Search by Drug Name or NDC
NDC 65841-0028-10 METFORMIN HYDROCHLORIDE 500 mg/1 Details
METFORMIN HYDROCHLORIDE 500 mg/1
METFORMIN HYDROCHLORIDE is a ORAL TABLET, FILM COATED in the HUMAN PRESCRIPTION DRUG category. It is labeled and distributed by Zydus Lifesciences Limited. The primary component is METFORMIN HYDROCHLORIDE.
MedlinePlus Drug Summary
Metformin is used alone or with other medications, including insulin, to treat type 2 diabetes (condition in which the body does not use insulin normally and, therefore, cannot control the amount of sugar in the blood). Metformin is in a class of drugs called biguanides. Metformin helps to control the amount of glucose (sugar) in your blood. It decreases the amount of glucose you absorb from your food and the amount of glucose made by your liver. Metformin also increases your body's response to insulin, a natural substance that controls the amount of glucose in the blood. Metformin is not used to treat type 1 diabetes (condition in which the body does not produce insulin and therefore cannot control the amount of sugar in the blood). Over time, people who have diabetes and high blood sugar can develop serious or life-threatening complications, including heart disease, stroke, kidney problems, nerve damage, and eye problems. Taking medication(s), making lifestyle changes (e.g., diet, exercise, quitting smoking), and regularly checking your blood sugar may help to manage your diabetes and improve your health. This therapy may also decrease your chances of having a heart attack, stroke, or other diabetes-related complications such as kidney failure, nerve damage (numb, cold legs or feet; decreased sexual ability in men and women), eye problems, including changes or loss of vision, or gum disease. Your doctor and other healthcare providers will talk to you about the best way to manage your diabetes.
Related Packages: 65841-0028-10Last Updated: 12/01/2022
MedLinePlus Full Drug Details: Metformin
Product Information
| NDC | 65841-0028 |
|---|---|
| Product ID | 65841-028_e88724a2-0bd6-435c-bc66-f7d05d85d667 |
| Associated GPIs | |
| GCN Sequence Number | n/a |
| GCN Sequence Number Description | n/a |
| HIC3 | n/a |
| HIC3 Description | n/a |
| GCN | n/a |
| HICL Sequence Number | n/a |
| HICL Sequence Number Description | n/a |
| Brand/Generic | n/a |
| Proprietary Name | METFORMIN HYDROCHLORIDE |
| Proprietary Name Suffix | n/a |
| Non-Proprietary Name | METFORMIN HYDROCHLORIDE |
| Product Type | HUMAN PRESCRIPTION DRUG |
| Dosage Form | TABLET, FILM COATED |
| Route | ORAL |
| Active Ingredient Strength | 500 |
| Active Ingredient Units | mg/1 |
| Substance Name | METFORMIN HYDROCHLORIDE |
| Labeler Name | Zydus Lifesciences Limited |
| Pharmaceutical Class | Biguanide [EPC], Biguanides [CS] |
| DEA Schedule | n/a |
| Marketing Category | ANDA |
| Application Number | ANDA077064 |
| Listing Certified Through | 2024-12-31 |
Package
Package Images





NDC 65841-0028-10 (65841002810)
| NDC Package Code | 65841-028-10 |
|---|---|
| Billing NDC | 65841002810 |
| Package | 1000 TABLET, FILM COATED in 1 BOTTLE (65841-028-10) |
| Marketing Start Date | 2005-09-28 |
| NDC Exclude Flag | N |
| Pricing Information | N/A |
Standard Product Labeling (SPL)/Prescribing Information SPL 5043d03e-3c9d-4c96-b48d-187f02a1d27c Details
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
NDC 65841-028-01 in bottle of 100 tablets
Metformin Hydrochloride Tablets USP, 500 mg
Rx only
100 tablets

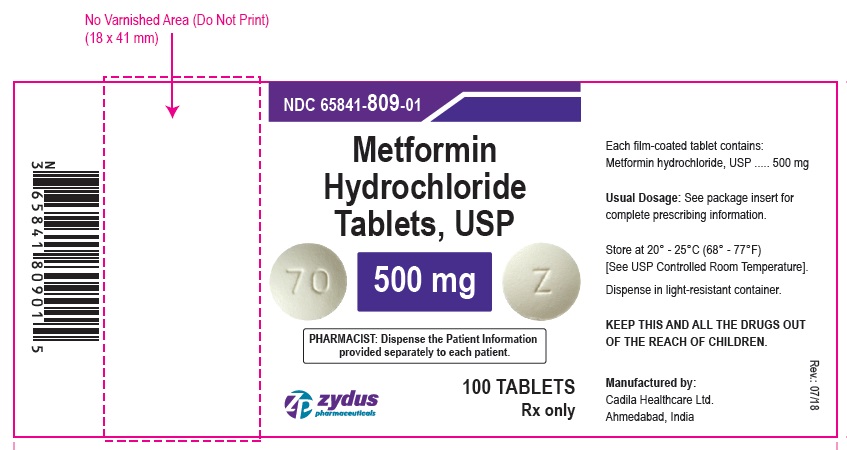
NDC 65841-809-01 in bottle of 100 tablets
Metformin Hydrochloride Tablets USP, 500 mg
Rx only
100 tablets

NDC 65841-029-01 in bottle of 100 tablets
Metformin Hydrochloride Tablets USP, 850 mg
Rx only
100 tablets

NDC 65841-810-01 in bottle of 100 tablets
Metformin Hydrochloride Tablets USP, 850 mg
Rx only
100 tablets

NDC 65841-030-01 in bottle of 100 tablets
Metformin Hydrochloride Tablets USP, 1000 mg
Rx only
100 tablets

NDC 65841-811-01 in bottle of 100 tablets
Metformin Hydrochloride Tablets USP, 1000 mg
Rx only
100 tablets

INGREDIENTS AND APPEARANCE
| METFORMIN HYDROCHLORIDE
metformin hydrochloride tablet, film coated |
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
| METFORMIN HYDROCHLORIDE
metformin hydrochloride tablet, film coated |
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
| METFORMIN HYDROCHLORIDE
metformin hydrochloride tablet, film coated |
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
| METFORMIN HYDROCHLORIDE
metformin hydrochloride tablet, film coated |
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
| METFORMIN HYDROCHLORIDE
metformin hydrochloride tablet, film coated |
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
| METFORMIN HYDROCHLORIDE
metformin hydrochloride tablet, film coated |
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
| Labeler - Zydus Lifesciences Limited (918596198) |
| Registrant - Zydus Lifesciences Limited (918596198) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Zydus Lifesciences Limited | 918596198 | ANALYSIS(65841-028, 65841-029, 65841-030, 65841-809, 65841-810, 65841-811) , MANUFACTURE(65841-028, 65841-029, 65841-030, 65841-809, 65841-810, 65841-811) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Zydus Lifesciences Limited | 863362789 | ANALYSIS(65841-028, 65841-029, 65841-030, 65841-809, 65841-810, 65841-811) , MANUFACTURE(65841-028, 65841-029, 65841-030, 65841-809, 65841-810, 65841-811) | |