogesterone Acetate Tablets USP contain medroxyprogesterone acetate, USP which is a derivative of progesterone. It is a white to off-white, odorless crystalline powder, stable in air, melting between 200 and 210°C. It is freely soluble in chloroform, soluble in acetone and in dioxane, sparingly soluble in alcohol and in methanol, slightly soluble in ether, and insoluble in water.
The chemical name for medroxyprogesterone acetate is pregn-4-ene-3, 20-dione, 17-(acetyloxy)-6-methyl-, (6α)-. The structural formula is:

- C24H34O4 M.W. 386.53
Each tablet, for oral administration, contains 2.5 mg, 5 mg or 10 mg of medroxyprogesterone acetate, USP and the following inactive ingredients: crospovidone, lactose monohydrate, magnesium stearate, methylcellulose, pregelatinized corn starch, and sodium lauryl sulfate.
CLINICAL PHARMACOLOGY
Medroxyprogesterone acetate (MPA) administered orally or parenterally in the recommended doses to women with adequate endogenous estrogen, transforms proliferative into secretory endometrium. Androgenic and anabolic effects have been noted, but the drug is apparently devoid of significant estrogenic activity. While parenterally administered MPA inhibits gonadotropin production, which in turn prevents follicular maturation and ovulation, available data indicate that this does not occur when the usually recommended oral dosage is given as single daily doses.
Pharmacokinetics
The pharmacokinetics of MPA were determined in 20 postmenopausal women following a single-dose administration of eight medroxyprogesterone acetate 2.5 mg tablets or a single administration of two medroxyprogesterone acetate 10 mg tablets under fasting conditions. In another study, the steady-state pharmacokinetics of MPA were determined under fasting conditions in 30 postmenopausal women following daily administration of one medroxyprogesterone acetate 10 mg tablet for 7 days. In both studies, MPA was quantified in serum using a validated gas chromatography-mass spectrometry (GC-MS) method. Estimates of the pharmacokinetic parameters of MPA after single and multiple doses of medroxyprogesterone acetate tablets were highly variable and are summarized in Table 1.
|
Tablet Strength |
Cmax (ng/mL) |
Tmax (h) |
Auc0-(∞) (ng·h/mL) |
t½ (h) |
Vd/f (L) |
CL/f (mL/min) |
|
Single Dose |
||||||
|
2 x 10 mg |
1.01 (0.599) |
2.65 (1.41) |
6.95 (3.39) |
12.1 (3.49) |
78024 (47220) |
64110 (42662) |
|
8 x 2.5 mg |
0.805 (0.413) |
2.22 (1.39) |
5.62 (2.79) |
11.6 (2.81) |
62748 (40146) |
74123 (35126) |
|
Multiple Dose |
||||||
|
10 mg * |
0.71 (0.35) |
2.83 (1.83) |
6.01 (3.16) |
16.6 (15) |
40564 (38256) |
41963 (38402) |
*Following Day 7 dose
A. Absorption
No specific investigation on the absolute bioavailability of MPA in humans has been conducted. MPA is rapidly absorbed from the gastrointestinal tract, and maximum MPA concentrations are obtained between 2 to 4 hours after oral administration.
Administration of medroxyprogesterone acetate with food increases the bioavailability of MPA. A 10 mg dose of medroxyprogesterone acetate, taken immediately before or after a meal, increased MPA Cmax (50 to 70%) and AUC (18 to 33%). The half-life of MPA was not changed with food.
B. Distribution
MPA is approximately 90% protein bound, primarily to albumin; no MPA binding occurs with sex hormone binding globulin.
C. Metabolism
Following oral dosing, MPA is extensively metabolized in the liver via hydroxylation, with subsequent conjugation and elimination in the urine.
D. Excretion
Most MPA metabolites are excreted in the urine as glucuronide conjugates with only minor amounts excreted as sulfates.
E. Specific Populations
Hepatic Insufficiency
MPA is almost exclusively eliminated via hepatic metabolism. In 14 patients with advanced liver disease, MPA disposition was significantly altered (reduced elimination). In patients with fatty liver, the mean percent dose excreted in the 24-hour urine as intact MPA after a 10 mg or 100 mg dose was 7.3% and 6.4%, respectively.
F. Drug Interactions
Medroxyprogesterone acetate (MPA) is metabolized in-vitro primarily by hydroxylation via the CYP3A4. Specific drug-drug interaction studies evaluating the clinical effects with CYP3A4 inducers or inhibitors on MPA have not been conducted. Inducers and/or inhibitors of CYP3A4 may affect the metabolism of MPA.
CLINICAL STUDIES
Effects on the Endometrium
In a 3-year, double-blind, placebo-controlled study of 356 nonhysterectomized, postmenopausal women between 45 and 64 years of age randomized to receive placebo (n=119), 0.625 mg conjugated estrogen only (n=119), or 0.625 mg conjugated estrogen plus cyclic medroxyprogesterone acetate (n=118), results showed a reduced risk of endometrial hyperplasia in the treatment group receiving 10 mg medroxyprogesterone acetate plus 0.625 mg conjugated estrogens compared to the group receiving 0.625 mg conjugated estrogens only. See Table 2.
|
Histological Results |
Placebo (n=119) |
CEE* (n=119) |
Medroxyprogesterone Acetate† + CEE (n=118) |
|
Normal/No hyperplasia (%) |
116 (97) |
45 (38) |
112 (95) |
|
Simple (cystic) hyperplasia (%) |
1 (1) |
33 (28) |
4 (3) |
|
Complex (adenomatous) hyperplasia (%) |
1 (1) |
27 (22) |
2 (2) |
|
Atypia (%) |
0 |
14 (12) |
0 |
|
Adenocarcinoma (%) |
1 (1) |
0 |
0 |
|
* Includes most extreme abnormal result |
|||
In a second 1-year study, 832 postmenopausal women between 45 and 65 years of age were treated with daily 0.625 mg conjugated estrogen (days 1 to 28), plus either 5 mg cyclic medroxyprogesterone acetate or 10 mg cyclic medroxyprogesterone acetate (days 15 to 28), or daily 0.625 mg conjugated estrogen only. The treatment groups receiving 5 or 10 mg cyclic medroxyprogesterone acetate (days 15 to 28) plus daily conjugated estrogens showed a significantly lower rate of hyperplasia as compared to the conjugated estrogens only group. See Table 3.
|
CEE* | |||
|
(n=283) |
MPA 5 mg (n=277) |
MPA 10 mg (n=272) |
|
|
Cystic hyperplasia (%) |
55 (19) |
3 (1) |
0 |
|
Adenomatous hyperplasia without atypia |
2 (1) |
0 |
0 |
Women’s Health Initiative Studies
The WHI enrolled approximately 27,000 predominantly healthy postmenopausal women in two substudies to assess the risks and benefits of daily oral CE (0.625 mg)-alone or in combination with MPA (2.5 mg) compared to placebo in the prevention of certain chronic diseases. The primary endpoint was the incidence of coronary heart disease (CHD) (defined as nonfatal MI, silent MI and CHD death), with invasive breast cancer as the primary adverse outcome. A “global index” included the earliest occurrence of CHD, invasive breast cancer, stroke, PE, endometrial cancer (only in the CE plus MPA substudy), colorectal cancer, hip fracture, or death due to other cause. These substudies did not evaluate the effects of CE-alone or CE plus MPA on menopausal symptoms.
WHI Estrogen Plus Progestin Substudy
The WHI estrogen plus progestin substudy was stopped early. According to the predefined stopping rule, after an average follow-up of 5.6 years of treatment, the increased risk of invasive breast cancer and cardiovascular events exceeded the specified benefits included in the “global index.” The absolute excess risk of events included in the “global index” was 19 per 10,000 women-years.
For those outcomes included in the WHI “global index” that reached statistical significance after 5.6 years of follow-up, the absolute excess risks per 10,000 women-years in the group treated with CE plus MPA were 7 more CHD events, 8 more strokes, 10 more PEs, and 8 more invasive breast cancers, while the absolute risk reduction per 10,000 women-years were 6 fewer colorectal cancers and 5 fewer hip fractures.
Results of the CE plus MPA substudy which included 16,608 women (average 63 years of age, range 50 to 79; 83.9 percent White, 6.8 percent Black, 5.4 percent Hispanic, 3.9 percent Other) are presented in Table 4. These results reflect centrally adjudicated data after an average follow-up of 5.6 years.
|
|||
|
Event |
Relative Risk CE/MPA vs placebo (95% nCI‡) |
CE/MPA n = 8,506 |
Placebo n = 8,102 |
|
Absolute Risk per 10,000 Women-Years |
|||
|
CHD events |
1.23 (0.99 to 1.53) 1.28 (1 to 1.63) 1.10 (0.70 to 1.75) |
41 31 8 |
34 25 8 |
|
All strokes |
1.31 (1.03 to 1.68) |
33 |
25 |
|
Ischemic stroke |
1.44 (1.09 to 1.90) |
26 |
18 |
|
Deep vein thrombosis§ |
1.95 (1.43 to 2.67) |
26 |
13 |
|
Pulmonary embolism |
2.13 (1.45 to 3.11) |
18 |
8 |
|
Invasive breast cancer¶ |
1.24 (1.01 to 1.54) |
41 |
33 |
|
Colorectal cancer |
0.61 (0.42 to 0.87) |
10 |
16 |
|
Endometrial cancer§ |
0.81 (0.48 to 1.36) |
6 |
7 |
|
Cervical Cancer§ |
1.44 (0.47 to 4.42) |
2 |
1 |
|
Hip fracture |
0.67 (0.47 to 0.96) |
11 |
16 |
|
Vertebral fractures§ |
0.65 (0.46 to 0.92) |
11 |
17 |
|
Lower arm/wrist fractures§ |
0.71 (0.59 to 0.85) |
44 |
62 |
|
Total fractures§ |
0.76 (0.69 to 0.83) |
152 |
199 |
|
Overall mortality# |
1 (0.83 to 1.19) |
52 |
52 |
|
Global IndexÞ |
1.13 (1.02 to 1.25) |
184 |
165 |
Timing of the initiation of estrogen plus progestin therapy relative to the start of menopause may affect the overall risk benefit profile. The WHI estrogen plus progestin substudy stratified by age showed in women 50 to 59 years of age a nonsignificant trend toward reduced risk in overall mortality [hazard ration (HR) 0.69 (95 percent CI, 0.44 to 1.07)].
Women's Health Initiative Memory Study
The WHIMS estrogen plus progestin ancillary study of WHI enrolled 4,532 predominantly healthy postmenopausal women 65 years of age and older (47 percent were aged 65 to 69 years of age, 35 percent were 70 to 74 years of age, and 18 percent were 75 years of age and older) to evaluate the effects of daily CE (0.625 mg) plus MPA (2.5 mg) on the incidence of probable dementia (primary outcome) compared to placebo.
After an average follow-up of 4 years, the relative risk of probable dementia for CE plus MPA versus placebo was 2.05 (95 percent CI, 1.21 to 3.48). The absolute risk of probable dementia for CE plus MPA versus placebo was 45 versus 33 per 10,000 women-years. Probable dementia as defined in this study included Alzheimer’s disease (AD), vascular dementia (VaD) and mixed type (having features of both AD and VaD). The most common classification of probable dementia in the treatment group and the placebo group was AD. Since the ancillary study was conducted in women 65 to 79 years of age, it is unknown whether these findings apply to younger postmenopausal women. (See WARNINGS,Probable Dementia and PRECAUTIONS, Geriatric Use).
INDICATIONS AND USAGE
Medroxyprogesterone Acetate Tablets USP are indicated for the treatment of secondary amenorrhea and abnormal uterine bleeding due to hormonal imbalance in the absence of organic pathology, such as fibroids or uterine cancer. They are also indicated for use in the prevention of endometrial hyperplasia in nonhysterectomized postmenopausal women who are receiving daily oral conjugated estrogens 0.625 mg tablets.
CONTRAINDICATIONS
Medroxyprogesterone acetate is contraindicated in women with any of the following conditions:
- Undiagnosed abnormal genital bleeding.
- Known, suspected, or history of breast cancer.
- Known or suspected estrogen- or progesterone-dependent neoplasia.
- Active DVT, PE, or a history of these conditions
- Active arterial thromboembolic disease (for example, stroke and MI), or a history of these conditions.
- Known anaphylactic reaction or angioedema to medroxyprogesterone acetate.
- Known liver impairment or disease.
- Known or suspected pregnancy.
WARNINGS
See BOXED WARNINGS.
1. Cardiovascular Disorders.
An increased risk of PE, DVT, stroke, and MI has been reported with estrogen plus progestin therapy. Should any of these events occur or be suspected, estrogen plus progestin therapy should be discontinued immediately.
Risk factors for arterial vascular disease (for example, hypertension, diabetes mellitus, tobacco use, hypercholesterolemia, and obesity) and/or venous thromboembolism (VTE) (for example, personal history or family history of VTE, obesity, and systemic lupus erythematosus) should be managed appropriately.
a. Stroke
In the WHI estrogen plus progestin substudy, a statistically significant increased risk of stroke was reported in women 50 to 79 years of age receiving CE (0.625 mg) plus MPA (2.5 mg) compared to women in the same age group receiving placebo (33 versus 25 per 10,000 women-years). (See CLINICAL STUDIES.) The increase in risk was demonstrated after the first year and persisted. Should a stroke occur or be suspected, estrogen plus progestin therapy should be discontinued immediately.
b. Coronary Heart Disease
In the WHI estrogen plus progestin substudy, there was a statistically non-significant increased risk of CHD events reported in women receiving daily CE (0.625 mg) plus MPA (2.5 mg) compared to women receiving placebo (41 versus 34 per 10,000 women-years). An increase in relative risk was demonstrated in year 1, and a trend toward decreasing relative risk was reported in years 2 through 5.
In postmenopausal women with documented heart disease (n = 2,763, average 66.7 years of age), in a controlled clinical trial of secondary prevention of cardiovascular disease (Heart and Estrogen/Progestin Replacement Study [HERS]), treatment with daily CE (0.625 mg) plus MPA (2.5mg) demonstrated no cardiovascular benefit. During an average follow-up of 4.1 years, treatment with CE plus MPA did not reduce the overall rate of CHD events in postmenopausal women with established coronary heart disease. There were more CHD events in the CE plus MPA-treated group than in the placebo group in year 1, but not during the subsequent years. Two thousand three hundred and twenty-one (2,321) women from the original HERS trial agreed to participate in an open label extension of HERS, HERS II. Average follow-up in HERS II was an additional 2.7 years, for a total of 6.8 years overall. Rates of CHD events were comparable among women in the CE plus MPA group and the placebo group in HERS, HERS II, and overall.
c. Venous Thromboembolism
In the WHI estrogen plus progestin substudy, a statistically significant 2-fold greater rate of VTE, (DVT and PE), was reported in women receiving daily CE (0.625 mg) plus MPA (2.5 mg) compared to women receiving placebo (35 versus 17 per 10,000 women-years). Statistically significant increases in risk for both DVT (26 versus 13 per 10,000 women-years) and PE (18 versus 8 per 10,000 women-years) were also demonstrated. The increase in VTE risk was demonstrated during the first year and persisted. (See CLINICAL STUDIES.) Should a VTE occur or be suspected, estrogen plus progestin therapy should be discontinued immediately.
If feasible, estrogens plus progestins should be discontinued at least 4 to 6 weeks before surgery of the type associated with an increased risk of thromboembolism, or during periods of prolonged immobilization.
2. Malignant Neoplasms
a. Breast Cancer
The most important randomized clinical trial providing information about breast cancer in estrogen plus progestin users is the WHI substudy of daily CE (0.625 mg) plus MPA (2.5 mg). After a mean follow-up of 5.6 years, the estrogen plus progestin substudy reported an increased risk of invasive breast cancer in women who took daily CE plus MPA.
In this substudy, prior use of estrogen-alone or estrogen plus progestin therapy was reported by 26 percent of the women. The relative risk of invasive breast cancer was 1.24, and the absolute risk was 41 versus 33 cases per 10,000 women-years, for CE plus MPA compared with placebo. Among women who reported prior use of hormone therapy, the relative risk of invasive breast cancer was 1.86, and the absolute risk was 46 versus 25 cases per 10,000 women-years, for CE plus MPA compared with placebo. Among women who reported no prior use of hormone therapy, the relative risk of invasive breast cancer was 1.09, and the absolute risk was 40 versus 36 cases per 10,000 women-years, for CE plus MPA compared with placebo. In the same substudy, invasive breast cancers were larger, were more likely to be node positive, and were diagnosed at a more advanced stage in the CE (0.625 mg) plus MPA (2.5 mg) group compared with the placebo group. Metastatic disease was rare with no apparent difference between the two groups. Other prognostic factors such as histologic subtype, grade, and hormone receptor status did not differ between the groups. (See CLINICAL STUDIES.)
Consistent with the WHI clinical trial, observational studies have also reported an increased risk of breast cancer for estrogen plus progestin therapy, and a smaller risk for estrogen-alone therapy, after several years of use. The risk increased with duration of use, and appeared to return to baseline over about 5 years after stopping treatment (only the observational studies have substantial data on risk after stopping). Observational studies also suggest that the risk of breast cancer was greater, and became apparent earlier, with estrogen plus progestin therapy as compared to estrogen-alone therapy. However, these studies have not found significant variation in the risk of breast cancer among different estrogen plus progestin combinations, or routes of administration.
The use of estrogen plus progestin has been reported to result in an increase in abnormal mammograms requiring further evaluation. All women should receive yearly breast examinations by a healthcare provider and perform monthly breast self-examinations. In addition, mammography examinations should be scheduled based on patient age, risk factors, and prior mammogram results.
b. Endometrial Cancer
An increased risk of endometrial cancer has been reported with the use of unopposed estrogen therapy in women with a uterus. The reported endometrial cancer risk among unopposed estrogen users is about 2 to 12 times greater than in non-users, and appears dependent on duration of treatment and on estrogen dose. Most studies show no significant increased risk associated with the use of estrogens for less than 1 year. The greatest risk appears associated with prolonged use, with increased risks of 15- to 24-fold for 5 to 10 years or more. This risk has been shown to persist for at least 8 to 15 years after estrogen therapy is discontinued.
Clinical surveillance of all women using estrogen plus progestin therapy is important. Adequate diagnostic measures, including endometrial sampling when indicated, should be undertaken to rule out malignancy in all cases of undiagnosed persistent or recurring abnormal genital bleeding. There is no evidence that the use of natural estrogens results in a different endometrial risk profile than synthetic estrogens of equivalent estrogen dose. Adding a progestin to estrogen therapy has been shown to reduce the risk of endometrial hyperplasia, which may be a precursor to endometrial cancer.
c. Ovarian Cancer
The WHI estrogen plus progestin substudy reported a statistically non-significant increased risk of ovarian cancer. After an average follow-up of 5.6 years, the relative risk for ovarian cancer for CE plus MPA versus placebo was 1.58 (95 percent CI, 0.77 to 3.24). The absolute risk for CE plus MPA was 4 versus 3 cases per 10,000 women-years. In some epidemiologic studies, the use of estrogen plus progestin and estrogen-only products, in particular for 5 or more years, has been associated with increased risk of ovarian cancer. However, the duration of exposure associated with increased risk is not consistent across all epidemiologic studies and some report no association.
3. Probable Dementia
In the WHIMS estrogen plus progestin ancillary study of WHI, a population of 4,532 postmenopausal women aged 65 to 79 years was randomized to daily CE (0.625 mg) plus MPA (2.5 mg) or placebo.
After an average follow-up of 4 years, 40 women in the CE plus MPA group and 21 women in the placebo group were diagnosed with probable dementia. The relative risk of probable dementia for CE plus MPA versus placebo was 2.05 (95 percent CI, 1.21 to 3.48). The absolute risk of probable dementia for CE plus MPA versus placebo was 45 versus 22 cases per 10,000 women-years. It is unknown whether these findings apply to younger postmenopausal women. (See CLINICAL STUDIES and PRECAUTIONS, Geriatric Use.)
4. Visual Abnormalities
Discontinue estrogen plus progestin therapy pending examination if there is sudden partial or complete loss of vision, or a sudden onset of proptosis, diplopia or migraine. If examination reveals papilledema or retinal vascular lesions, estrogen plus progestin therapy should be permanently discontinued.
PRECAUTIONS
A. General
- Addition of a progestin when a woman has not had a hysterectomy
- Studies of the addition of a progestin for 10 or more days of a cycle of estrogen administration, or daily with estrogen in a continuous regimen, have reported a lowered incidence of endometrial hyperplasia than would be induced by estrogen treatment alone. Endometrial hyperplasia may be a precursor to endometrial cancer.
- There are, however, possible risks that may be associated with the use of progestins with estrogens compared to estrogen-alone regimens. These include an increased risk of breast cancer.
- Unexpected abnormal vaginal bleeding
- In cases of unexpected abnormal vaginal bleeding, adequate diagnostic measures are indicated.
- Elevated blood pressure
- Blood pressure should be monitored at regular intervals with estrogen plus progestin therapy.
- Hypertriglyceridemia
- In women with pre-existing hypertriglyceridemia, estrogen plus progestin therapy may be associated with elevations of plasma triglycerides leading to pancreatitis. Consider discontinuation of treatment if pancreatitis occurs.
- Hepatic Impairment and/or past history of cholestatic jaundice
- Estrogens plus progestins may be poorly metabolized in women with impaired liver function. For women with a history of cholestatic jaundice associated with past estrogen use or with pregnancy, caution should be exercised, and in the case of recurrence, medication should be discontinued.
- Fluid Retention
- Progestins may cause some degree of fluid retention. Women who have conditions which might be influenced by this factor, such as cardiac or renal impairment, warrant careful observation when estrogen plus progestin are prescribed.
- Hypocalcemia
- Estrogen plus progestin therapy should be used with caution in women with hypoparathyroidism as estrogen-induced hypocalcemia may occur.
- Exacerbation of other conditions
- Estrogen plus progestin therapy may cause an exacerbation of asthma, diabetes mellitus, epilepsy, migraine, porphyria, systemic lupus erythematosus, and hepatic hemangiomas and should be used with caution in women with these conditions.
B. Patient Information
Physicians are advised to discuss the Patient Information leaflet with women for whom they prescribe medroxyprogesterone acetate.
There may be an increased risk of minor birth defects in children whose mothers are exposed to progestins during the first trimester of pregnancy. The possible risk to the male baby is hypospadias, a condition in which the opening of the penis is on the underside rather than the tip of the penis. This condition occurs naturally in approximately 5 to 8 per 1000 male births. The risk may be increased with exposure to medroxyprogesterone acetate. Enlargement of the clitoris and fusion of the labia may occur in female babies. However, a clear association between hypospadias, clitoral enlargement and labial fusion with use of medroxyprogesterone acetate has not been established.
Inform the patient of the importance of reporting exposure to medroxyprogesterone acetate in early pregnancy.
C. Drug-Laboratory Test Interactions
The following laboratory results may be altered by the use of estrogen plus progestin therapy:
- Accelerated prothrombin time, partial thromboplastin time, and platelet aggregation time; increased platelet count; increased factors II, VII antigen, VIII antigen, VIII coagulant activity, IX, X, XII, VII-X complex, II-VII-X complex, and beta-thromboglobulin; decreased levels of anti-factor Xa and antithrombin III, decreased antithrombin III activity; increased levels of fibrinogen and fibrinogen activity; increased plasminogen antigen and activity.
- Increased thyroid-binding globulin (TBG) levels leading to increased circulating total thyroid hormone levels as measured by protein-bound iodine (PBI), T4 levels (by column or by radioimmunoassay) or T3 levels by radioimmunoassay, T3 resin uptake is decreased, reflecting the elevated TBG. Free T4 and free T3 concentrations are unaltered. Women on thyroid replacement therapy may require higher doses of thyroid hormone.
- Other binding proteins may be elevated in serum for example, corticosteroid binding globulin (CBG), sex hormone binding globulin (SHBG) leading to increased circulating corticosteroid and sex steroids, respectively. Free hormone concentrations, such as testosterone and estradiol, may be decreased. Other plasma proteins may be increased (angiotensinogen/renin substrate, alpha-1-antitrypsin, ceruloplasmin).
- Increased plasma high-density lipoprotein (HDL) and HDL2 cholesterol subfraction concentrations, reduced low-density lipoprotein (LDL) cholesterol concentration, increased triglycerides levels.
- Impaired glucose tolerance.
D. Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenicity: Long-term intramuscular administration of medroxyprogesterone acetate has been shown to produce mammary tumors in beagle dogs. There was no evidence of a carcinogenic effect associated with the oral administration of medroxyprogesterone acetate to rats and mice.
Long-term continuous administration of estrogen plus progestin therapy has shown an increased risk of breast cancer and ovarian cancer. (See WARNINGS and PRECAUTIONS.)
Genotoxicity: Medroxyprogesterone acetate was not mutagenic in a battery of in vitro or in vivo genetic toxicity assays.
Fertility: Medroxyprogesterone acetate at high doses is an antifertility drug and high doses would be expected to impair fertility until the cessation of treatment.
E. Pregnancy
Teratogenic Effects
Pregnancy Category X
Medroxyprogesterone acetate should not be used during pregnancy. (See CONTRAINDICATIONS.)
There may be increased risks for hypospadias, clitoral enlargement and labial fusion in children whose mothers are exposed to medroxyprogesterone acetate during the first trimester of pregnancy. However, a clear association between these conditions with use of medroxyprogesterone acetate has not been established.
F. Nursing Mothers
Medroxyprogesterone acetate should not be used during lactation. Detectable amounts of progestin have been identified in the breast milk of nursing mothers receiving progestins.
G. Pediatric Use
Medroxyprogesterone acetate tablets are not indicated in children. Clinical studies have not been conducted in the pediatric population.
H. Geriatric Use
There have not been sufficient numbers of geriatric women involved in clinical studies utilizing medroxyprogesterone acetate alone to determine whether those over 65 years of age differ from younger subjects in their response to medroxyprogesterone acetate alone.
The Women’s Health Initiative Studies
In the WHI estrogen plus progestin substudy (daily CE [0.625 mg] plus MPA [2.5 mg] versus placebo), there was a higher relative risk of nonfatal stroke and invasive breast cancer in women greater than 65 years of age. (See CLINICAL STUDIES.)
The Women’s Health Initiative Memory Study
In the WHIMS ancillary studies of postmenopausal women 65 to 79 years of age, there was an increased risk of developing probable dementia in women receiving estrogen- alone or estrogen plus progestin when compared to placebo. (See WARNINGS, Probable Dementia.)
Since both ancillary studies were conducted in women 65 to 79 years of age, it is unknown whether these findings apply to younger postmenopausal women. (See WARNINGS, Probable Dementia.)
ADVERSE REACTIONS
See BOXED WARNINGS,WARNINGS, and PRECAUTIONS.
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The following adverse reactions have been reported in women taking medroxyprogesterone acetate tablets, without concomitant estrogens treatment:
1. Genitourinary system
Abnormal uterine bleeding (irregular, increase, decrease), change in menstrual flow, breakthrough bleeding, spotting, amenorrhea, changes in cervical erosion and cervical secretions.
2. Breasts
Breast tenderness, mastodynia or galactorrhea has been reported.
3. Cardiovascular
Thromboembolic disorders including thrombophlebitis and pulmonary embolism have been reported.
4. Gastrointestinal
Nausea, cholestatic jaundice.
5. Skin
Sensitivity reactions consisting of urticaria, pruritus, edema and generalized rash have occurred. Acne, alopecia and hirsutism have been reported.
6. Eyes
Neuro-ocular lesions, for example, retinal thrombosis, and optic neuritis.
7. Central nervous system
Mental depression, insomnia, somnolence, dizziness, headache, nervousness.
8. Miscellaneous
Hypersensitivity reactions (for example, anaphylaxis and anaphylactoid reactions, angioedema), rash (allergic) with and without pruritus, change in weight (increase or decrease), pyrexia, edema/fluid retention, fatigue, decreased glucose tolerance.
The following adverse reactions have been reported with estrogen plus progestin therapy.
1. Genitourinary system
Abnormal uterine bleeding/spotting, or flow; breakthrough bleeding; spotting; dysmenorrheal/pelvic pain; increase in size of uterine leiomyomata; vaginitis, including vaginal candidiasis; change in amount of cervical secretion; changes in cervical ectropion; ovarian cancer; endometrial hyperplasia; endometrial cancer.
2. Breasts
Tenderness, enlargement, pain, nipple discharge, galactorrhea; fibrocystic breast changes; breast cancer.
3. Cardiovascular
Deep and superficial venous thrombosis; pulmonary embolism; thrombophlebitis; myocardial infarction; stroke; increase in blood pressure.
4. Gastrointestinal
Nausea, vomiting; abdominal cramps, bloating; cholestatic jaundice; increased incidence of gallbladder disease; pancreatitis; enlargement of hepatic hemangiomas.
5. Skin
Chloasma or melasma that may persist when drug is discontinued; erythema multiforme; erythema nodosum; hemorrhagic eruption; loss of scalp hair; hirsutism; pruritus, rash.
6. Eyes
Retinal vascular thrombosis, intolerance to contact lenses.
7. Central nervous system
Headache; migraine; dizziness; mental depression; chorea; nervousness; mood disturbances; irritability; exacerbation of epilepsy, dementia.
8. Miscellaneous
Increase or decrease in weight; reduced carbohydrate tolerance; aggravation of porphyria; edema; arthalgias; leg cramps; changes in libido; urticaria, angioedema, anaphylactoid/anaplylactic reactions; hypocalcemia; exacerbation of asthma; increased triglycerides.
OVERDOSAGE
Overdosage of estrogen plus progestin therapy may cause nausea and vomiting, breast tenderness, dizziness, abdominal pain, drowsiness/fatigue and withdrawal bleeding may occur in women. Treatment of overdose consists of discontinuation of CE plus MPA together with institution of appropriate symptomatic care.
DOSAGE AND ADMINISTRATION
Secondary Amenorrhea
Medroxyprogesterone acetate tablets may be given in dosages of 5 or 10 mg daily for 5 to 10 days. A dose for inducing an optimum secretory transformation of an endometrium that has been adequately primed with either endogenous or exogenous estrogen is 10 mg of medroxyprogesterone acetate daily for 10 days. In cases of secondary amenorrhea, therapy may be started at any time. Progestin withdrawal bleeding usually occurs within three to seven days after discontinuing medroxyprogesterone acetate therapy.
Abnormal Uterine Bleeding Due to Hormonal Imbalance in the Absence of Organic Pathology
Beginning on the calculated 16th or 21st day of the menstrual cycle, 5 or 10 mg of medroxyprogesterone acetate may be given daily for 5 to 10 days. To produce an optimum secretory transformation of an endometrium that has been adequately primed with either endogenous or exogenous estrogen, 10 mg of medroxyprogesterone acetate daily for 10 days beginning on the 16th day of the cycle is suggested. Progestin withdrawal bleeding usually occurs within three to seven days after discontinuing therapy with medroxyprogesterone acetate. Patients with a past history of recurrent episodes of abnormal uterine bleeding may benefit from planned menstrual cycling with medroxyprogesterone acetate.
Reduction of Endometrial Hyperplasia in Postmenopausal Women Receiving Daily 0.625 mg Conjugated Estrogens
When estrogen is prescribed for a postmenopausal woman with a uterus, a progestin should also be initiated to reduce the risk of endometrial cancer. A woman without a uterus does not need progestin. Use of estrogen, alone or in combination with a progestin, should be with the lowest effective dose and for the shortest duration consistent with treatment goals and risks for the individual woman. Patients should be re-evaluated periodically as clinically appropriate (for example, 3 to 6 month intervals) to determine if treatment is still necessary (see WARNINGS). For women who have a uterus, adequate diagnostic measures, such as endometrial sampling, when indicated, should be undertaken to rule out malignancy in cases of undiagnosed persistent or recurring abnormal vaginal bleeding.
Medroxyprogesterone acetate tablets may be given in dosages of 5 or 10 mg daily for 12 to 14 consecutive days per month, in postmenopausal women receiving daily 0.625 mg conjugated estrogens, either beginning on the 1st day of the cycle or the 16th day of the cycle.
Patients should be started at the lowest dose.
The lowest effective dose of medroxyprogesterone acetate has not been determined.
HOW SUPPLIED
Medroxyprogesterone Acetate Tablets USP are available as:
5 mg: White, round, scored, biconvex tablet. Debossed with 555/873 on the scored side and stylized b on the other side, available in bottles of 100 (NDC: 63629-8826-1).
Dispense in a tight, light-resistant container as defined in the USP, with a child-resistant closure (as required).
Store at 20° to 25°C (68° to 77°F) [See USP Controlled Room Temperature].
KEEP THIS AND ALL MEDICATIONS OUT OF THE REACH OF CHILDREN.
NDC: 63629-8826-1: 100 Tablets in a BOTTLE
PATIENT INFORMATION
MedroxyPROGESTERone Acetate Tablets USP
Rx only
Read this Patient Information before you start taking medroxyprogesterone acetate and read what you get each time you refill your medroxyprogesterone acetate prescription. There may be new information. This information does not take the place of talking to your healthcare provider about your medical condition or your treatment.
What is the most important information I should know about medroxyprogesterone acetate (a progestin hormone)?
- Do not use estrogens with progestins to prevent heart disease, heart attacks, strokes, or dementia (decline in brain function).
- Using estrogens with progestins may increase your chance of getting heart attacks, strokes, breast cancer, and blood clots.
- Using estrogens with progestins may increase your chance of getting dementia, based on a study of women age 65 years or older.
- You and your healthcare provider should talk regularly about whether you still need treatment with medroxyprogesterone acetate.
What is medroxyprogesterone acetate?
Medroxyprogesterone acetate is a medicine that contains medroxyprogesterone acetate, a progestin hormone.
What is medroxyprogesterone acetate used for?
Medroxyprogesterone acetate is used to:
- Treat menstrual periods that have stopped or to treat abnormal uterine bleeding. Women with a uterus who are not pregnant, who stop having regular menstrual periods or who begin to have irregular menstrual periods may have a drop in their progesterone level. Talk with your healthcare provider about whether medroxyprogesterone acetate is right for you.
- Reduce your chances of getting cancer of the uterus (womb). In postmenopausal women with a uterus who use estrogens, taking progestin in combination with estrogen will reduce your chance of getting cancer of the uterus (womb).
Who should not take medroxyprogesterone acetate?
Do not start taking medroxyprogesterone acetate if you:
- have unusual vaginal bleeding
-
currently have or have had certain cancers
Estrogen plus progestin may increase your chance of getting certain types of cancers, including cancer of the breast. If you have or have had cancer, talk with your healthcare provider about whether you should use medroxyprogesterone acetate. - had a stroke or heart attack
- currently have or have had blood clots
- currently have or have had liver problems
-
are allergic to medroxyprogesterone acetate tablets or any of its ingredients
See the list of ingredients inmedroxyprogesterone acetate tablets at the end of this leaflet. -
think you may be pregnant
Medroxyprogesterone acetate is not for pregnant women. If you think you may be pregnant, you should have a pregnancy test and know the results. Do not use medroxyprogesterone acetate if the test is positive and talk to your healthcare provider. There may be an increased risk of minor birth defects in children whose mothers take medroxyprogesterone acetate during the first 4 months of pregnancy.Medroxyprogesterone acetate should not be used as a test for pregnancy.
What should I tell my healthcare provider before taking medroxyprogesterone acetate? Before you take medroxyprogesterone acetate, tell your healthcare provider if you:
-
have any other medical problems
Your healthcare provider may need to check you more carefully if you have certain conditions such as asthma (wheezing), epilepsy (seizures), diabetes, migraine, endometriosis (severe pelvic pain), lupus, or problems with your heart, liver, thyroid, kidneys, or have high calcium in your blood. -
are going to have surgery or will be on bed rest
Your healthcare provider will let you know if you need to stop taking medroxyprogesterone acetate. -
are breast feeding
The hormone in medroxyprogesterone acetate can pass into your breast milk.
Tell your healthcare provider about all the medicines you take including prescription and nonprescription medicines, vitamins, and herbal supplements. Some medicines may affect how medroxyprogesterone acetate works. Medroxyprogesterone acetate may also affect how other medicines work.
How should I take medroxyprogesterone acetate?
Start at the lowest dose and talk to your healthcare provider about how well that dose is working for you. The lowest effective dose of medroxyprogesterone acetate has not been determined. You and your healthcare provider should talk regularly (every 3 to 6 months) about the dose you are taking and whether you still need treatment with medroxyprogesterone acetate.
- Absence of menstrual period: Medroxyprogesterone acetate may be given in doses ranging from 5 to 10 mg daily for 5 to 10 days.
- Abnormal Uterine Bleeding: Medroxyprogesterone acetate may be given in doses ranging from 5 to 10 mg daily for 5 to 10 days.
- Overgrowth of the lining of the uterus: When used in combination with oral conjugated estrogens in postmenopausal women with a uterus, medroxyprogesterone acetate may be given in doses ranging from 5 or 10 mg daily for 12 to 14 straight days per month.
What are the possible side effects of medroxyprogesterone acetate?
The following side effects have been reported with the use of medroxyprogesterone acetate alone:
- breast tenderness
- breast milk secretion
- breakthrough bleeding
- spotting (minor vaginal bleeding)
- irregular periods
- amenorrhea (absence of menstrual periods)
- vaginal secretions
- headaches
- nervousness
- dizziness
- depression
- insomnia, sleepiness, fatigue
- premenstrual syndrome-like symptoms
- thrombophlebitis (inflamed veins)
- blood clot
- itching, hives, skin rash
- acne
- hair loss, hair growth
- abdominal discomfort
- nausea
- bloating
- fever
- increase in weight
- swelling
- changes in vision and sensitivity to contact lenses
Call your healthcare provider right away if you get hives, problems breathing, swelling of the face, mouth, tongue or neck.
The following side effects have been reported with the use of medroxyprogesterone acetate with an estrogen.
Side effects are grouped by how serious they are and how often they happen when you are treated.
Serious, but less common side effects include:
- heart attack
- stroke
- blood clots
- dementia
- breast cancer
- cancer of the uterus
- cancer of the ovary
- high blood pressure
- high blood sugar
- gallbladder disease
- liver problems
- changes in your thyroid hormone levels
- enlargements of benign tumors (“fibroids”)
Call your healthcare provider right away if you get any of the following warning signs or any other unusual symptoms that concern you:
- new breast lumps
- unusual vaginal bleeding
- changes in vision and speech
- sudden new severe headaches
- severe pains in your chest or legs with or without shortness of breath, weakness and fatigue
- memory loss or confusion
Less serious but common side effects include:
- headache
- breast pain
- irregular vaginal bleeding or spotting
- stomach or abdominal cramps, bloating
- nausea and vomiting
- hair loss
- fluid retention
- vaginal yeast infection
These are not all the possible side effects of medroxyprogesterone acetate with or without estrogen. For more information, ask your healthcare provider or pharmacist for advice about side effects. Tell your healthcare provider if you have side effect that bothers you or does not go away. You may report side effects to Teva at 1-866-832-8537 or FDA at 1-800-FDA-1088.
What can I do to lower my chances of a serious side effect with medroxyprogesterone acetate?
- Talk with your healthcare provider regularly about whether you should continue taking medroxyprogesterone acetate. The addition of a progestin is generally recommended for women with a uterus to reduce the chance of getting cancer of the uterus (womb).
- See your healthcare provider right away if you get vaginal bleeding while taking medroxyprogesterone acetate.
- Have a pelvic exam, breast exam and mammogram (breast X-ray) every year unless your healthcare provider tells you something else. If members of your family have had breast cancer or if you have ever had breast lumps or an abnormal mammogram, you may need to have breast exams more often.
- If you have high blood pressure, high cholesterol (fat in the blood), diabetes, are overweight, or if you use tobacco, you may have a higher chance of getting heart disease. Ask your healthcare provider for ways to lower your chance of getting heart disease.
General information about safe and effective use of medroxyprogesterone acetate
- Medicines are sometimes prescribed for conditions that are not mentioned in patient information leaflets.
- Do not take medroxyprogesterone acetate for conditions for which it was not prescribed.
- Do not give medroxyprogesterone acetate to other people, even if they have the same symptoms you have. It may harm them.
Keep medroxyprogesterone acetate out of the reach of children.
This leaflet provides a summary of the most important information about medroxyprogesterone acetate. If you would like more information, talk with your health care provider or pharmacist. You can ask for information about medroxyprogesterone acetate that is written for health professionals. You can get more information by calling the toll-free number, 1-888-838-2872.
What are the ingredients in medroxyprogesterone acetate?
Each medroxyprogesterone acetate tablet for oral administration contains 2.5 mg, 5 mg or 10 mg of medroxyprogesterone acetate.
Inactive ingredients: crospovidone, lactose monohydrate, magnesium stearate, methylcellulose, pregelatinized corn starch, and sodium lauryl sulfate.
TEVA PHARMACEUTICALS USA, INC.
North Wales, PA 19454
Rev. A 5/2015
INGREDIENTS AND APPEARANCE
| MEDROXYPROGESTERONE ACETATE
medroxyprogesterone acetate tablet |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Labeler - Bryant Ranch Prepack (171714327) |
| Registrant - Bryant Ranch Prepack (171714327) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Bryant Ranch Prepack | 171714327 | REPACK(63629-8826) , RELABEL(63629-8826) | |