Search by Drug Name or NDC
NDC 65862-0160-99 Zolpidem Tartrate 10 mg/1 Details
Zolpidem Tartrate 10 mg/1
Zolpidem Tartrate is a ORAL TABLET, FILM COATED in the HUMAN PRESCRIPTION DRUG category. It is labeled and distributed by Aurobindo Pharma Limited. The primary component is ZOLPIDEM TARTRATE.
MedlinePlus Drug Summary
Zolpidem is used to treat insomnia (difficulty falling asleep or staying asleep). Zolpidem belongs to a class of medications called sedative-hypnotics. It works by slowing activity in the brain to allow sleep.
Related Packages: 65862-0160-99Last Updated: 12/01/2022
MedLinePlus Full Drug Details: Zolpidem
Product Information
| NDC | 65862-0160 |
|---|---|
| Product ID | 65862-160_74c16acb-ed05-4b1b-934b-faf9d5bc8c70 |
| Associated GPIs | 60204080100315 |
| GCN Sequence Number | 019188 |
| GCN Sequence Number Description | zolpidem tartrate TABLET 10 MG ORAL |
| HIC3 | H2E |
| HIC3 Description | SEDATIVE-HYPNOTICS,NON-BARBITURATE |
| GCN | 00871 |
| HICL Sequence Number | 007842 |
| HICL Sequence Number Description | ZOLPIDEM TARTRATE |
| Brand/Generic | Generic |
| Proprietary Name | Zolpidem Tartrate |
| Proprietary Name Suffix | n/a |
| Non-Proprietary Name | Zolpidem Tartrate |
| Product Type | HUMAN PRESCRIPTION DRUG |
| Dosage Form | TABLET, FILM COATED |
| Route | ORAL |
| Active Ingredient Strength | 10 |
| Active Ingredient Units | mg/1 |
| Substance Name | ZOLPIDEM TARTRATE |
| Labeler Name | Aurobindo Pharma Limited |
| Pharmaceutical Class | Central Nervous System Depression [PE], GABA A Agonists [MoA], Pyridines [CS], gamma-Aminobutyric Acid-ergic Agonist [EPC] |
| DEA Schedule | CIV |
| Marketing Category | ANDA |
| Application Number | ANDA078413 |
| Listing Certified Through | 2024-12-31 |
Package
Package Images


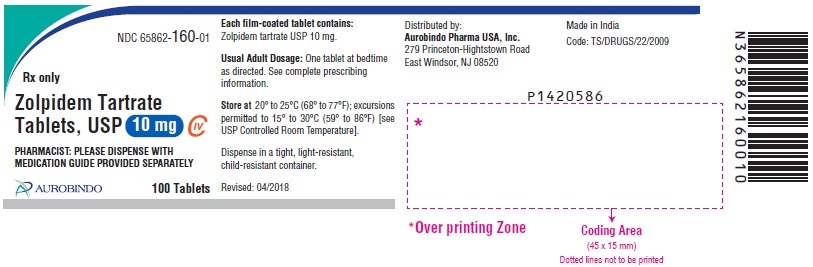

NDC 65862-0160-99 (65862016099)
| NDC Package Code | 65862-160-99 |
|---|---|
| Billing NDC | 65862016099 |
| Package | 1000 TABLET, FILM COATED in 1 BOTTLE (65862-160-99) |
| Marketing Start Date | 2007-05-04 |
| NDC Exclude Flag | N |
| Pricing Information | N/A |
Standard Product Labeling (SPL)/Prescribing Information SPL ea2067bd-1622-4d98-8012-2c09405aa88d Details
HIGHLIGHTS OF PRESCRIBING INFORMATION
ZOLPIDEM TARTRATE tablets, for oral use, CIV
Initial U.S. Approval: 1992
WARNING: COMPLEX SLEEP BEHAVIORS
See full prescribing information for complete boxed warning.
Complex sleep behaviors including sleep-walking, sleep-driving, and engaging in other activities while not fully awake may occur following use of zolpidem tartrate. Some of these events may result in serious injuries, including death. Discontinue zolpidem tartrate immediately if a patient experiences a complex sleep behavior. (4, 5.1)
RECENT MAJOR CHANGES
INDICATIONS AND USAGE
Zolpidem tartrate tablets, a gamma-aminobutyric acid (GABA) A receptor positive modulator, is indicated for the short-term treatment of insomnia characterized by difficulties with sleep initiation. (1)
DOSAGE AND ADMINISTRATION
- Use the lowest dose effective for the patient and must not exceed a total of 10 mg daily (2.1)
- Treatment should be as short as possible (2.1)
- Recommended initial dose is a single dose of 5 mg for women and a single dose of 5 or 10 mg for men, immediately before bedtime with at least 7 to 8 hours remaining before the planned time of awakening (2.1)
- Geriatric patients and patients with mild to moderate hepatic impairment: Recommended dose is 5 mg for men and women (2.2)
- Lower doses of CNS depressants may be necessary when taken concomitantly with zolpidem tartrate tablets (2.3)
- The effect of zolpidem tartrate tablets may be slowed if taken with or immediately after a meal (2.4)
DOSAGE FORMS AND STRENGTHS
5 mg and 10 mg tablets. Tablets not scored. (3)
CONTRAINDICATIONS
WARNINGS AND PRECAUTIONS
- CNS-Depressant Effects: Impaired alertness and motor coordination including risk of morning impairment. Risk increases with dose and use with other CNS depressants and alcohol. Caution patients against driving and other activities requiring mental alertness the morning after use. Instruct patients on correct use. (5.2)
- Need to Evaluate for Comorbid Diagnoses: Reevaluate if insomnia persists after 7 to 10 days of use. (5.3)
- Severe Anaphylactic/Anaphylactoid Reactions: Angioedema and anaphylaxis have been reported. Do not rechallenge if such reactions occur. (5.4)
- Abnormal Thinking and Behavioral Changes: Changes including decreased inhibition, bizarre behavior, agitation, and depersonalization have been reported. Immediately evaluate any new onset behavioral changes. (5.5)
- Depression: Worsening of depression or suicidal thinking may occur. Prescribe the least amount of tablets feasible to avoid intentional overdose. (5.6)
- Respiratory Depression: Consider this risk before prescribing in patients with compromised respiratory function. (5.7)
- Hepatic Impairment: Avoid zolpidem tartrate use in patients with severe hepatic impairment. (5.8)
- Withdrawal Effects: Symptoms may occur with rapid dose reduction or discontinuation. (5.9, 9.3)
ADVERSE REACTIONS
Most commonly observed adverse reactions were:
Short-term (<10 nights): Drowsiness, dizziness, and diarrhea
Long-term (28 to 35 nights): Dizziness and drugged feelings (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Aurobindo Pharma USA, Inc. at 1-866-850-2876 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS
- CNS depressants, including alcohol: Possible adverse additive CNS-depressant effects (5.2, 7.1)
- Opioids: Concomitant use may increase risk of respiratory depression (5.7, 7.1)
- Imipramine: Decreased alertness observed (7.1)
- Chlorpromazine: Impaired alertness and psychomotor performance observed (7.1)
- CYP3A4 inducers (rifampin or St. John’s wort): Combination use may decrease effect (7.2)
- Ketoconazole: Combination use may increase effect (7.2)
USE IN SPECIFIC POPULATIONS
- Pregnancy: May cause respiratory depression and sedation in neonates with exposure late in the third trimester. (8.1)
- Lactation: A lactating woman may pump and discard breast milk during treatment and for 23 hours after zolpidem tartrate administration. (8.2)
- Pediatric use: Safety and effectiveness not established. Hallucinations (incidence rate 7%) and other psychiatric and/or nervous system adverse reactions were observed frequently in a study of pediatric patients with Attention-Deficit/Hyperactivity Disorder. (5.5, 8.4)
See 17 for PATIENT COUNSELING INFORMATION and Medication Guide.
Revised: 4/2022
Table Of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
WARNING: COMPLEX SLEEP BEHAVIORS
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Dosage in Adults
2.2 Special Populations
2.3 Use with CNS Depressants
2.4 Administration
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Complex Sleep Behaviors
5.2 CNS-Depressant Effects and Next-Day Impairment
5.3 Need to Evaluate for Comorbid Diagnoses
5.4 Severe Anaphylactic and Anaphylactoid Reactions
5.5 Abnormal Thinking and Behavioral Changes
5.6 Use in Patients with Depression
5.7 Respiratory Depression
5.8 Precipitation of Hepatic Encephalopathy
5.9 Withdrawal Effects
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Postmarketing Experience
7 DRUG INTERACTIONS
7.1 CNS-Active Drugs
7.2 Drugs that Affect Drug Metabolism via Cytochrome P450
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Gender Difference in Pharmacokinetics
8.7 Hepatic Impairment
9 DRUG ABUSE AND DEPENDENCE
9.1 Controlled Substance
9.2 Abuse
9.3 Dependence
10 OVERDOSAGE
10.1 Signs and Symptoms
10.2 Recommended Treatment
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
14.1 Transient Insomnia
14.2 Chronic Insomnia
14.3 Studies Pertinent to Safety Concerns for Sedative/Hypnotic Drugs
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- *
- Sections or subsections omitted from the full prescribing information are not listed.
WARNING
Complex sleep behaviors including sleep-walking, sleep-driving, and engaging in other activities while not fully awake may occur following use of zolpidem tartrate. Some of these events may result in serious injuries, including death. Discontinue zolpidem tartrate immediately if a patient experiences a complex sleep behavior [see Contraindications (4) and Warnings and Precautions (5.1)].
1 INDICATIONS AND USAGE
Zolpidem tartrate tablets are indicated for the short-term treatment of insomnia characterized by difficulties with sleep initiation. Zolpidem tartrate tablets have been shown to decrease sleep latency for up to 35 days in controlled clinical studies [see Clinical Studies (14)].
The clinical trials performed in support of efficacy were 4 to 5 weeks in duration with the final formal assessments of sleep latency performed at the end of treatment.
2 DOSAGE AND ADMINISTRATION
2.1 Dosage in Adults
Use the lowest effective dose for the patient. The recommended initial dose is 5 mg for women and either 5 or 10 mg for men, taken only once per night immediately before bedtime with at least 7 to 8 hours remaining before the planned time of awakening. If the 5 mg dose is not effective, the dose can be increased to 10 mg. In some patients, the higher morning blood levels following use of the 10 mg dose increase the risk of next-day impairment of driving and other activities that require full alertness [see Warnings and Precautions (5.2)]. The total dose of zolpidem tartrate tablets should not exceed 10 mg once daily immediately before bedtime. Zolpidem tartrate tablets should be taken as a single dose and should not be readministered during the same night.
The recommended initial doses for women and men are different because zolpidem clearance is lower in women.
Long-term use of zolpidem tartrate tablets is not recommended. Treatment should be as short as possible. Extended treatment should not take place without re-evaluation of the patient's status because the risk of abuse and dependence increases with the duration of treatment [see Drug Abuse and Dependence (9.3)].
2.2 Special Populations
Elderly or debilitated patients may be especially sensitive to the effects of zolpidem tartrate. The recommended dose of zolpidem tartrate in these patients is 5 mg once daily immediately before bedtime [see Warnings and Precautions (5.2), Use in Specific Populations (8.5)].
Patients with mild to moderate hepatic impairment do not clear the drug as rapidly as normal subjects. The recommended dose of zolpidem tartrate in these patients is 5 mg once daily immediately before bedtime. Avoid zolpidem tartrate use in patients with severe hepatic impairment as it may contribute to encephalopathy [see Warnings and Precautions (5.8), Use in Specific Populations (8.7), Clinical Pharmacology (12.3)].
2.3 Use with CNS Depressants
Dosage adjustment may be necessary when zolpidem tartrate tablets are combined with other CNS-depressant drugs because of the potentially additive effects [see Warnings and Precautions (5.2, 5.7)].
3 DOSAGE FORMS AND STRENGTHS
Zolpidem tartrate is available in 5 mg and 10 mg strength tablets for oral administration. Tablets are not scored.
Zolpidem tartrate tablets USP 5 mg are white to off-white, circular, biconvex, film-coated tablets, debossed with “E” on one side and “78” on the other side.
Zolpidem tartrate tablets USP 10 mg are white to off-white, oval shaped, biconvex, film-coated tablets, debossed with “E” on one side and “79” on the other side.
4 CONTRAINDICATIONS
Zolpidem tartrate tablets are contraindicated in patients
- who have experienced complex sleep behaviors after taking zolpidem tartrate tablets [see Warnings and Precautions (5.1)].
- with known hypersensitivity to zolpidem. Observed reactions include anaphylaxis and angioedema [see Warnings and Precautions (5.4)].
5 WARNINGS AND PRECAUTIONS
5.1 Complex Sleep Behaviors
Complex sleep behaviors, including sleep-walking, sleep-driving, and engaging in other activities while not fully awake, may occur following the first or any subsequent use of zolpidem tartrate. Patients can be seriously injured or injure others during complex sleep behaviors. Such injuries may result in a fatal outcome. Other complex sleep behaviors (e.g., preparing and eating food, making phone calls, or having sex) have also been reported. Patients usually do not remember these events. Postmarketing reports have shown that complex sleep behaviors may occur with zolpidem tartrate alone at recommended doses, with or without the concomitant use of alcohol or other central nervous system (CNS) depressants [see Drug Interactions (7.1)]. Discontinue zolpidem tartrate immediately if a patient experiences a complex sleep behavior [see Contraindications (4)].
5.2 CNS-Depressant Effects and Next-Day Impairment
Zolpidem tartrate, like other sedative-hypnotic drugs, has CNS-depressant effects. Coadministration with other CNS depressants (e.g., benzodiazepines, opioids, tricyclic antidepressants, alcohol) increases the risk of CNS depression [see Drug Interactions (7.1)]. Dosage adjustments of zolpidem tartrate and of other concomitant CNS depressants may be necessary when zolpidem tartrate is administered with such agents because of the potentially additive effects. The use of zolpidem tartrate with other sedative-hypnotics (including other zolpidem products) at bedtime or the middle of the night is not recommended [see Dosage and Administration (2.3)].
The risk of next-day psychomotor impairment, including impaired driving, is increased if zolpidem tartrate is taken with less than a full night of sleep remaining (7 to 8 hours); if a higher than the recommended dose is taken; if coadministered with other CNS depressants or alcohol; or if coadministered with other drugs that increase the blood levels of zolpidem. Patients should be warned against driving and other activities requiring complete mental alertness if zolpidem tartrate is taken in these circumstances [see Dosage and Administration (2), Clinical Studies (14.3)].
Vehicle drivers and machine operators should be warned that, as with other hypnotics, there may be a possible risk of adverse reactions including drowsiness, prolonged reaction time, dizziness, sleepiness, blurred/double vision, reduced alertness, and impaired driving the morning after therapy. In order to minimize this risk a full night of sleep (7 to 8 hours) is recommended.
Because zolpidem tartrate can cause drowsiness and a decreased level of consciousness, patients, particularly the elderly, are at higher risk of falls.
5.3 Need to Evaluate for Comorbid Diagnoses
Because sleep disturbances may be the presenting manifestation of a physical and/or psychiatric disorder, symptomatic treatment of insomnia should be initiated only after a careful evaluation of the patient. The failure of insomnia to remit after 7 to 10 days of treatment may indicate the presence of a primary psychiatric and/or medical illness that should be evaluated. Worsening of insomnia or the emergence of new thinking or behavior abnormalities may be the consequence of an unrecognized psychiatric or physical disorder. Such findings have emerged during the course of treatment with sedative/hypnotic drugs, including zolpidem.
5.4 Severe Anaphylactic and Anaphylactoid Reactions
Cases of angioedema involving the tongue, glottis or larynx have been reported in patients after taking the first or subsequent doses of sedative-hypnotics, including zolpidem. Some patients have had additional symptoms such as dyspnea, throat closing or nausea and vomiting that suggest anaphylaxis. Some patients have required medical therapy in the emergency department. If angioedema involves the throat, glottis or larynx, airway obstruction may occur and be fatal. Patients who develop angioedema after treatment with zolpidem should not be rechallenged with the drug.
5.5 Abnormal Thinking and Behavioral Changes
Abnormal thinking and behavior changes have been reported in patients treated with sedative/hypnotics, including zolpidem tartrate. Some of these changes included decreased inhibition (e.g., aggressiveness and extroversion that seemed out of character), bizarre behavior, agitation and depersonalization. Visual and auditory hallucinations have been reported.
In controlled trials of zolpidem tartrate 10 mg taken at bedtime <1% of adults with insomnia reported hallucinations. In a clinical trial, 7% of pediatric patients treated with zolpidem tartrate 0.25 mg/kg taken at bedtime reported hallucinations versus 0% treated with placebo [see Use in Specific Populations (8.4)]. There have been postmarketing reports of delirium with zolpidem use [Adverse Reactions (6.2)].
It can rarely be determined with certainty whether a particular instance of the abnormal behaviors listed above is drug induced, spontaneous in origin, or a result of an underlying psychiatric or physical disorder. Nonetheless, the emergence of any new behavioral sign or symptom of concern requires careful and immediate evaluation.
5.6 Use in Patients with Depression
In primarily depressed patients treated with sedative-hypnotics, worsening of depression, and suicidal thoughts and actions (including completed suicides), have been reported. Suicidal tendencies may be present in such patients and protective measures may be required. Intentional overdosage is more common in this group of patients; therefore, the lowest number of tablets that is feasible should be prescribed for the patient at any one time.
5.7 Respiratory Depression
Although studies with 10 mg zolpidem tartrate did not reveal respiratory depressant effects at hypnotic doses in healthy subjects or in patients with mild to moderate chronic obstructive pulmonary disease (COPD), a reduction in the Total Arousal Index, together with a reduction in lowest oxygen saturation and increase in the times of oxygen desaturation below 80% and 90%, was observed in patients with mild to moderate sleep apnea when treated with zolpidem compared to placebo. Since sedative-hypnotics have the capacity to depress respiratory drive, precautions should be taken if zolpidem tartrate is prescribed to patients with compromised respiratory function or concomitant use with opioids or other CNS depressants. Postmarketing reports of respiratory insufficiency in patients receiving 10 mg of zolpidem tartrate, most of whom had pre-existing respiratory impairment, have been reported. The risk of respiratory depression should be considered prior to prescribing zolpidem tartrate in patients with respiratory impairment including sleep apnea and myasthenia gravis or with concomitant opioid use [see Dosage and Administration (2.3), Drug Interactions (7.1)].
5.8 Precipitation of Hepatic Encephalopathy
Drugs affecting GABA receptors, such as zolpidem tartrate, have been associated with precipitation of hepatic encephalopathy in patients with hepatic insufficiency. In addition, patients with hepatic insufficiency do not clear zolpidem tartrate as rapidly as patients with normal hepatic function. Avoid zolpidem tartrate use in patients with severe hepatic impairment as it may contribute to encephalopathy [see Dosage and Administration (2.2), Use in Specific Populations (8.7), Clinical Pharmacology (12.3)].
6 ADVERSE REACTIONS
The following serious adverse reactions are discussed in greater detail in other sections of the labeling:
- Complex Sleep Behaviors [see Warnings and Precautions (5.1)]
- CNS-Depressant Effects and Next-Day Impairment [see Warnings and Precautions (5.2)]
- Severe Anaphylactic and Anaphylactoid Reactions [see Warnings and Precautions (5.4)]
- Abnormal Thinking and Behavior Changes [see Warnings and Precautions (5.5)]
- Withdrawal Effects [see Warnings and Precautions (5.9)]
6.1 Clinical Trials Experience
Associated with Discontinuation of Treatment
Approximately 4% of 1,701 patients who received zolpidem at all doses (1.25 to 90 mg) in U.S. premarketing clinical trials discontinued treatment because of an adverse reaction. Reactions most commonly associated with discontinuation from U.S. trials were daytime drowsiness (0.5%), dizziness (0.4%), headache (0.5%), nausea (0.6%), and vomiting (0.5%).
Approximately 4% of 1,959 patients who received zolpidem at all doses (1 to 50 mg) in similar foreign trials discontinued treatment because of an adverse reaction. Reactions most commonly associated with discontinuation from these trials were daytime drowsiness (1.1%), dizziness/vertigo (0.8%), amnesia (0.5%), nausea (0.5%), headache (0.4%), and falls (0.4%).
Data from a clinical study in which selective serotonin reuptake inhibitor (SSRI)-treated patients were given zolpidem revealed that four of the seven discontinuations during double-blind treatment with zolpidem (n=95) were associated with impaired concentration, continuing or aggravated depression, and manic reaction; one patient treated with placebo (n=97) was discontinued after an attempted suicide.
Most Commonly Observed Adverse Reactions in Controlled Trials
During short-term treatment (up to 10 nights) with zolpidem tartrate at doses up to 10 mg, the most commonly observed adverse reactions associated with the use of zolpidem and seen at statistically significant differences from placebo-treated patients were drowsiness (reported by 2% of zolpidem patients), dizziness (1%), and diarrhea (1%). During longer-term treatment (28 to 35 nights) with zolpidem at doses up to 10 mg, the most commonly observed adverse reactions associated with the use of zolpidem and seen at statistically significant differences from placebo-treated patients were dizziness (5%) and drugged feelings (3%).
Adverse Reactions Observed at an Incidence of ≥1% in Controlled Trials
The following tables enumerate treatment-emergent adverse reactions frequencies that were observed at an incidence equal to 1% or greater among patients with insomnia who received zolpidem tartrate and at a greater incidence than placebo in U.S. placebo-controlled trials. Events reported by investigators were classified utilizing a modified World Health Organization (WHO) dictionary of preferred terms for the purpose of establishing event frequencies. The prescriber should be aware that these figures cannot be used to predict the incidence of side effects in the course of usual medical practice, in which patient characteristics and other factors differ from those that prevailed in these clinical trials. Similarly, the cited frequencies cannot be compared with figures obtained from other clinical investigators involving related drug products and uses, since each group of drug trials is conducted under a different set of conditions. However, the cited figures provide the physician with a basis for estimating the relative contribution of drug and nondrug factors to the incidence of side effects in the population studied.
The following table was derived from results of 11 placebo-controlled short-term U.S. efficacy trials involving zolpidem in doses ranging from 1.25 to 20 mg. The table is limited to data from doses up to and including 10 mg, the highest dose recommended for use.
| * Reactions reported by at least 1% of patients treated with zolpidem tartrate and at a greater frequency than placebo. | ||
| Body System Adverse Reaction* | Zolpidem
(≤10 mg) (N=685) | Placebo
(N=473) |
| Central and Peripheral Nervous System
| | |
| Headache | 7 | 6 |
| Drowsiness | 2 | - |
| Dizziness | 1 | - |
| Gastrointestinal System
| | |
| Diarrhea | 1 | - |
The following table was derived from results of three placebo-controlled long-term efficacy trials involving zolpidem tartrate. These trials involved patients with chronic insomnia who were treated for 28 to 35 nights with zolpidem at doses of 5, 10, or 15 mg. The table is limited to data from doses up to and including 10 mg, the highest dose recommended for use. The table includes only adverse events occurring at an incidence of at least 1% for zolpidem patients.
| * Reactions reported by at least 1% of patients treated with zolpidem tartrate and at a greater frequency than placebo. | ||
| Body System
Adverse Event* | Zolpidem
(≤10 mg) (N=152) | Placebo
(N=161) |
| Autonomic Nervous System
| | |
| Dry mouth | 3 | 1 |
| Body as a Whole
| | |
| Allergy | 4 | 1 |
| Back Pain | 3 | 2 |
| Influenza-like symptoms | 2 | - |
| Chest pain | 1 | - |
| Cardiovascular System
| | |
| Palpitation | 2 | - |
| Central and Peripheral Nervous System
| | |
| Drowsiness | 8 | 5 |
| Dizziness | 5 | 1 |
| Lethargy | 3 | 1 |
| Drugged feeling | 3 | - |
| Lightheadedness | 2 | 1 |
| Depression | 2 | 1 |
| Abnormal dreams | 1 | - |
| Amnesia | 1 | - |
| Sleep disorder | 1 | - |
| Gastrointestinal System
| | |
| Diarrhea | 3 | 2 |
| Abdominal pain | 2 | 2 |
| Constipation | 2 | 1 |
| Respiratory System
| | |
| Sinusitis | 4 | 2 |
| Pharyngitis | 3 | 1 |
| Skin and Appendages
| ||
| Rash | 2 | 1 |
Dose Relationship for Adverse Reactions
There is evidence from dose comparison trials suggesting a dose relationship for many of the adverse reactions associated with zolpidem use, particularly for certain CNS and gastrointestinal adverse events.
Adverse Event Incidence Across the Entire Preapproval Database
Zolpidem tartrate was administered to 3,660 subjects in clinical trials throughout the U.S., Canada, and Europe. Treatment-emergent adverse events associated with clinical trial participation were recorded by clinical investigators using terminology of their own choosing. To provide a meaningful estimate of the proportion of individuals experiencing treatment-emergent adverse events, similar types of untoward events were grouped into a smaller number of standardized event categories and classified utilizing a modified World Health Organization (WHO) dictionary of preferred terms.
The frequencies presented, therefore, represent the proportions of the 3,660 individuals exposed to zolpidem, at all doses, who experienced an event of the type cited on at least one occasion while receiving zolpidem. All reported treatment-emergent adverse events are included, except those already listed in the table above of adverse events in placebo-controlled studies, those coding terms that are so general as to be uninformative, and those events where a drug cause was remote. It is important to emphasize that, although the events reported did occur during treatment with zolpidem tartrate, they were not necessarily caused by it.
Adverse events are further classified within body system categories and enumerated in order of decreasing frequency using the following definitions: frequent adverse events are defined as those occurring in greater than 1/100 subjects; infrequent adverse events are those occurring in 1/100 to 1/1,000 patients; rare events are those occurring in less than 1/1,000 patients.
Autonomic nervous system: Infrequent: increased sweating, pallor, postural hypotension, syncope. Rare: abnormal accommodation, altered saliva, flushing, glaucoma, hypotension, impotence, increased saliva, tenesmus.
Body as a whole: Frequent: asthenia. Infrequent: edema, falling, fatigue, fever, malaise, trauma. Rare: allergic reaction, allergy aggravated, anaphylactic shock, face edema, hot flashes, increased ESR, pain, restless legs, rigors, tolerance increased, weight decrease.
Cardiovascular system: Infrequent: cerebrovascular disorder, hypertension, tachycardia. Rare: angina pectoris, arrhythmia, arteritis, circulatory failure, extrasystoles, hypertension aggravated, myocardial infarction, phlebitis, pulmonary embolism, pulmonary edema, varicose veins, ventricular tachycardia.
Central and peripheral nervous system: Frequent: ataxia, confusion, euphoria, headache, insomnia, vertigo. Infrequent: agitation, anxiety, decreased cognition, detached, difficulty concentrating, dysarthria, emotional lability, hallucination, hypoesthesia, illusion, leg cramps, migraine, nervousness, paresthesia, sleeping (after daytime dosing), speech disorder, stupor, tremor. Rare: abnormal gait, abnormal thinking, aggressive reaction, apathy, appetite increased, decreased libido, delusion, dementia, depersonalization, dysphasia, feeling strange, hypokinesia, hypotonia, hysteria, intoxicated feeling, manic reaction, neuralgia, neuritis, neuropathy, neurosis, panic attacks, paresis, personality disorder, somnambulism, suicide attempts, tetany, yawning.
Gastrointestinal system: Frequent: dyspepsia, hiccup, nausea. Infrequent: anorexia, constipation, dysphagia, flatulence, gastroenteritis, vomiting. Rare: enteritis, eructation, esophagospasm, gastritis, hemorrhoids, intestinal obstruction, rectal hemorrhage, tooth caries.
Hematologic and lymphatic system: Rare: anemia, hyperhemoglobinemia, leukopenia, lymphadenopathy, macrocytic anemia, purpura, thrombosis.
Immunologic system: Infrequent: infection. Rare: abscess herpes simplex herpes zoster, otitis externa, otitis media.
Liver and biliary system: Infrequent: abnormal hepatic function, increased SGPT. Rare: bilirubinemia, increased SGOT.
Metabolic and nutritional: Infrequent: hyperglycemia, thirst. Rare: gout, hypercholesteremia, hyperlipidemia, increased alkaline phosphatase, increased BUN, periorbital edema.
Musculoskeletal system: Frequent: arthralgia, myalgia. Infrequent: arthritis. Rare: arthrosis, muscle weakness, sciatica, tendinitis.
Reproductive system: Infrequent: menstrual disorder, vaginitis. Rare: breast fibroadenosis, breast neoplasm, breast pain.
Respiratory system: Frequent: upper respiratory infection, lower respiratory infection. Infrequent: bronchitis, coughing, dyspnea, rhinitis. Rare: bronchospasm, respiratory depression, epistaxis, hypoxia, laryngitis, pneumonia.
Skin and appendages: Infrequent: pruritus. Rare: acne, bullous eruption, dermatitis, furunculosis, injection-site inflammation, photosensitivity reaction, urticaria.
Special senses: Frequent: diplopia, vision abnormal. Infrequent: eye irritation, eye pain, scleritis, taste perversion, tinnitus. Rare: conjunctivitis, corneal ulceration, lacrimation abnormal, parosmia, photopsia.
Urogenital system: Frequent: urinary tract infection. Infrequent: cystitis, urinary incontinence. Rare: acute renal failure, dysuria, micturition frequency, nocturia, polyuria, pyelonephritis, renal pain, urinary retention.
6.2 Postmarketing Experience
The following adverse reactions have been identified during postapproval use of zolpidem tartrate. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Liver and biliary system: acute hepatocellular, cholestatic or mixed liver injury with or without jaundice (i.e., bilirubin >2 x ULN, alkaline phosphatase ≥2 x ULN, transaminase ≥5 x ULN).
Psychiatric disorders: delirium
7 DRUG INTERACTIONS
7.1 CNS-Active Drugs
CNS Depressants
Coadministration of zolpidem with other CNS depressants increases the risk of CNS depression. Concomitant use of zolpidem with these drugs may increase drowsiness and psychomotor impairment, including impaired driving ability [see Warnings and Precautions (5.1, 5.2)]. Zolpidem tartrate was evaluated in healthy volunteers in single-dose interaction studies for several CNS drugs.
Alcohol
An additive adverse effect on psychomotor performance between alcohol and oral zolpidem was demonstrated [see Warnings and Precautions (5.1, 5.2)].
Opioids
The concomitant use of zolpidem tartrate with opioids may increase the risk of respiratory depression. Limit dosage and duration of concomitant use of zolpidem tartrate and opioids [see Dosage and Administration (2.3), Warnings and Precautions (5.7)].
Imipramine, Chlorpromazine
Imipramine in combination with zolpidem produced no pharmacokinetic interaction other than a 20% decrease in peak levels of imipramine, but there was an additive effect of decreased alertness. Similarly, chlorpromazine in combination with zolpidem produced no pharmacokinetic interaction, but there was an additive effect of decreased alertness and psychomotor performance [see Clinical Pharmacology (12.3)].
Sertraline
Concomitant administration of zolpidem and sertraline increases exposure to zolpidem [see Clinical Pharmacology (12.3)].
Fluoxetine
After multiple doses of zolpidem tartrate and fluoxetine an increase in the zolpidem half-life (17%) was observed. There was no evidence of an additive effect in psychomotor performance [see Clinical Pharmacology (12.3)].
Haloperidol
A study involving haloperidol and zolpidem revealed no effect of haloperidol on the pharmacokinetics or pharmacodynamics of zolpidem. The lack of a drug interaction following single-dose administration does not predict the absence of an effect following chronic administration [see Clinical Pharmacology (12.3)].
7.2 Drugs that Affect Drug Metabolism via Cytochrome P450
Some compounds known to induce or inhibit CYP3A may affect exposure to zolpidem. The effect of drugs that induce or inhibit other P450 enzymes on the exposure to zolpidem is not known.
CYP3A4 Inducers
Rifampin
Rifampin, a CYP3A4 inducer, significantly reduced the exposure to and the pharmacodynamic effects of zolpidem. Use of Rifampin in combination with zolpidem may decrease the efficacy of zolpidem and is not recommended [see Clinical Pharmacology (12.3)].
St. John’s wort
Use of St. John’s wort, a CYP3A4 inducer, in combination with zolpidem may decrease blood levels of zolpidem and is not recommended.
CYP3A4 Inhibitors
Ketoconazole
Ketoconazole, a potent CYP3A4 inhibitor, increased the exposure to and pharmacodynamic effects of zolpidem. Consideration should be given to using a lower dose of zolpidem when a potent CYP3A4 inhibitor and zolpidem are given together [see Clinical Pharmacology (12.3)].
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
Neonates born to mothers using zolpidem late in the third trimester of pregnancy have been reported to experience symptoms of respiratory depression and sedation [see Clinical Considerations and Data]. Published data on the use of zolpidem during pregnancy have not reported a clear association with zolpidem and major birth defects [see Data]. Oral administration of zolpidem to pregnant rats and rabbits did not indicate a risk for adverse effects on fetal development at clinically relevant doses [see Data].
The estimated background risk of major birth defects and miscarriage for the indicated populations are unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively.
Clinical Considerations
Fetal/neonatal adverse reactions
Zolpidem crosses the placenta and may produce respiratory depression and sedation in neonates. Monitor neonates exposed to zolpidem tartrate during pregnancy and labor for signs of excess sedation, hypotonia, and respiratory depression and manage accordingly.
Data
Human data
Published data from observational studies, birth registries, and case reports on the use of zolpidem during pregnancy do not report a clear association with zolpidem and major birth defects.
There are limited postmarketing reports of severe to moderate cases of respiratory depression that occurred after birth in neonates whose mothers had taken zolpidem during pregnancy. These cases required artificial ventilation or intratracheal intubation. The majority of neonates recovered within hours to a few weeks after birth once treated.
Zolpidem has been shown to cross the placenta.
Animal data
Oral administration of zolpidem to pregnant rats during the period of organogenesis at 4, 20, and 100 mg base/kg/day, which are approximately 5, 25, and 120 times the maximum recommended human dose (MRHD) of 10 mg/day (8 mg zolpidem base) based on mg/m2 body surface area, caused delayed fetal development (incomplete fetal skeletal ossification) at maternally toxic (ataxia) doses 25 and 120 times the MRHD based on mg/m2 body surface area.
Oral administration of zolpidem to pregnant rabbits during the period of organogenesis at 1, 4, and 16 mg base/kg/day, which are approximately 2.5, 10, and 40 times the MRHD of 10 mg/day (8 mg zolpidem base) based on mg/m2 body surface area caused embryo-fetal death and delayed fetal development (incomplete fetal skeletal ossification) at a maternally toxic (decreased body weight gain) dose 40 times the MRHD based on mg/m2 body surface area.
Oral administration of zolpidem to pregnant rats from day 15 of gestation through lactation at 4, 20, and 100 mg base/kg/day, which are approximately 5, 25, and 120 times the MRHD of 10 mg/day (8 mg zolpidem base) based on mg/m2 body surface area, delayed offspring growth and decreased survival at doses 25 and 120 times, respectively, the MRHD based on mg/m2 body surface area.
8.2 Lactation
Risk Summary
Limited data from published literature report the presence of zolpidem in human milk. There are reports of excess sedation in infants exposed to zolpidem through breastmilk [see Clinical Considerations]. There is no information on the effects of zolpidem on milk production. The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for zolpidem tartrate and any potential adverse effects on the breastfed infant from zolpidem tartrate or from the underlying maternal condition.
Clinical Considerations
Infants exposed to zolpidem tartrate through breastmilk should be monitored for excess sedation, hypotonia, and respiratory depression. A lactating woman may consider interrupting breastfeeding and pumping and discarding breast milk during treatment and for 23 hours (approximately 5 elimination half-lives) after zolpidem tartrate administration in order to minimize drug exposure to a breast fed infant.
8.4 Pediatric Use
Zolpidem tartrate is not recommended for use in children. Safety and effectiveness of zolpidem in pediatric patients below the age of 18 years have not been established.
In an 8-week study in pediatric patients (aged 6 to 17 years) with insomnia associated with attention-deficit/hyperactivity disorder (ADHD) an oral solution of zolpidem tartrate dosed at 0.25 mg/kg at bedtime did not decrease sleep latency compared to placebo. Psychiatric and nervous system disorders comprised the most frequent (>5%) treatment emergent adverse reactions observed with zolpidem versus placebo and included dizziness (23.5% vs 1.5%), headache (12.5% vs 9.2%), and hallucinations were reported in 7% of the pediatric patients who received zolpidem; none of the pediatric patients who received placebo reported hallucinations [see Warnings and Precautions (5.5)]. Ten patients on zolpidem (7.4%) discontinued treatment due to an adverse reaction.
8.5 Geriatric Use
A total of 154 patients in U.S. controlled clinical trials and 897 patients in non-U.S. clinical trials who received zolpidem were ≥60 years of age. For a pool of U.S. patients receiving zolpidem at doses of ≤10 mg or placebo, there were three adverse reactions occurring at an incidence of at least 3% for zolpidem and for which the zolpidem incidence was at least twice the placebo incidence (i.e., they could be considered drug related).
| Adverse Event | Zolpidem | Placebo |
|---|---|---|
| Dizziness | 3% | 0% |
| Drowsiness | 5% | 2% |
| Diarrhea | 3% | 1% |
A total of 30/1,959 (1.5%) non-U.S. patients receiving zolpidem reported falls, including 28/30 (93%) who were ≥70 years of age. Of these 28 patients, 23 (82%) were receiving zolpidem doses >10 mg. A total of 24/1,959 (1.2%) non-U.S. patients receiving zolpidem reported confusion, including 18/24 (75%) who were ≥70 years of age. Of these 18 patients, 14 (78%) were receiving zolpidem doses >10 mg.
The dose of zolpidem tartrate in elderly patients is 5 mg to minimize adverse effects related to impaired motor and/or cognitive performance and unusual sensitivity to sedative/hypnotic drugs [see Warnings and Precautions (5.2)].
8.6 Gender Difference in Pharmacokinetics
Women clear zolpidem tartrate from the body at a lower rate than men. Cmax and AUC parameters of zolpidem were approximately 45% higher at the same dose in female subjects compared with male subjects. Given the higher blood levels of zolpidem tartrate in women compared to men at a given dose, the recommended initial dose of zolpidem tartrate for adult women is 5 mg, and the recommended dose for adult men is 5 or 10 mg.
In geriatric patients, clearance of zolpidem is similar in men and women. The recommended dose of zolpidem tartrate in geriatric patients is 5 mg regardless of gender.
8.7 Hepatic Impairment
The recommended dose of zolpidem tartrate in patients with mild to moderate hepatic impairment is 5 mg once daily immediately before bedtime. Avoid zolpidem tartrate use in patients with severe hepatic impairment as it may contribute to encephalopathy [see Dosage and Administration (2.2), Warnings and Precautions (5.8), Clinical Pharmacology (12.3)].
9 DRUG ABUSE AND DEPENDENCE
9.1 Controlled Substance
Zolpidem tartrate is classified as a Schedule IV controlled substance by federal regulation.
9.2 Abuse
Abuse and addiction are separate and distinct from physical dependence and tolerance. Abuse is characterized by misuse of the drug for non-medical purposes, often in combination with other psychoactive substances. Tolerance is a state of adaptation in which exposure to a drug induces changes that result in a diminution of one or more of the drug effects over time. Tolerance may occur to both desired and undesired effects of drugs and may develop at different rates for different effects.
Addiction is a primary, chronic, neurobiological disease with genetic, psychosocial, and environmental factors influencing its development and manifestations. It is characterized by behaviors that include one or more of the following: impaired control over drug use, compulsive use, continued use despite harm, and craving. Drug addiction is a treatable disease, using a multidisciplinary approach, but relapse is common.
Studies of abuse potential in former drug abusers found that the effects of single doses of zolpidem tartrate 40 mg were similar, but not identical, to diazepam 20 mg, while zolpidem tartrate 10 mg was difficult to distinguish from placebo.
Because persons with a history of addiction to, or abuse of, drugs or alcohol are at increased risk for misuse, abuse and addiction of zolpidem, they should be monitored carefully when receiving zolpidem or any other hypnotic.
9.3 Dependence
Use of zolpidem tartrate may lead to the development of physical and/or psychological dependence. The risk of dependence increases with dose and duration of treatment. The risk of abuse and dependence is also greater in patients with a history of alcohol or drug abuse. Zolpidem tartrate should be used with extreme caution in patients with current or past alcohol or drug abuse
Physical dependence is a state of adaptation that is manifested by a specific withdrawal syndrome that can be produced by abrupt cessation, rapid dose reduction, decreasing blood level of the drug, and/or administration of an antagonist.
Sedative/hypnotics have produced withdrawal signs and symptoms following abrupt discontinuation. These reported symptoms range from mild dysphoria and insomnia to a withdrawal syndrome that may include abdominal and muscle cramps, vomiting, sweating, tremors, convulsions, and delirium.
The following adverse events, which are considered to meet the DSM-III-R criteria for uncomplicated sedative/hypnotic withdrawal, were reported during clinical trials with zolpidem tartrate following placebo substitution occurring within 48 hours following last zolpidem treatment: fatigue, nausea, flushing, lightheadedness, uncontrolled crying, emesis, stomach cramps, panic attack, nervousness, and abdominal discomfort. These reported adverse events occurred at an incidence of 1% or less. However, available data cannot provide a reliable estimate of the incidence, if any, of dependence during treatment at recommended doses. There have been postmarketing reports of abuse, dependence and withdrawal with zolpidem.
10 OVERDOSAGE
10.1 Signs and Symptoms
In postmarketing experience of overdose with zolpidem tartrate alone, or in combination with CNS-depressant agents, impairment of consciousness ranging from somnolence to coma, cardiovascular and/or respiratory compromise, and fatal outcomes have been reported.
10.2 Recommended Treatment
General symptomatic and supportive measures should be used along with immediate gastric lavage where appropriate. Intravenous fluids should be administered as needed. Zolpidem’s sedative hypnotic effect was shown to be reduced by flumazenil and therefore may be useful; however, flumazenil administration may contribute to the appearance of neurological symptoms (convulsions). As in all cases of drug overdose, respiration, pulse, blood pressure, and other appropriate signs should be monitored and general supportive measures employed. Hypotension and CNS depression should be monitored and treated by appropriate medical intervention. Sedating drugs should be withheld following zolpidem overdosage, even if excitation occurs. The value of dialysis in the treatment of overdosage has not been determined, although hemodialysis studies in patients with renal failure receiving therapeutic doses have demonstrated that zolpidem is not dialyzable.
As with the management of all overdosage, the possibility of multiple drug ingestion should be considered. The physician may wish to consider contacting a poison control center for up-to-date information on the management of hypnotic drug product overdosage.
11 DESCRIPTION
Zolpidem tartrate USP is a gamma-aminobutyric acid (GABA) A receptor positive modulator of the imidazopyridine class. Zolpidem tartrate USP is available in 5 mg and 10 mg strength tablets for oral administration.
Chemically, zolpidem is N,N,6-trimethyl-2-p-tolylimidazo[1,2-a] pyridine-3-acetamide L-(+)-tartrate (2:1). It has the following structure:
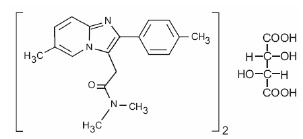
Zolpidem tartrate USP is a white to off-white crystalline powder that is sparingly soluble in water, alcohol, and propylene glycol. It has a molecular weight of 764.88.
Each zolpidem tartrate tablet, USP includes the following inactive ingredients: lactose monohydrate, microcrystalline cellulose, sodium starch glycolate, magnesium stearate, hypromellose, polyethylene glycol, and titanium dioxide.
Meets USP Dissolution Test-3.
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Zolpidem is a GABA A receptor positive modulator presumed to exert its therapeutic effects in the short-term treatment of insomnia through binding to the benzodiazepine site of α1 subunit containing GABA A receptors, increasing the frequency of chloride channel opening resulting in the inhibition of neuronal excitation.
12.2 Pharmacodynamics
Zolpidem binds to GABA A receptors with greater affinity for α1 subunit relative to α2 and α3 subunit containing receptors. Zolpidem has no appreciable binding affinity for α5 subunit containing GABA A receptors. This binding profile may explain the relative absence of myorelaxant effects in animal studies. Zolpidem has no appreciable binding affinity for dopaminergic D2, serotonergic 5HT2, adrenergic, histaminergic or muscarinic receptors.
12.3 Pharmacokinetics
The pharmacokinetic profile of zolpidem tartrate is characterized by rapid absorption from the gastrointestinal tract and a short elimination half-life (T1/2) in healthy subjects.
In a single-dose crossover study in 45 healthy subjects administered 5 and 10 mg zolpidem tartrate tablets, the mean peak concentrations (Cmax) were 59 (range: 29 to 113) and 121 (range: 58 to 272) ng/mL, respectively, occurring at a mean time (Tmax) of 1.6 hours for both. The mean zolpidem tartrate elimination half-life was 2.6 (range: 1.4 to 4.5) and 2.5 (range: 1.4 to 3.8) hours, for the 5 and 10 mg tablets, respectively. Zolpidem tartrate is converted to inactive metabolites that are eliminated primarily by renal excretion. Zolpidem tartrate demonstrated linear kinetics in the dose range of 5 to 20 mg. Total protein binding was found to be 92.5 ± 0.1% and remained constant, independent of concentration between 40 and 790 ng/mL. Zolpidem did not accumulate in young adults following nightly dosing with 20 mg zolpidem tartrate tablets for 2 weeks.
A food-effect study in 30 healthy male subjects compared the pharmacokinetics of zolpidem tartrate 10 mg when administered while fasting or 20 minutes after a meal. Results demonstrated that with food, mean AUC and Cmax were decreased by 15% and 25%, respectively, while mean Tmax was prolonged by 60% (from 1.4 to 2.2 hr). The half-life remained unchanged. These results suggest that, for faster sleep onset, zolpidem tartrate should not be administered with or immediately after a meal.
Special Populations
Elderly
In the elderly, the dose for zolpidem tartrate should be 5 mg [see Warnings and Precautions (5), Dosage and Administration (2)]. This recommendation is based on several studies in which the mean Cmax, T1/2, and AUC were significantly increased when compared to results in young adults. In one study of eight elderly subjects (>70 years), the means for Cmax, T1/2, and AUC significantly increased by 50% (255 vs 384 ng/mL), 32% (2.2 vs 2.9 hr), and 64% (955 vs 1,562 ng•hr/mL), respectively, as compared to younger adults (20 to 40 years) following a single 20 mg oral dose. Zolpidem tartrate did not accumulate in elderly subjects following nightly oral dosing of 10 mg for 1 week.
Hepatic impairment
The pharmacokinetics of zolpidem tartrate in eight patients with chronic hepatic insufficiency was compared to results in healthy subjects. Following a single 20 mg oral zolpidem tartrate dose, mean Cmax and AUC were found to be two times (250 vs 499 ng/mL) and five times (788 vs 4,203 ng•hr/mL) higher, respectively, in hepatically compromised patients. Tmax did not change. The mean half-life in cirrhotic patients of 9.9 hr (range: 4.1 to 25.8 hr) was greater than that observed in normal subjects of 2.2 hr (range: 1.6 to 2.4 hr) [see Dosage and Administration (2.2), Warnings and Precautions (5.8), Use in Specific Populations (8.7)].
Renal impairment
The pharmacokinetics of zolpidem tartrate was studied in 11 patients with end-stage renal failure (mean ClCr = 6.5 ± 1.5 mL/min) undergoing hemodialysis three times a week, who were dosed with zolpidem tartrate 10 mg orally each day for 14 or 21 days. No statistically significant differences were observed for Cmax, Tmax, half-life, and AUC between the first and last day of drug administration when baseline concentration adjustments were made. Zolpidem was not hemodialyzable. No accumulation of unchanged drug appeared after 14 or 21 days. Zolpidem pharmacokinetics was not significantly different in renally impaired patients. No dosage adjustment is necessary in patients with compromised renal function.
Drug Interactions
CNS depressants
Coadministration of zolpidem with other CNS depressants increases the risk of CNS depression [see Warnings and Precautions (5.2)]. Zolpidem tartrate was evaluated in healthy volunteers in single-dose interaction studies for several CNS drugs. Imipramine in combination with zolpidem produced no pharmacokinetic interaction other than a 20% decrease in peak levels of imipramine, but there was an additive effect of decreased alertness. Similarly, chlorpromazine in combination with zolpidem produced no pharmacokinetic interaction, but there was an additive effect of decreased alertness and psychomotor performance.
A study involving haloperidol and zolpidem revealed no effect of haloperidol on the pharmacokinetics or pharmacodynamics of zolpidem. The lack of a drug interaction following single-dose administration does not predict the absence of an effect following chronic administration.
An additive adverse effect on psychomotor performance between alcohol and oral zolpidem was demonstrated [see Warnings and Precautions (5.2)].
Following five consecutive nightly doses at bedtime of oral zolpidem tartrate 10 mg in the presence of sertraline 50 mg (17 consecutive daily doses, at 7:00 am, in healthy female volunteers), zolpidem Cmax was significantly higher (43%) and Tmax was significantly decreased (-53%). Pharmacokinetics of sertraline and N-desmethylsertraline were unaffected by zolpidem.
A single-dose interaction study with zolpidem tartrate 10 mg and fluoxetine 20 mg at steady-state levels in male volunteers did not demonstrate any clinically significant pharmacokinetic or pharmacodynamic interactions. When multiple doses of zolpidem and fluoxetine were given at steady state and the concentrations evaluated in healthy females, an increase in the zolpidem half-life (17%) was observed. There was no evidence of an additive effect in psychomotor performance.
Drugs that affect drug metabolism via cytochrome P450
Some compounds known to inhibit CYP3A may increase exposure to zolpidem. The effect of inhibitors of other P450 enzymes on the pharmacokinetics of zolpidem is unknown.
A single-dose interaction study with zolpidem tartrate 10 mg and itraconazole 200 mg at steady-state levels in male volunteers resulted in a 34% increase in AUC0-∞ of zolpidem tartrate. There were no pharmacodynamic effects of zolpidem detected on subjective drowsiness, postural sway, or psychomotor performance.
A single-dose interaction study with zolpidem tartrate 10 mg and rifampin 600 mg at steady-state levels in female subjects showed significant reductions of the AUC (-73%), Cmax (-58%), and T1/2 (-36%) of zolpidem together with significant reductions in the pharmacodynamic effects of zolpidem tartrate. Rifampin, a CYP3A4 inducer, significantly reduced the exposure to and the pharmacodynamic effects of zolpidem [see Drug Interactions (7.2)].
Similarly, St. John's wort, a CYP3A4 inducer, may also decrease the blood levels of zolpidem.
A single-dose interaction study with zolpidem tartrate 5 mg and ketoconazole, a potent CYP3A4 inhibitor, given as 200 mg twice daily for 2 days increased Cmax of zolpidem (30%) and the total AUC of zolpidem (70%) compared to zolpidem alone and prolonged the elimination half-life (30%) along with an increase in the pharmacodynamic effects of zolpidem [see Drug Interactions (7.2)].
Additionally, fluvoxamine (a strong inhibitor of CYP1A2 and a weak inhibitor of CYP3A4 and CYP2C9) and ciprofloxacin (a strong inhibitor of CYP1A2 and a moderate inhibitor of CYP3A4) are also likely to inhibit zolpidem’s metabolic pathways, potentially leading to an increase in zolpidem exposure.
Other drugs with no interactions with zolpidem
A study involving cimetidine/zolpidem tartrate and ranitidine/zolpidem tartrate combinations revealed no effect of either drug on the pharmacokinetics or pharmacodynamics of zolpidem.
Zolpidem tartrate had no effect on digoxin pharmacokinetics and did not affect prothrombin time when given with warfarin in healthy subjects.
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis
Zolpidem was administered to mice and rats for 2 years at oral doses of 4, 18, and 80 mg base/kg/day. In mice, these doses are approximately 2.5, 10, and 50 times the MRHD of 10 mg/day (8 mg zolpidem base) based on mg/m2 body surface area and in rats, these doses are approximately 5, 20, and 100 times the MRHD based on mg/m2 body surface area. No evidence of carcinogenic potential was observed in mice. In rats, renal tumors (lipoma, liposarcoma) were seen at the mid and high doses.
Mutagenesis
Zolpidem was negative in in vitro (bacterial reverse mutation, mouse lymphoma, and chromosomal aberration) and in vivo (mouse micronucleus) genetic toxicology assays.
Impairment of Fertility
Zolpidem was administered to rats at 4, 20, and 100 mg base/kg/day, which are approximately 5, 25, and 120 times the MRHD of 10 mg/day (8 mg zolpidem base) based on mg/m2 body surface area, prior to and during mating, and continuing in females through postpartum day 25. Zolpidem caused irregular estrus cycles and prolonged precoital intervals at the highest dose tested, which is approximately 120 times the MRHD based on mg/m2 body surface area. The NOAEL for these effects is 25 times the MRHD based on a mg/m2 body surface area. There was no impairment of fertility at any dose tested.
14 CLINICAL STUDIES
14.1 Transient Insomnia
Normal adults experiencing transient insomnia (n=462) during the first night in a sleep laboratory were evaluated in a double-blind, parallel group, single-night trial comparing two doses of zolpidem (7.5 and 10 mg) and placebo. Both zolpidem doses were superior to placebo on objective (polysomnographic) measures of sleep latency, sleep duration, and number of awakenings.
Normal elderly adults (mean age 68) experiencing transient insomnia (n=35) during the first two nights in a sleep laboratory were evaluated in a double-blind, crossover, 2-night trial comparing four doses of zolpidem (5, 10, 15 and 20 mg) and placebo. All zolpidem doses were superior to placebo on the two primary PSG parameters (sleep latency and efficiency) and all four subjective outcome measures (sleep duration, sleep latency, number of awakenings, and sleep quality).
14.2 Chronic Insomnia
Zolpidem was evaluated in two controlled studies for the treatment of patients with chronic insomnia (most closely resembling primary insomnia, as defined in the APA Diagnostic and Statistical Manual of Mental Disorders, DSM-IV™). Adult outpatients with chronic insomnia (n=75) were evaluated in a double-blind, parallel group, 5-week trial comparing two doses of zolpidem tartrate and placebo. On objective (polysomnographic) measures of sleep latency and sleep efficiency, zolpidem 10 mg was superior to placebo on sleep latency for the first 4 weeks and on sleep efficiency for weeks 2 and 4. Zolpidem was comparable to placebo on number of awakenings at both doses studied.
Adult outpatients (n=141) with chronic insomnia were also evaluated, in a double-blind, parallel group, 4-week trial comparing two doses of zolpidem and placebo. Zolpidem 10 mg was superior to placebo on a subjective measure of sleep latency for all 4 weeks, and on subjective measures of total sleep time, number of awakenings, and sleep quality for the first treatment week.
Increased wakefulness during the last third of the night as measured by polysomnography has not been observed in clinical trials with zolpidem tartrate.
14.3 Studies Pertinent to Safety Concerns for Sedative/Hypnotic Drugs
Next-Day Residual Effects
Next-day residual effects of zolpidem tartrate were evaluated in seven studies involving normal subjects. In three studies in adults (including one study in a phase advance model of transient insomnia) and in one study in elderly subjects, a small but statistically significant decrease in performance was observed in the Digit Symbol Substitution Test (DSST) when compared to placebo. Studies of zolpidem tartrate in non-elderly patients with insomnia did not detect evidence of next-day residual effects using the DSST, the Multiple Sleep Latency Test (MSLT), and patient ratings of alertness.
Rebound Effects
There was no objective (polysomnographic) evidence of rebound insomnia at recommended doses seen in studies evaluating sleep on the nights following discontinuation of zolpidem tartrate. There was subjective evidence of impaired sleep in the elderly on the first post-treatment night at doses above the recommended elderly dose of 5 mg.
Memory Impairment
Controlled studies in adults utilizing objective measures of memory yielded no consistent evidence of next-day memory impairment following the administration of zolpidem tartrate. However, in one study involving zolpidem doses of 10 and 20 mg, there was a significant decrease in next-morning recall of information presented to subjects during peak drug effect (90 minutes post dose), i.e., these subjects experienced anterograde amnesia. There was also subjective evidence from adverse event data for anterograde amnesia occurring in association with the administration of zolpidem tartrate, predominantly at doses above 10 mg.
Effects on Sleep Stages
In studies that measured the percentage of sleep time spent in each sleep stage, zolpidem tartrate has generally been shown to preserve sleep stages. Sleep time spent in stages 3 and 4 (deep sleep) was found comparable to placebo with only inconsistent, minor changes in REM (paradoxical) sleep at the recommended dose.
16 HOW SUPPLIED/STORAGE AND HANDLING
Zolpidem Tartrate Tablets USP, 5 mg are white to off-white, circular, biconvex, film-coated tablets, debossed with “E” on one side and “78” on the other side.
Bottles of 100 NDC 65862-159-01
Bottles of 500 NDC 65862-159-05
Bottles of 1,000 NDC 65862-159-99
10 x 10 Unit-dose Tablets NDC 65862-159-10
Zolpidem Tartrate Tablets USP, 10 mg are white to off-white, oval shaped, biconvex, film-coated tablets, debossed with “E” on one side and “79” on the other side.
Bottles of 100 NDC 65862-160-01
Bottles of 500 NDC 65862-160-05
Bottles of 1,000 NDC 65862-160-99
10 x 10 Unit-dose Tablets NDC 65862-160-10
Store at 20° to 25°C (68° to 77°F); excursions permitted to 15° to 30°C (59° to 86°F) [see USP Controlled Room Temperature].
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Medication Guide).
Inform patients and their families about the benefits and risks of treatment with zolpidem tartrate. Inform patients of the availability of a Medication Guide and instruct them to read the Medication Guide prior to initiating treatment with zolpidem tartrate and with each prescription refill. Review the zolpidem tartrate Medication Guide with every patient prior to initiation of treatment. Instruct patients or caregivers that zolpidem tartrate should be taken only as prescribed.
Complex Sleep Behaviors
Instruct patients and their families that zolpidem tartrate may cause complex sleep behaviors, including sleep-walking, sleep-driving, preparing and eating food, making phone calls, or having sex while not being fully awake. Serious injuries and death have occurred during complex sleep behavior episodes. Tell patients to discontinue zolpidem tartrate and notify their healthcare provider immediately if they develop any of these symptoms [see Boxed Warning, Warnings and Precautions (5.1)].
CNS-Depressant Effects and Next-Day Impairment
Tell patients that zolpidem tartrate has the potential to cause next-day impairment, and that this risk is increased if dosing instructions are not carefully followed. Tell patients to wait for at least 8 hours after dosing before driving or engaging in other activities requiring full mental alertness. Inform patients that impairment can be present despite feeling fully awake. Advise patients that increased drowsiness and decreased consciousness may increase the risk of falls in some patients [see Warnings and Precautions (5.2)].
Severe Anaphylactic and Anaphylactoid Reactions
Inform patients that severe anaphylactic and anaphylactoid reactions have occurred with zolpidem. Describe the signs/symptoms of these reactions and advise patients to seek medical attention immediately if any of them occur [see Warnings and Precautions (5.4)].
Suicide
Tell patients to immediately report any suicidal thoughts.
Alcohol and other Drugs
Ask patients about alcohol consumption, medicines they are taking, and drugs they may be taking without a prescription. Advise patients not to use zolpidem tartrate if they drank alcohol that evening or before bed.
Concomitant Use with Opioids
Inform patients and caregivers that potentially serious additive effects may occur if zolpidem tartrate is used with opioids and not to use such drugs concomitantly unless supervised by a healthcare provider [see Warnings and Precautions (5.2, 5.7), Drug Interactions (7.1)].
Tolerance, Abuse, and Dependence
Tell patients not to increase the dose of zolpidem tartrate on their own, and to inform you if they believe the drug “does not work.”
Administration Instructions
Patients should be counseled to take zolpidem tartrate right before they get into bed and only when they are able to stay in bed a full night (7 to 8 hours) before being active again. Zolpidem tartrate tablets should not be taken with or immediately after a meal. Advise patients NOT to take zolpidem tartrate if they drank alcohol that evening.
Pregnancy
Advise patients to notify their healthcare provider if they become pregnant or intend to become pregnant during treatment with zolpidem tartrate. Advise patients that use of zolpidem tartrate late in the third trimester may cause respiratory depression and sedation in neonates. Advise mothers who used zolpidem tartrate during the late third trimester of pregnancy to monitor neonates for signs of sleepiness (more than usual), breathing difficulties, or limpness [see Use in Specific Populations (8.1)].
Lactation
Advise breastfeeding mothers using zolpidem tartrate to monitor infants for increased sleepiness, breathing difficulties, or limpness. Instruct breastfeeding mothers to seek immediate medical care if they notice these signs. A lactating woman may consider pumping and discarding breastmilk during treatment and for 23 hours after zolpidem tartrate administration to minimize drug exposure to a breastfed infant [see Use in Specific Populations (8.2)].
Dispense with Medication Guide available at: www.aurobindousa.com/medication-guides
MEDICATION GUIDE
This Medication Guide has been approved by the U.S. Food and Drug Administration.
Revised: 04/2022
PACKAGE LABEL-PRINCIPAL DISPLAY PANEL - 5 mg (100 Tablets Bottle)
PACKAGE LABEL-PRINCIPAL DISPLAY PANEL - 5 mg Blister Carton (10 x 10 Unit-dose)
PACKAGE LABEL-PRINCIPAL DISPLAY PANEL - 10 mg (100 Tablets Bottle)
PACKAGE LABEL-PRINCIPAL DISPLAY PANEL - 10 mg Blister Carton (10 x 10 Unit-dose)
INGREDIENTS AND APPEARANCE
| ZOLPIDEM TARTRATE
zolpidem tartrate tablet, film coated |
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
| ZOLPIDEM TARTRATE
zolpidem tartrate tablet, film coated |
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
| Labeler - Aurobindo Pharma Limited (650082092) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Aurobindo Pharma Limited | 918917642 | ANALYSIS(65862-159, 65862-160) , MANUFACTURE(65862-159, 65862-160) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Aurobindo Pharma Limited | 650381903 | ANALYSIS(65862-159, 65862-160) , MANUFACTURE(65862-159, 65862-160) | |