Search by Drug Name or NDC
NDC 63629-9321-01 NALOXONE HYDROCHLORIDE 4 mg/.1mL Details
NALOXONE HYDROCHLORIDE 4 mg/.1mL
NALOXONE HYDROCHLORIDE is a NASAL SPRAY in the HUMAN PRESCRIPTION DRUG category. It is labeled and distributed by Bryant Ranch Prepack. The primary component is NALOXONE HYDROCHLORIDE.
MedlinePlus Drug Summary
Naloxone nasal spray is used along with emergency medical treatment to reverse the life-threatening effects of a known or suspected opiate (narcotic) overdose in adults and children. Naloxone nasal spray is in a class of medications called opiate antagonists. It works by blocking the effects of opiates to relieve dangerous symptoms caused by high levels of opiates in the blood.
Related Packages: 63629-9321-01Last Updated: 12/01/2022
MedLinePlus Full Drug Details: Naloxone Nasal Spray
Product Information
| NDC | 63629-9321 |
|---|---|
| Product ID | 63629-9321_9c47fac5-40e9-43ae-b7d1-9a12ad1f8b3f |
| Associated GPIs | |
| GCN Sequence Number | 075222 |
| GCN Sequence Number Description | naloxone HCl SPRAY 4 MG NASAL |
| HIC3 | H3T |
| HIC3 Description | OPIOID ANTAGONISTS |
| GCN | 40233 |
| HICL Sequence Number | 001874 |
| HICL Sequence Number Description | NALOXONE HCL |
| Brand/Generic | Generic |
| Proprietary Name | NALOXONE HYDROCHLORIDE |
| Proprietary Name Suffix | n/a |
| Non-Proprietary Name | naloxone hydrochloride |
| Product Type | HUMAN PRESCRIPTION DRUG |
| Dosage Form | SPRAY |
| Route | NASAL |
| Active Ingredient Strength | 4 |
| Active Ingredient Units | mg/.1mL |
| Substance Name | NALOXONE HYDROCHLORIDE |
| Labeler Name | Bryant Ranch Prepack |
| Pharmaceutical Class | Opioid Antagonist [EPC], Opioid Antagonists [MoA] |
| DEA Schedule | n/a |
| Marketing Category | ANDA |
| Application Number | ANDA211951 |
| Listing Certified Through | 2023-12-31 |
Package
Package Images

NDC 63629-9321-01 (63629932101)
| NDC Package Code | 63629-9321-1 |
|---|---|
| Billing NDC | 63629932101 |
| Package | 2 VIAL, SINGLE-DOSE in 1 CARTON (63629-9321-1) / .1 mL in 1 VIAL, SINGLE-DOSE |
| Marketing Start Date | 2022-07-07 |
| NDC Exclude Flag | N |
| Pricing Information | N/A |
Standard Product Labeling (SPL)/Prescribing Information SPL 73edcc70-d988-4f66-a09a-8d23042283c3 Details
HIGHLIGHTS OF PRESCRIBING INFORMATION
NALOXONE HYDROCHLORIDE nasal spray
Initial U.S. Approval: 1971
INDICATIONS AND USAGE
Naloxone HCl Nasal Spray is an opioid antagonist indicated for the emergency treatment of known or suspected opioid overdose, as manifested by respiratory and/or central nervous system depression. (1)
Naloxone HCl Nasal Spray is intended for immediate administration as emergency therapy in settings where opioids may be present. (1)
Naloxone HCl Nasal Spray is not a substitute for emergency medical care. (1)
DOSAGE AND ADMINISTRATION
- •
- Naloxone HCl Nasal Spray is for intranasal use only. (2.1)
- •
- Seek emergency medical care immediately after use. (2.1)
- •
- Administration of a single spray of Naloxone HCl Nasal Spray intranasally into one nostril. (2.2)
- •
- Administer additional doses of Naloxone HCl Nasal Spray, using a new nasal spray with each dose, if the patient does not respond or responds and then relapses into respiratory depression, additional doses of Naloxone HCl Nasal Spray may be given every 2 to 3 minutes until emergency medical assistance arrives. (2.2)
- •
- Additional supportive and/or resuscitative measures may be helpful while awaiting emergency medical assistance. (2.2)
DOSAGE FORMS AND STRENGTHS
Nasal spray: 4 mg of naloxone hydrochloride in 0.1 mL. (3) (3)
CONTRAINDICATIONS
Hypersensitivity to naloxone hydrochloride. (4)
WARNINGS AND PRECAUTIONS
- •
- Risk of Recurrent Respiratory and CNS Depression: Due to the duration of action of naloxone relative to the opioid, keep patient under continued surveillance and administer repeat doses of naloxone using a new nasal spray with each dose, as necessary, while awaiting emergency medical assistance. (5.1)
- •
- Risk of Limited Efficacy with Partial Agonists or Mixed Agonists/Antagonists: Reversal of respiratory depression caused by partial agonists or mixed agonists/antagonists, such as buprenorphine and pentazocine, may be incomplete. Larger or repeat doses may be required. (5.2)
- •
- Precipitation of Severe Opioid Withdrawal: Use in patients who are opioid dependent may precipitate opioid withdrawal. In neonates, opioid withdrawal may be life-threatening if not recognized and properly treated. Monitor for the development of opioid withdrawal. (5.3)
- •
- Risk of Cardiovascular (CV) Effects: Abrupt postoperative reversal of opioid depression may result in adverse CV effects. These events have primarily occurred in patients who had pre-existing CV disorders or received other drugs that may have similar adverse CV effects. Monitor these patients closely in an appropriate healthcare setting after use of naloxone hydrochloride. (5.3)
ADVERSE REACTIONS
The following adverse reactions were observed in a naloxone hydrochloride nasal spray clinical study: increased blood pressure, constipation, toothache, muscle spasms, musculoskeletal pain, headache, nasal dryness, nasal edema, nasal congestion, nasal inflammation, rhinalgia and xeroderma. (6)
To report SUSPECTED ADVERSE REACTIONS, contact Perrigo at 1-866-634-9120 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 7/2022
Table Of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Important Administration Instructions
2.2 Dosing in Adults and Pediatric Patients
2.3 Dosing Modifications due to Partial Agonists or Mixed Agonist/Antagonists
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Risk of Recurrent Respiratory and Central Nervous System Depression
5.2 Risk of Limited Efficacy with Partial Agonists or Mixed Agonist/Antagonists
5.3 Precipitation of Severe Opioid Withdrawal
6 ADVERSE REACTIONS
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- *
- Sections or subsections omitted from the full prescribing information are not listed.
1 INDICATIONS AND USAGE
Naloxone HCl Nasal Spray is indicated for the emergency treatment of known or suspected opioid overdose, as manifested by respiratory and/or central nervous system depression.
Naloxone HCl Nasal Spray is intended for immediate administration as emergency therapy in settings where opioids may be present.
Naloxone HCl Nasal Spray is not a substitute for emergency medical care.
Limitations of Use:
Restrict prescription of naloxone hydrochloride nasal spray 2 mg to opioid-dependent patients expected to be at risk for severe opioid withdrawal in situations where there is a low risk for accidental or intentional opioid exposure by household contacts.
2 DOSAGE AND ADMINISTRATION
2.1 Important Administration Instructions
Naloxone HCl Nasal Spray is for intranasal use only.
No additional device assembly is required.
Because treatment of suspected opioid overdose must be performed by someone other than the patient, instruct the prescription recipient to inform those around them about the presence of Naloxone HCl Nasal Spray and the Instructions for Use.
Instruct the patient or caregiver to read the Instructions for Use at the time they receive a prescription for Naloxone HCl Nasal Spray. Emphasize the following instructions to the patient or caregiver:
- •
- Administer Naloxone HCl Nasal Spray as quickly as possible because prolonged respiratory depression may result in damage to the central nervous system or death. Since the duration of action of most opioids exceeds that of naloxone hydrochloride and the suspected opioid overdose may occur outside of supervised medical settings, seek immediate emergency medical assistance, keep the patient under continued surveillance until emergency personnel arrive, and administer repeated doses of Naloxone HCl Nasal Spray, as necessary. Always seek emergency medical assistance in the event of a suspected, potentially life-threatening opioid emergency after administration of the first dose of Naloxone HCl Nasal Spray.
- •
- Additional doses of Naloxone HCl Nasal Spray may be required until emergency medical assistance becomes available.
- •
- Do not attempt to reuse Naloxone HCl Nasal Spray. Each Naloxone HCl Nasal Spray contains a single dose of naloxone and cannot be reused.
- •
- Re-administer Naloxone HCl Nasal Spray, using a new nasal spray, every 2 to 3 minutes if the patient does not respond or responds and then relapses into respiratory depression.
- •
- Administer Naloxone HCl Nasal Spray in alternate nostrils with each dose.
- •
- Administer Naloxone HCl Nasal Spray according to the printed instructions on the device label and the Instructions for Use.
- •
- Place the patient in the supine position. Prior to administration, be sure the device nozzle is inserted in either nostril of the patient, and provide support to the back of the neck to allow the head to tilt back. Do not prime or test the device prior to administration.
- •
- To administer the dose, press firmly on the device plunger.
- •
- Remove the device nozzle from the nostril after use.
- •
- Turn patient on their side as shown in the Instructions for Use and call for emergency medical assistance immediately after administration of the first dose of Naloxone HCl Nasal Spray.
2.2 Dosing in Adults and Pediatric Patients
Initial Dosing
The recommended initial dose of Naloxone HCl Nasal Spray in adults and pediatric patients is one spray delivered by intranasal administration into one nostril.
Repeat Dosing
Seek emergency medical assistance as soon as possible after administering the first dose of Naloxone HCl Nasal Spray.
The requirement for repeat doses of Naloxone HCl Nasal Spray depends upon the amount, type, and route of administration of the opioid being antagonized.
Administer Naloxone HCl Nasal Spray in alternate nostrils with each dose.
If the patient responds to Naloxone HCl Nasal Spray and relapses back into respiratory depression before emergency assistance arrives, administer an additional dose of Naloxone HCl Nasal Spray using a new Naloxone HCl Nasal Spray and continue surveillance of the patient.
If the desired response is not obtained after 2 or 3 minutes, administer an additional dose of Naloxone HCl Nasal Spray using a new Naloxone HCl Nasal Spray. If there is still no response and additional doses are available, administer additional doses of Naloxone HCl Nasal Spray every 2 to 3 minutes using a new Naloxone HCl Nasal Spray with each dose until emergency medical assistance arrives.
Additional supportive and/or resuscitative measures may be helpful while awaiting emergency medical assistance.
2.3 Dosing Modifications due to Partial Agonists or Mixed Agonist/Antagonists
Reversal of respiratory depression by partial agonists or mixed agonist/antagonists, such as buprenorphine and pentazocine, may be incomplete and require higher doses of naloxone hydrochloride or repeated administration of Naloxone HCl Nasal Spray using a new nasal spray [see Warnings and Precautions (5.2)].
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Risk of Recurrent Respiratory and Central Nervous System Depression
The duration of action of most opioids may exceed that of Naloxone HCl Nasal Spray resulting in a return of respiratory and/or central nervous system depression after an initial improvement in symptoms. Therefore, it is necessary to seek emergency medical assistance immediately after administration of the first dose of Naloxone HCl Nasal Spray and to keep the patient under continued surveillance. Administer additional doses of Naloxone HCl Nasal Spray if the patient is not adequately responding or responds and then relapses back into respiratory depression, as necessary [see Dosage and Administration (2.2)]. Additional supportive and/or resuscitative measures may be helpful while awaiting emergency medical assistance.
5.2 Risk of Limited Efficacy with Partial Agonists or Mixed Agonist/Antagonists
Reversal of respiratory depression by partial agonists or mixed agonist/antagonists such as buprenorphine and pentazocine, may be incomplete. Larger or repeat doses of naloxone hydrochloride may be required to antagonize buprenorphine because the latter has a long duration of action due to its slow rate of binding and subsequent slow dissociation from the opioid receptor [see Dosage and Administration (2.3)]. Buprenorphine antagonism is characterized by a gradual onset of the reversal effects and a decreased duration of action of the normally prolonged respiratory depression.
5.3 Precipitation of Severe Opioid Withdrawal
The use of Naloxone HCl Nasal Spray in patients who are opioid-dependent may precipitate opioid withdrawal characterized by the following signs and symptoms: body aches, diarrhea, tachycardia, fever, runny nose, sneezing, piloerection, sweating, yawning, nausea or vomiting, nervousness, restlessness or irritability, shivering or trembling, abdominal cramps, weakness, and increased blood pressure. In neonates, opioid withdrawal may be life-threatening if not recognized and properly treated and may include the following signs and symptoms: convulsions, excessive crying, and hyperactive reflexes. Monitor the patient for the development of the signs and symptoms of opioid withdrawal.
There are limited data to inform if the 2 mg dose of naloxone hydrochloride nasal spray will avoid precipitation of severe opioid withdrawal in the setting of opioid dependence. However, the 2 mg dose may not provide an adequate and timely reversal in persons who may be exposed to an overdose of a potent or very high dose of opioids.
Abrupt postoperative reversal of opioid depression after using naloxone hydrochloride may result in nausea, vomiting, sweating, tremulousness, tachycardia, hypotension, hypertension, seizures, ventricular tachycardia and fibrillation, pulmonary edema, and cardiac arrest. Death, coma, and encephalopathy have been reported as sequelae of these events.
These events have primarily occurred in patients who had pre-existing cardiovascular disorders or received other drugs that may have similar adverse cardiovascular effects. Although a direct cause and effect relationship has not been established, after use of naloxone hydrochloride, monitor patients with pre-existing cardiac disease or patients who have received medications with potential adverse cardiovascular effects for hypotension, ventricular tachycardia or fibrillation, and pulmonary edema in an appropriate healthcare setting. It has been suggested that the pathogenesis of pulmonary edema associated with the use of naloxone hydrochloride is similar to neurogenic pulmonary edema, i.e., a centrally mediated massive catecholamine response leading to a dramatic shift of blood volume into the pulmonary vascular bed resulting in increased hydrostatic pressures.
There may be clinical settings, particularly the postpartum period in neonates with known or suspected exposure to maternal opioid use, where it is preferable to avoid the abrupt precipitation of opioid withdrawal symptoms. In these settings, consider use of an alternative, naloxone-containing product that can be titrated to effect and, where applicable, dosed according to weight. [see Use in Specific Populations (8.4)].
6 ADVERSE REACTIONS
The following serious adverse reactions are discussed elsewhere in the labeling:
- •
- Precipitation of Severe Opioid Withdrawal [see Warnings and Precautions (5.3)]
Because clinical studies are conducted under widely varying conditions, adverse reaction rates observed in the clinical studies of a drug cannot be directly compared to the rates in the clinical studies of another drug and may not reflect the rates observed in practice.
The following adverse reactions were observed in a naloxone hydrochloride nasal spray clinical study.
In a pharmacokinetic study of 30 healthy adult volunteers exposed to one spray of naloxone hydrochloride nasal spray in one nostril or two sprays of naloxone hydrochloride nasal spray, one in each nostril, the most common adverse reactions were: increased blood pressure, constipation, toothache, muscle spasms, musculoskeletal pain, headache, nasal dryness, nasal edema, nasal congestion, nasal inflammation, rhinalgia, and xeroderma.
The following adverse reactions have been identified primarily during post-approval use of naloxone hydrochloride in the post-operative setting. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure: Hypotension, hypertension, ventricular tachycardia and fibrillation, dyspnea, pulmonary edema, and cardiac arrest. Death, coma, and encephalopathy have been reported as sequelae of these events. Excessive doses of naloxone hydrochloride in post-operative patients have resulted in significant reversal of analgesia, and have caused agitation.
Abrupt reversal of opioid effects in persons who were physically dependent on opioids has precipitated an acute withdrawal syndrome. Signs and symptoms have included: body aches, fever, sweating, runny nose, sneezing, piloerection, yawning, weakness, shivering or trembling, nervousness, restlessness or irritability, diarrhea, nausea or vomiting, abdominal cramps, increased blood pressure, tachycardia. In some patients, there may be aggressive behavior upon abrupt reversal of an opioid overdose. In the neonate, opioid withdrawal signs and symptoms also included convulsions, excessive crying, and hyperactive reflexes.
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
The limited available data on naloxone use in pregnant women are not sufficient to inform a drug- associated risk. However, there are clinical considerations [see Clinical Considerations]. In animal reproduction studies, no embryotoxic or teratogenic effects were observed in mice and rats treated with naloxone hydrochloride during the period of organogenesis at doses equivalent to 6-times and 12-times, respectively, a human dose of 8 mg/day (two Naloxone HCl Nasal Sprays) based on body surface area comparison [see Data].
The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively.
Clinical Considerations
Fetal/Neonatal adverse reactions
Naloxone hydrochloride crosses the placenta, and may precipitate withdrawal in the fetus, as well as in the opioid-dependent mother [see Warnings and Precautions (5.3)]. The fetus should be evaluated for signs of distress after Naloxone HCl Nasal Spray is used. Careful monitoring is needed until the fetus and mother are stabilized.
Data
Animal Data
Naloxone hydrochloride was administered during organogenesis to mice and rats at subcutaneous doses up to 10 mg/kg/day (equivalent to 6-times and 12-times, respectively, a human dose of 8 mg (two Naloxone HCl Nasal Sprays) based on body surface area comparison). These studies demonstrated no embryotoxic or teratogenic effects due to naloxone hydrochloride.
Pregnant female rats were administered 2 or 10 mg/kg naloxone subcutaneously from Gestation Day 15 to Postnatal day 21. There were no adverse effects on the offspring (up to 12-times a human dose of 8 mg/day (two Naloxone HCl Nasal Sprays) based on body surface area comparison).
8.2 Lactation
Risk Summary
There is no information regarding the presence of naloxone in human milk, or the effects of naloxone on the breastfed infant or on milk production. Studies in nursing mothers have shown that naloxone does not affect prolactin or oxytocin hormone levels. Naloxone is minimally orally bioavailable.
8.4 Pediatric Use
The safety and effectiveness of Naloxone HCl Nasal Spray have been established in pediatric patients of all ages for known or suspected opioid overdose as manifested by respiratory and/or central nervous system depression. Use of naloxone hydrochloride in all pediatric patients is supported by adult bioequivalence studies coupled with evidence from the safe and effective use of other naloxone hydrochloride drug products. No pediatric studies were conducted for Naloxone HCl Nasal Spray.
Absorption of naloxone hydrochloride following intranasal administration in pediatric patients may be erratic or delayed. Even when the opiate-intoxicated pediatric patient responds appropriately to naloxone hydrochloride, he/she must be carefully monitored for at least 24 hours, as a relapse may occur as naloxone hydrochloride is metabolized.
In opioid-dependent pediatric patients, (including neonates), administration of naloxone hydrochloride may result in an abrupt and complete reversal of opioid effects, precipitating an acute opioid withdrawal syndrome. Neonatal opioid withdrawal syndrome, unlike opioid withdrawal syndrome in adults, may be life-threatening, if not recognized, and should be treated according to protocols developed by neonatology experts [see Warnings and Precautions (5.3)].
In settings such as in neonates with known or suspected exposure to maternal opioid use, where it may be preferable to avoid the abrupt precipitation of opioid withdrawal symptoms, consider use of an alternate naloxone-containing product that can be dosed according to weight and titrated to effect.
Also, in situations where the primary concern is for infants at risk for opioid overdose, consider whether the availability of alternate naloxone-containing products may be better suited than Naloxone HCl Nasal Spray.
8.5 Geriatric Use
Geriatric patients have a greater frequency of decreased hepatic, renal, or cardiac function and of concomitant disease or other drug therapy. Therefore, the systemic exposure of naloxone hydrochloride can be higher in these patients.
Clinical studies of naloxone hydrochloride did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients.
11 DESCRIPTION
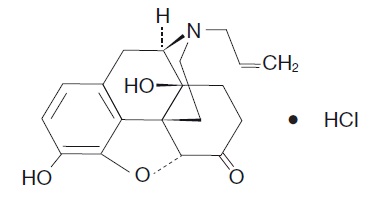
Naloxone HCl Nasal Spray is a pre-filled, single dose intranasal spray. Chemically, naloxone hydrochloride is the hydrochloride salt of 17-Allyl-4,5α-epoxy-3,14-dihydroxymorphinan-6-one hydrochloride with the following structure:
C19H21NO4●HCl
M.W. 363.84
Naloxone hydrochloride, an opioid antagonist, occurs as a white to slightly off-white powder, and is soluble in water, in dilute acids, and in strong alkali; slightly soluble in alcohol; practically insoluble in ether and in chloroform.
Each Naloxone HCl Nasal Spray contains a 4 mg single dose of naloxone hydrochloride (equivalent to 3.6 mg of naloxone) in a 0.1 mL (100 microliter) aqueous solution.
Inactive ingredients include benzalkonium chloride (preservative), disodium ethylenediaminetetraacetate (chelating agent), sodium chloride, hydrochloric acid to adjust pH, and purified water. The pH range is 3.5 to 5.5.
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Naloxone hydrochloride is an opioid antagonist that antagonizes opioid effects by competing for the same receptor sites.
Naloxone hydrochloride reverses the effects of opioids, including respiratory depression, sedation, and hypotension. It can also reverse the psychotomimetic and dysphoric effects of agonist-antagonists such as pentazocine.
12.2 Pharmacodynamics
When naloxone hydrochloride is administered intravenously, the onset of action is generally apparent within two minutes. The time to onset of action is shorter for intravenous compared to subcutaneous or intramuscular routes of administration. The duration of action is dependent upon the dose and route of administration of naloxone hydrochloride.
12.3 Pharmacokinetics
In a pharmacokinetic study in 30 healthy adult subjects, the relative bioavailability (BA) of one nasal spray in one nostril, consisting of a 2 mg total dose (0.1 mL of 20 mg/mL naloxone hydrochloride solution) and a 4 mg total dose (0.1 mL of 40 mg/mL naloxone hydrochloride solution), and two nasal sprays administered as one nasal spray in each nostril, consisting of a 4 mg total dose (0.1 mL of 20 mg/mL naloxone hydrochloride solution in each nostril) and an 8 mg total dose (0.1 mL of 40 mg/mL naloxone hydrochloride solution in each nostril), were compared to a single dose of 0.4 mg naloxone hydrochloride intramuscular injection. For intranasal administration, the subjects were instructed not to breathe through the nose during administration of the nasal spray, and remained fully supine for approximately one hour post-dose. For intramuscular administration, naloxone was administered as a single injection in the gluteus maximus muscle. The pharmacokinetic parameters obtained in the study are shown in Table 1.
Table 1 Mean Pharmacokinetic Parameters (CV%) for Naloxone Following Naloxone HCl Nasal Spray and Intramuscular Injection of Naloxone HCl to Healthy Subjects
|
Parameter |
2 mg – One Nasal Spray in one nostril 20 mg/mL (N=29) |
4 mg – Two Nasal Sprays, one in each nostril 20 mg/mL (N=29) |
4 mg – One Nasal Spray in one nostril 40 mg/mL (N=29) |
8 mg – Two Nasal Sprays, one in each nostril 40 mg/mL (N=29) |
0.4 mg Intramuscular Injection (N=29) |
|
tmax (h)† |
0.33 (0.25, 1.00) |
0.33 (0.17, 0.57) |
0.50 (0.17, 1.00) |
0.33 (0.17, 1.00) |
0.38 (0.08, 2.05) |
|
Cmax (ng/mL) |
2.91 (35) |
6.30 (34) |
4.83 (43) |
9.70 (36) |
0.88 (31) |
|
AUCt (hr ng/mL) |
4.60 (27) |
9.64 (24) |
7.87 (37) |
15.3 (23) |
1.75 (23) |
|
AUC0-inf (h*ng/mL) |
4.66 (27) |
9.74 (24) |
7.95 (37) |
15.5 (23) |
1.79 (23) |
|
t1/2 (h) |
1.85 (33) |
2.19 (33) |
2.08 (30) |
2.10 (32) |
1.24 (26) |
|
Dose normalized Relative BA (%) vs. IM |
51.7 (22) |
54.0 (23) |
44.2 (31)†† |
43.1 (24) |
100 |
† tmax reported as median (minimum, maximum)
††N=28 for Relative BA.
Figure 1 Mean + SD Plasma Concentration of Naloxone, (a) 0-6 h and (b) 0-1h
Following Intranasal Administration and Intramuscular Injection
(a)
(b)
The median naloxone tmax after intranasal administration of naloxone hydrochloride nasal spray, one nasal spray in one nostril (2 mg or 4 mg) or two nasal sprays as one spray in each nostril (4 mg or 8 mg), was not significantly different compared to the 0.4 mg dose of naloxone hydrochloride intramuscular injection (Table 1).
The dose normalized relative bioavailability of one dose (2 mg or 4 mg) or two doses (4 mg or 8 mg) of naloxone hydrochloride nasal spray as compared to the 0.4 mg dose of naloxone hydrochloride administered by intramuscular injection was 52%, 44%, 54%, and 43%, respectively.
Distribution
Following parenteral administration, naloxone is distributed in the body and readily crosses the placenta. Plasma protein binding occurs but is relatively weak. Plasma albumin is the major binding constituent, but significant binding of naloxone also occurs to plasma constituents other than albumin. It is not known whether naloxone is excreted into human milk.
Elimination
Following a single intranasal administration of naloxone hydrochloride nasal spray (2 mg or 4 mg dose of naloxone hydrochloride), the mean plasma half-life of naloxone in healthy adults was approximately 1.85 (33% CV) hours and 2.08 (30% CV) hours; respectively, which was longer than that observed after administrations of a 0.4 mg naloxone hydrochloride intramuscular injection, where the half-life was 1.24 hours (26% CV). In a neonatal study of naloxone hydrochloride injection, the mean (± SD) plasma half-life was observed to be 3.1 (± 0.5) hours.
Metabolism
Naloxone hydrochloride is metabolized in the liver, primarily by glucuronide conjugation, with naloxone-3-glucoronide as the major metabolite.
Excretion
After an oral or intravenous dose, about 25-40% of naloxone is excreted as metabolites in urine within 6 hours, about 50% in 24 hours, and 60-70% in 72 hours.
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis
Long-term animal studies to evaluate the carcinogenic potential of naloxone have not been completed.
Mutagenesis
Naloxone was weakly positive in the Ames mutagenicity and in the in vitro human lymphocyte chromosome aberration test but was negative in the in vitro Chinese hamster V79 cell HGPRT mutagenicity assay and in the in vivo rat bone marrow chromosome aberration study.
Impairment of Fertility
Male rats were treated with 2 or 10 mg/kg naloxone for 60 days prior to mating. Female rats treated for 14-days prior to mating and throughout gestation with the same doses of naloxone (up to 12-times a human dose of 8 mg/day (two naloxone hydrochloride nasal sprays) based on body surface area comparison). There was no adverse effect on fertility.
17 PATIENT COUNSELING INFORMATION
Advise the patient and family members or caregivers to read the FDA-approved patient labeling (Patient Information and Instructions for Use).
Recognition of Opioid Overdose
Inform patients and their family members or caregivers about how to recognize the signs and symptoms of an opioid overdose such as the following:
- •
- Extreme somnolence - inability to awaken a patient verbally or upon a firm sternal rub.
- •
- Respiratory depression - this can range from slow or shallow respiration to no respiration in a patient who is unarousable.
- •
- Other signs and symptoms that may accompany somnolence and respiratory depression include the following:
- ▪
- Miosis.
- ▪
- Bradycardia and/or hypotension.
Risk of Recurrent Respiratory and Central Nervous System Depression
Instruct patients and their family members or caregivers that, since the duration of action of most opioids may exceed that of Naloxone HCl Nasal Spray, they must seek immediate emergency medical assistance after the first dose of Naloxone HCl Nasal Spray and keep the patient under continued surveillance [see Dosage and Administration (2.2), Warnings and Precautions (5.3)].
Limited Efficacy for/with Partial Agonists or Mixed Agonist/Antagonists
Instruct patients and their family members or caregivers that the reversal of respiratory depression caused by partial agonists or mixed agonist/antagonists, such as buprenorphine and pentazocine, may be incomplete and may require higher doses of naloxone hydrochloride or repeated administration of Naloxone HCl Nasal Spray, using a new nasal spray each time [see Dosage and Administration (2.3), Warnings and Precautions (5.2)].
Precipitation of Severe Opioid Withdrawal
Instruct patients and their family members or caregivers that the use of Naloxone HCl Nasal Spray in patients who are opioid dependent may precipitate opioid withdrawal [see Warnings and Precautions (5.3), Adverse Reactions (6)].
Administration Instructions
Instruct patients and their family members or caregivers to:
- •
- Ensure Naloxone HCl Nasal Spray is present whenever persons may be intentionally or accidentally exposed to an opioid overdose (i.e., opioid emergencies).
- •
- Administer Naloxone HCl Nasal Spray as quickly as possible if a patient is unresponsive and an opioid overdose is suspected, even when in doubt, because prolonged respiratory depression may result in damage to the central nervous system or death. Naloxone HCl Nasal Spray is not a substitute for emergency medical care[see Dosage and Administration (2.1)].
- •
- Lay the patient on their back and administer Naloxone HCl Nasal Spray into one nostril while providing support to the back of the neck to allow the head to tilt back [see Dosage and Administration (2.1)].
- •
- Use each nasal spray only one time [see Dosage and Administration (2.1)].
- •
- Turn patient on their side as shown in the Instructions for Use and call for emergency medical assistance immediately after administration of the first dose of Naloxone HCl Nasal Spray. Additional supportive and/or resuscitative measures may be helpful while awaiting emergency medical assistance [see Dosage and Administration (2.1)].
- •
- Monitor patients and re-administer Naloxone HCl Nasal Spray using a new Naloxone HCl Nasal Spray every 2 to 3 minutes, if the patient is not responding or responds and then relapses back into respiratory depression. Administer Naloxone HCl Nasal Spray in alternate nostrils with each dose [see Dosage and Administration (2.1)].
- •
- Replace Naloxone HCl Nasal Spray before its expiration date.
Made in Israel
Manufactured by Perrigo
Yeruham, Israel
Distributed by Perrigo
Allegan, MI 49010
2Y200 RC P J2
Rev. 11-20
PATIENT INFORMATION
|
PATIENT INFORMATION Naloxone (nal-OKS-one) Hydrochloride Nasal Spray |
|
You and your family members or caregivers should read this Patient Information leaflet before an opioid emergency happens. This information does not take the place of talking with your healthcare provider about your medical condition or your treatment. |
|
What is the most important information I should know about Naloxone HCl Nasal Spray? Naloxone HCl Nasal Spray is used to temporarily reverse the effects of opioid medicines. The medicine in Naloxone HCl Nasal Spray has no effect in people who are not taking opioid medicines. Always carry Naloxone HCl Nasal Spray with you in case of an opioid emergency. 1. Use Naloxone HCl Nasal Spray right away if you or your caregiver think signs or symptoms of an opioid emergency are present, even if you are not sure, because an opioid emergency can cause severe injury or death. Signs and symptoms of an opioid emergency may include:
2. Family members, caregivers, or other people who may have to use Naloxone HCl Nasal Spray in an opioid emergency should know where Naloxone HCl Nasal Spray is stored and how to give Naloxone HCl Nasal Spray before an opioid emergency happens. 3. Get emergency medical help right away after giving the first dose of Naloxone HCl Nasal Spray. Rescue breathing or CPR (cardiopulmonary resuscitation) may be given while waiting for emergency medical help. 4. The signs and symptoms of an opioid emergency can return after Naloxone HCl Nasal Spray is given. If this happens, give another dose after 2 to 3 minutes using a new Naloxone HCl Nasal Spray and watch the person closely until emergency help is received. |
|
What is Naloxone HCl Nasal Spray?
|
|
Who should not use Naloxone HCl Nasal Spray? Do not use Naloxone HCl Nasal Spray if you are allergic to naloxone hydrochloride or any of the ingredients in Naloxone HCl Nasal Spray. See the end of this leaflet for a complete list of ingredients in Naloxone HCl Nasal Spray. |
|
What should I tell my healthcare provider before using Naloxone HCl Nasal Spray? Before using Naloxone HCl Nasal Spray, tell your healthcare provider about all of your medical conditions, including if you:
Tell your healthcare provider about the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. |
|
How should I use Naloxone HCl Nasal Spray? Read the “Instructions for Use” at the end of this Patient Information leaflet for detailed information about the right way to use Naloxone HCl Nasal Spray.
|
|
What are the possible side effects of Naloxone HCl Nasal Spray? Naloxone HCl Nasal Spray may cause serious side effects, including:
In infants under 4 weeks old who have been receiving opioids regularly, sudden opioid withdrawal may be life-threatening if not treated the right way. Signs and symptoms include: seizures, crying more than usual, and increased reflexes. These are not all of the possible side effects of Naloxone HCl Nasal Spray. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088. |
|
How should I store Naloxone HCl Nasal Spray?
Keep Naloxone HCl Nasal Spray and all medicines out of the reach of children. |
|
General information about the safe and effective use of Naloxone HCl Nasal Spray. Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use Naloxone HCl Nasal Spray for a condition for which it was not prescribed. You can ask your pharmacist or healthcare provider for information about Naloxone HCl Nasal Spray that is written for health professionals. |
|
What are the ingredients in Naloxone HCl Nasal Spray? Active ingredient: naloxone hydrochloride Inactive ingredients: benzalkonium chloride (preservative), disodium ethylenediaminetetraacetate (chelating agent), sodium chloride, hydrochloric acid to adjust pH and sterile water. Naloxone HCl Nasal Spray is not made with natural rubber latex. Made in Israel Manufactured by Perrigo Yeruham, Israel Distributed by Perrigo Allegan, MI 49010 For more information, go to www.perrigorx.com or call 1-866-634-9120. |
This Patient Information has been approved by the U.S. Food and Drug Administration.
Instructions for Use
Instructions for Use
Naloxone (nal-OKS-one) Hydrochloride Nasal Spray
You and your family members or caregivers should read the Instructions for Use that comes with Naloxone HCl Nasal Spray before using it. Talk to your healthcare provider if you and your family members or caregivers have any questions about the use of Naloxone HCl Nasal Spray.
Use Naloxone HCl Nasal Spray for known or suspected opioid overdose in adults and children.
Important: For use in the nose only.
- •
- Do not remove or test the Naloxone HCl Nasal Spray until ready to use.
- •
- Each Naloxone HCl Nasal Spray has 1 dose and cannot be reused.
- •
- You do not need to prime Naloxone HCl Nasal Spray.
How to use Naloxone HCl Nasal Spray:
Step 1. Lay the person on their back to receive a dose of Naloxone HCl Nasal Spray.
Step 2. Remove Naloxone HCl Nasal Spray from the box. Peel back the tab with the circle to open the Naloxone HCl Nasal Spray.
Note: Naloxone HCl Nasal Spray freezes at temperatures below 5°F (-15°C). If this happens, the device will not spray. Get emergency medical help right away if this happens. Do not wait for Naloxone HCl Nasal Spray to thaw. Naloxone HCl Nasal Spray may still be used if it has been thawed after being previously frozen.
Step 3. Hold the Naloxone HCl Nasal Spray with your thumb on the bottom of the plunger and your first and middle fingers on either side of the nozzle.
Step 4. Tilt the person’s head back and provide support under the neck with your hand. Gently insert the tip of the nozzle into one nostril until your fingers on either side of the nozzle are against the bottom of the person’s nose.
Step 5. Press the plunger firmly to give the dose of Naloxone HCl Nasal Spray.
Step 6. Remove the Naloxone HCl Nasal Spray from the nostril after giving the dose.
What to do after Naloxone HCl Nasal Spray has been used:
Step 7.
Get emergency medical help right away.
- •
- Move the person on their side (recovery position) after giving Naloxone HCl Nasal Spray.
- •
- Watch the person closely.
- •
- If the person does not respond by waking up, to voice or touch, or breathing normally another dose may be given. Naloxone HCl Nasal Spray may be dosed every 2 to 3 minutes, if available.
- •
- Repeat Steps 2 through 6 using a new Naloxone HCl Nasal Spray to give another dose in the other nostril. If additional Naloxone HCl Nasal Sprays are available, Steps 2 through 6 may be repeated every 2 to 3 minutes until the person responds or emergency medical help is received.
Step 8. Put the used Naloxone HCl Nasal Spray back into its box.
Step 9. Throw away (dispose of) the used Naloxone HCl Nasal Spray in a place that is away from children.
How should I store Naloxone HCl Nasal Spray?
- •
- Store below 77°F (25°C).
- •
- Excursions permitted up to 104°F (40°C).
- •
- Do not freeze or expose to excessive heat above 104°F (40°C).
- •
- Keep Naloxone HCl Nasal Spray in the box until ready to use. Protect from light.
- •
- Replace Naloxone HCl Nasal Spray before the expiration date on the box.
Keep Naloxone HCl Nasal Spray and all medicines out of the reach of children.
This Instructions for Use has been approved by the U.S. Food and Drug Administration.
For more information, go to www.perrigorx.com or call 1-866-634-9120.
Made in Israel
Manufactured by Perrigo
Yeruham, Israel
Distributed by Perrigo
Allegan, MI 49010
2Y200 RC P J2 Rev. 11-20
QUICK START GUIDE
Naloxone HCl Nasal Spray 4 mg
QUICK START GUIDE
Opioid Overdose Response Instructions
Use Naloxone Hydrochloride Nasal Spray for known or suspected opioid overdose in adults and children.
Important: For use in the nose only.
Do not remove or test the Naloxone HCl Nasal Spray until ready to use.
1 Identify Opioid Overdose and Check for Response
Ask person if he or she is okay and shout name.
Shake shoulders and firmly rub the middle of their chest.
Check for signs of opioid overdose:
- •
- Will not wake up or respond to your voice or touch
- •
- Breathing is very slow, irregular, or has stopped
- •
- Center part of their eye is very small, sometimes called “pinpoint pupils”
Lay the person on their back to receive a dose of Naloxone HCl Nasal Spray
2 Give Naloxone HCl Nasal Spray
Remove Naloxone HCl Nasal Spray from the box.
Peel back the tab with the circle to open the Naloxone HCl Nasal Spray.
Hold the Naloxone HCl Nasal Spray with your thumb on the bottom of the plunger and your first and middle fingers on either side of the nozzle.
Gently insert the tip of the nozzle into either nostril.
- •
- Tilt the person’s head back and provide support under the neck with your hand. Gently insert the tip of the nozzle into one nostril, until your fingers on either side of the nozzle are against the bottom of the person’s nose.
Press the plunger firmly to give the dose of Naloxone HCl Nasal Spray.
- •
- Remove the Naloxone HCl Nasal Spray from the nostril after giving the dose.
3 Call for emergency medical help, Evaluate, and Support
Get emergency medical help right away.
Move the person on their side (recovery position) after giving Naloxone HCl Nasal Spray.
Watch the person closely.
If the person does not respond by waking up, to voice or touch, or breathing normally another dose may be given. Naloxone HCl Nasal Spray may be dosed every 2 to 3 minutes, if available.
Repeat Step 2 using a new Naloxone HCl Nasal Spray to give another dose in the other nostril.
If additional Naloxone HCl Nasal Sprays are available, repeat step 2 every 2 to 3 minutes until the person
responds or emergency medical help is received.
For more information, go to www.perrigorx.com or call 1-866-634-9120.
2Y200 RC P C2
Rev 02-21
INGREDIENTS AND APPEARANCE
| NALOXONE HYDROCHLORIDE
naloxone hydrochloride spray |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Bryant Ranch Prepack (171714327) |
| Registrant - Bryant Ranch Prepack (171714327) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Bryant Ranch Prepack | 171714327 | REPACK(63629-9321) , RELABEL(63629-9321) | |