Search by Drug Name or NDC
NDC 67877-0199-38 Amlodipine Besylate 10 mg/1 Details
Amlodipine Besylate 10 mg/1
Amlodipine Besylate is a ORAL TABLET in the HUMAN PRESCRIPTION DRUG category. It is labeled and distributed by Ascend Laboratories, LLC. The primary component is AMLODIPINE BESYLATE.
MedlinePlus Drug Summary
Amlodipine is used alone or in combination with other medications to treat high blood pressure in adults and children 6 years and older. It is also used to treat certain types of angina (chest pain) and coronary artery disease (narrowing of the blood vessels that supply blood to the heart). Amlodipine is in a class of medications called calcium channel blockers. It lowers blood pressure by relaxing the blood vessels so the heart does not have to pump as hard. It controls chest pain by increasing the supply of blood to the heart. If taken regularly, amlodipine controls chest pain, but it does not stop chest pain once it starts. Your doctor may prescribe a different medication to take when you have chest pain. High blood pressure is a common condition and when not treated, can cause damage to the brain, heart, blood vessels, kidneys and other parts of the body. Damage to these organs may cause heart disease, a heart attack, heart failure, stroke, kidney failure, loss of vision, and other problems. In addition to taking medication, making lifestyle changes will also help to control your blood pressure. These changes include eating a diet that is low in fat and salt, maintaining a healthy weight, exercising at least 30 minutes most days, not smoking, and using alcohol in moderation.
Related Packages: 67877-0199-38Last Updated: 12/01/2022
MedLinePlus Full Drug Details: Amlodipine
Product Information
| NDC | 67877-0199 |
|---|---|
| Product ID | 67877-199_e2555e66-d1b9-4ce3-80ef-9214e159a3aa |
| Associated GPIs | 34000003100340 |
| GCN Sequence Number | 016927 |
| GCN Sequence Number Description | amlodipine besylate TABLET 10 MG ORAL |
| HIC3 | A9A |
| HIC3 Description | CALCIUM CHANNEL BLOCKING AGENTS |
| GCN | 02682 |
| HICL Sequence Number | 006494 |
| HICL Sequence Number Description | AMLODIPINE BESYLATE |
| Brand/Generic | Generic |
| Proprietary Name | Amlodipine Besylate |
| Proprietary Name Suffix | n/a |
| Non-Proprietary Name | Amlodipine besylate |
| Product Type | HUMAN PRESCRIPTION DRUG |
| Dosage Form | TABLET |
| Route | ORAL |
| Active Ingredient Strength | 10 |
| Active Ingredient Units | mg/1 |
| Substance Name | AMLODIPINE BESYLATE |
| Labeler Name | Ascend Laboratories, LLC |
| Pharmaceutical Class | Calcium Channel Antagonists [MoA], Calcium Channel Blocker [EPC], Cytochrome P450 3A Inhibitors [MoA], Dihydropyridine Calcium Channel Blocker [EPC], Dihydropyridines [CS] |
| DEA Schedule | n/a |
| Marketing Category | ANDA |
| Application Number | ANDA078925 |
| Listing Certified Through | 2024-12-31 |
Package
Package Images



NDC 67877-0199-38 (67877019938)
| NDC Package Code | 67877-199-38 |
|---|---|
| Billing NDC | 67877019938 |
| Package | 10 BLISTER PACK in 1 CARTON (67877-199-38) / 10 TABLET in 1 BLISTER PACK |
| Marketing Start Date | 2010-10-20 |
| NDC Exclude Flag | N |
| Pricing Information | N/A |
Standard Product Labeling (SPL)/Prescribing Information SPL 3f4703c0-0b9e-41a0-8b70-da26683d9ed2 Details
HIGHLIGHTS OF PRESCRIBING INFORMATION
AMLODIPINE besylate tablets for oral administration
Initial U.S. Approval: 1992
INDICATIONS AND USAGE
Amlodipine besylate tablets is a calcium channel blocker and may be used alone or in combination with other antihypertensive and antianginal agents for the treatment of:
• Hypertension (1.1)
- Amlodipine besylate tablets is indicated for the treatment of hypertension, to lower blood pressure. Lowering blood pressure reduces the risk of fatal and nonfatal cardiovascular events, primarily strokes and myocardial infarctions.
• Coronary Artery Disease (1.2)
- Chronic Stable Angina
- Vasospastic Angina (Prinzmetal’s or Variant Angina)
- Angiographically Documented Coronary Artery Disease in patients without heart failure or an ejection fraction < 40%
DOSAGE AND ADMINISTRATION
- Adult recommended starting dose: 5 mg once daily with maximum dose 10 mg once daily. (2.1)
- Small, fragile, or elderly patients, or patients with hepatic insufficiency may be started on 2.5 mg once daily. (2.1)
- Pediatric starting dose: 2.5 mg to 5 mg once daily. (2.2)
Important Limitation: Doses in excess of 5 mg daily have not been studied in pediatric patients. (2.2)
DOSAGE FORMS AND STRENGTHS
- Tablets: 2.5 mg, 5 mg, and 10 mg (3)
CONTRAINDICATIONS
- Known sensitivity to amlodipine (4)
WARNINGS AND PRECAUTIONS
• Symptomatic hypotension is possible, particularly in patients with severe aortic stenosis. However, acute hypotension is unlikely. (5.1)
• Worsening angina and acute myocardial infarction can develop after starting or increasing the dose of amlodipine particularly in patients with severe obstructive coronary artery disease. (5.2)
• Titrate slowly in patients with severe hepatic impairment. (5.3)
ADVERSE REACTIONS
Most common adverse reaction to amlodipine is edema which occurred in a dose related manner. Other adverse experiences not dose related but reported with an incidence >1.0% are fatigue, nausea, abdominal pain, and somnolence. (6)
To report SUSPECTED ADVERSE REACTIONS, contact Ascend Laboratories, LLC at 1-877-ASC-RX01 (877-272-7901) or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch
DRUG INTERACTIONS
- Do not exceed doses greater than 20 mg daily of simvastatin. (7.2)
USE IN SPECIFIC POPULATIONS
See 17 for FDA-approved patient labeling.
Revised: 10/2021
Table Of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS & USAGE
1.1 Hypertension
1.2 Coronary Artery Disease (CAD)
2 DOSAGE & ADMINISTRATION
2.1 Adults
2.2 Children
3 DOSAGE FORMS & STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Hypotension
5.2 Increased Angina or Myocardial Infarction
5.3 Patients with Hepatic Failure
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Postmarketing Experience
7 DRUG INTERACTIONS
7.1 Impact of Other Drugs on Amlodipine
7.2 Impact of Amlodipine on Other Drugs
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
12.4 Pediatric Patients
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
14.1 Effects in Hypertension
14.2 Effects in Chronic Stable Angina
14.3 Effects in Vasospastic Angina
14.4 Effects in Documented Coronary Artery Disease
14.5 Studies in Patients with Heart Failure
16 HOW SUPPLIED/STORAGE AND HANDLING
- *
- Sections or subsections omitted from the full prescribing information are not listed.
1 INDICATIONS & USAGE
1.1 Hypertension
Amlodipine besylate tablets is indicated for the treatment of hypertension, to lower blood pressure. Lowering blood pressure reduces the risk of fatal and nonfatal cardiovascular events, primarily strokes and myocardial infarctions. These benefits have been seen in controlled trials of antihypertensive drugs from a wide variety of pharmacologic classes including Amlodipine besylate tablets.
Control of high blood pressure should be part of comprehensive cardiovascular risk management, including, as appropriate, lipid control, diabetes management, antithrombotic therapy, smoking cessation, exercise, and limited sodium intake. Many patients will require more than one drug to achieve blood pressure goals. For specific advice on goals and management, see published guidelines, such as those of the National High Blood Pressure Education Program’s Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC).
Numerous antihypertensive drugs, from a variety of pharmacologic classes and with different mechanisms of action, have been shown in randomized controlled trials to reduce cardiovascular morbidity and mortality, and it can be concluded that it is blood pressure reduction, and not some other pharmacologic property of the drugs, that is largely responsible for those benefits. The largest and most consistent cardiovascular outcome benefit has been a reduction in the risk of stroke, but reductions in myocardial infarction and cardiovascular mortality also have been seen regularly.
Elevated systolic or diastolic pressure causes increased cardiovascular risk, and the absolute risk increase per mmHg is greater at higher blood pressures, so that even modest reductions of severe hypertension can provide substantial benefit. Relative risk reduction from blood pressure reduction is similar across populations with varying absolute risk, so the absolute benefit is greater in patients who are at higher risk independent of their hypertension (for example, patients with diabetes or hyperlipidemia), and such patients would be expected to benefit from more aggressive treatment to a lower blood pressure goal.
Some antihypertensive drugs have smaller blood pressure effects (as monotherapy) in black patients, and many antihypertensive drugs have additional approved indications and effects (e.g., on angina, heart failure, or diabetic kidney disease). These considerations may guide selection of therapy.
Amlodipine besylate tablets may be used alone or in combination with other antihypertensive agents.
1.2 Coronary Artery Disease (CAD)
Chronic Stable Angina
Amlodipine besylate tablets is indicated for the symptomatic treatment of chronic stable angina. Amlodipine besylate tablets may be used alone or in combination with other antianginal agents.
Vasospastic Angina (Prinzmetal’s or Variant Angina)
Amlodipine besylate tablets is indicated for the treatment of confirmed or suspected vasospastic angina. Amlodipine besylate tablets may be used as monotherapy or in combination with other antianginal agents.
Angiographically Documented CAD
In patients with recently documented CAD by angiography and without heart failure or an ejection fraction <40%, Amlodipine besylate tablets is indicated to reduce the risk of hospitalization for angina and to reduce the risk of a coronary revascularization procedure.
2 DOSAGE & ADMINISTRATION
2.1 Adults
The usual initial antihypertensive oral dose of Amlodipine besylate tablets is 5 mg once daily, and the maximum dose is 10 mg once daily.
Small, fragile, or elderly patients, or patients with hepatic insufficiency may be started on 2.5 mg once daily and this dose may be used when adding Amlodipine besylate tablets to other antihypertensive therapy.
Adjust dosage according to blood pressure goals. In general, wait 7 to 14 days between titration steps. Titrate more rapidly, however, if clinically warranted, provided the patient is assessed frequently.
Angina: The recommended dose for chronic stable or vasospastic angina is 5 to 10 mg, with the lower dose suggested in the elderly and in patients with hepatic insufficiency. Most patients will require 10 mg for adequate effect.
Coronary artery disease: The recommended dose range for patients with coronary artery disease is 5 to 10 mg once daily. In clinical studies, the majority of patients required 10 mg [see Clinical Studies (14.4)].
2.2 Children
The effective antihypertensive oral dose in pediatric patients ages 6 to 17 years is 2.5 mg to 5 mg once daily. Doses in excess of 5 mg daily have not been studied in pediatric patients [see Clinical Pharmacology (12.4), Clinical Studies (14.1)].
3 DOSAGE FORMS & STRENGTHS
Tablets: 2.5 mg white to off white, round, flat-faced, beveled edge tablets '211' debossed on one side and plain on the other side.
Tablets: 5 mg white to off white, round, flat-faced, beveled edge tablets '210' debossed on one side and plain on the other side.
Tablets: 10 mg white to off white, round, flat-faced, beveled edge tablets '209' debossed on one side and plain on the other side.
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Hypotension
Symptomatic hypotension is possible, particularly in patients with severe aortic stenosis. Because of the gradual onset of action, acute hypotension is unlikely.
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Amlodipine has been evaluated for safety in more than 11,000 patients in U.S. and foreign clinical trials. In general, treatment with amlodipine was well-tolerated at doses up to 10 mg daily. Most adverse reactions reported during therapy with amlodipine were of mild or moderate severity. In controlled clinical trials directly comparing amlodipine (N=1730) at doses up to 10 mg to placebo (N=1250), discontinuation of amlodipine because of adverse reactions was required in only about 1.5% of patients and was not significantly different from placebo (about 1%). The most commonly reported side effects more frequent than placebo are reflected in the table below. The incidence (%) of side effects that occurred in a dose related manner are as follows:
| 2.5mg N=275 | Amlodipine 5mg N=296 | 10mg N=268 | Placebo N=520 |
|
| Edema | 1.8 | 3.0 | 10.8 | 0.6 |
| Dizziness | 1.1 | 3.4 | 3.4 | 1.5 |
| Flushing | 0.7 | 1.4 | 2.6 | 0.0 |
| Palpitation | 0.7 | 1.4 | 4.5 | 0.6 |
Other adverse reactions that were not clearly dose related but were reported with an incidence greater than 1.0% in placebo-controlled clinical trials include the following:
| | Amlodipine (%) (N=1730) | Placebo (%) (N=1250) |
| Fatigue | 4.5 | 2.8 |
| Nausea | 2.9 | 1.9 |
| Abdominal Pain | 1.6 | 0.3 |
| Somnolence | 1.4 | 0.6 |
For several adverse experiences that appear to be drug and dose related, there was a greater incidence in women than men associated with amlodipine treatment as shown in the following table:
| | Amlodipine | Placebo |
||
| | Male=% (N=1218) | Female=% (N=512) | Male=% (N=914) | Female=% (N=336) |
| Edema | 5.6 | 14.6 | 1.4 | 5.1 |
| Flushing | 1.5 | 4.5 | 0.3 | 0.9 |
| Palpitations | 1.4 | 3.3 | 0.9 | 0.9 |
| Somnolence | 1.3 | 1.6 | 0.8 | 0.3 |
The following events occurred in <1% but >0.1% of patients in controlled clinical trials or under conditions of open trials or marketing experience where a causal relationship is uncertain; they are listed to alert the physician to a possible relationship:
Cardiovascular: arrhythmia (including ventricular tachycardia and atrial fibrillation), bradycardia, chest pain, peripheral ischemia, syncope, tachycardia, vasculitis.
Central and Peripheral Nervous System: hypoesthesia, neuropathy peripheral, paresthesia, tremor, vertigo.
Gastrointestinal: anorexia, constipation, dysphagia, diarrhea, flatulence, pancreatitis, vomiting, gingival hyperplasia.
General: allergic reaction, asthenia,1 back pain, hot flushes, malaise, pain, rigors, weight gain, weight decrease.
Musculoskeletal System: arthralgia, arthrosis, muscle cramps,1 myalgia.
Psychiatric: sexual dysfunction (male1 and female), insomnia, nervousness, depression, abnormal dreams, anxiety, depersonalization.
Respiratory System: dyspnea,1 epistaxis.
Skin and Appendages: angioedema, erythema multiforme, pruritus,1 rash,1 rash erythematous, rash maculopapular.
Special Senses: abnormal vision, conjunctivitis, diplopia, eye pain, tinnitus.
Urinary System: micturition frequency, micturition disorder, nocturia.
Autonomic Nervous System: dry mouth, sweating increased.
Metabolic and Nutritional: hyperglycemia, thirst.
Hemopoietic: leukopenia, purpura, thrombocytopenia.
1 These events occurred in less than 1% in placebo-controlled trials, but the incidence of these side effects was between 1% and 2% in all multiple dose studies.
Amlodipine therapy has not been associated with clinically significant changes in routine laboratory tests. No clinically relevant changes were noted in serum potassium, serum glucose, total triglycerides, total cholesterol, HDL cholesterol, uric acid, blood urea nitrogen, or creatinine.
In the CAMELOT and PREVENT studies [see Clinical Studies (14.4)], the adverse event profile was similar to that reported previously (see above), with the most common adverse event being peripheral edema.
6.2 Postmarketing Experience
Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
The following postmarketing event has been reported infrequently where a causal relationship is uncertain: gynecomastia. In postmarketing experience, jaundice and hepatic enzyme elevations (mostly consistent with cholestasis or hepatitis), in some cases severe enough to require hospitalization, have been reported in association with use of amlodipine.
Postmarketing reporting has also revealed a possible association between extrapyramidal disorder and amlodipine.
Amlodipine has been used safely in patients with chronic obstructive pulmonary disease, well-compensated congestive heart failure, coronary artery disease, peripheral vascular disease, diabetes mellitus, and abnormal lipid profiles.
7 DRUG INTERACTIONS
7.1 Impact of Other Drugs on Amlodipine
CYP3A Inhibitors
Co-administration with CYP3A inhibitors (moderate and strong) results in increased systemic exposure to amlodipine and may require dose reduction. Monitor for symptoms of hypotension and edema when amlodipine is co-administered with CYP3A inhibitors to determine the need for dose adjustment [see Clinical Pharmacology (12.3)].
CYP3A Inducers
No information is available on the quantitative effects of CYP3A inducers on amlodipine. Blood pressure should be closely monitored when amlodipine is co-administered with CYP3A inducers.
Sildenafil
Monitor for hypotension when sildenafil is co-administered with amlodipine [see Clinical Pharmacology (12.2)
7.2 Impact of Amlodipine on Other Drugs
Simvastatin
Co-administration of simvastatin with amlodipine increases the systemic exposure of simvastatin. Limit the dose of simvastatin in patients on amlodipine to 20 mg daily [seeClinical Pharmacology (12.3)].
Immunosuppressants
Amlodipine may increase the systemic exposure of cyclosporine or tacrolimus when co-administered. Frequent monitoring of trough blood levels of cyclosporine and tacrolimus is recommended and adjust the dose when appropriate [see Clinical Pharmacology (12.3)].
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
The limited available data based on post-marketing reports with amlodipine use in pregnant women are not sufficient to inform a drug-associated risk for major birth defects and miscarriage. There are risks to the mother and fetus associated with poorly controlled hypertension in pregnancy [see Clinical Considerations]. In animal reproduction studies, there was no evidence of adverse developmental effects when pregnant rats and rabbits were treated orally with amlodipine maleate during organogenesis at doses approximately 10 and 20-times the maximum recommended human dose (MRHD), respectively. However for rats, litter size was significantly decreased (by about 50%) and the number of intrauterine deaths was significantly increased (about 5-fold). Amlodipine has been shown to prolong both the gestation period and the duration of labor in rats at this dose [see Data].
The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a background risk of birth defect, loss or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2%-4% and 15%-20%, respectively.
Clinical Considerations
Disease-associated maternal and/or embryo/fetal risk
Hypertension in pregnancy increases the maternal risk for pre-eclampsia, gestational diabetes, premature delivery, and delivery complications (e.g., need for cesarean section and post-partum hemorrhage). Hypertension increases the fetal risk for intrauterine growth restriction and intrauterine death. Pregnant women with hypertension should be carefully monitored and managed accordingly.
Data
Animal Data
No evidence of teratogenicity or other embryo/fetal toxicity was found when pregnant rats and rabbits were treated orally with amlodipine maleate at doses up to 10 mg amlodipine/kg/day (approximately 10 and 20 times the MRHD based on body surface area, respectively) during their respective periods of major organogenesis. However for rats, litter size was significantly decreased (by about 50%) and the number of intrauterine deaths was significantly increased (about 5-fold)in rats receiving amlodipine maleate at a dose equivalent to 10 mg amlodipine/kg/day for 14 days before mating and throughout mating and gestation. Amlodipine maleate has been shown to prolong both the gestation period and the duration of labor in rats at this dose.
8.2 Lactation
Risk Summary
Limited available data from a published clinical lactation study reports that amlodipine is present in human milk at an estimated median relative infant dose of 4.2%. No adverse effects of amlodipine on the breastfed infant have been observed. There is no available information on the effects of amlodipine on milk product
8.4 Pediatric Use
Amlodipine besylate tablets (2.5 to 5 mg daily) is effective in lowering blood pressure in patients 6 to 17 years [see Clinical Studies (14.1)]. Effect of amlodipine on blood pressure in patients less than 6 years of age is not known.
8.5 Geriatric Use
Clinical studies of amlodipine did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy. Elderly patients have decreased clearance of amlodipine with a resulting increase of AUC of approximately 40 to 60%, and a lower initial dose may be required [see Dosage and Administration (2.1)].
10 OVERDOSAGE
Overdosage might be expected to cause excessive peripheral vasodilation with marked hypotension and possibly a reflex tachycardia. In humans, experience with intentional overdosage of amlodipine is limited.
Single oral doses of amlodipine maleate equivalent to 40 mg amlodipine/kg and 100 mg amlodipine/kg in mice and rats, respectively, caused deaths. Single oral amlodipine maleate doses equivalent to 4 or more mg amlodipine/kg or higher in dogs (11 or more times the maximum recommended human dose on a mg/m2 basis) caused a marked peripheral vasodilation and hypotension.
If massive overdose should occur, initiate active cardiac and respiratory monitoring. Frequent blood pressure measurements are essential. Should hypotension occur, provide cardiovascular support including elevation of the extremities and the judicious administration of fluids. If hypotension remains unresponsive to these conservative measures, consider administration of vasopressors (such as phenylephrine) with attention to circulating volume and urine output. As amlodipine is highly protein bound, hemodialysis is not likely to be of benefit.
11 DESCRIPTION
Amlodipine besylate is the besylate salt of amlodipine, a long-acting calcium channel blocker.
Amlodipine besylate is chemically described as 3-Ethyl-5-methyl(±)-2- [(2-aminoethoxy)methyl]-4-(2-chlorophenyl)-1,4-dihydro-6- methyl-3,5-pyridinedicarboxylate, monobenzenesulphonate. Its molecular formula is C20H25ClN2O5•C6H6O3S, and its structural formula is:

Amlodipine besylate is a white crystalline powder with a molecular weight of 567.1. It is slightly soluble in water and sparingly soluble in ethanol. Amlodipine besylate tablets, USP are formulated as white tablets equivalent to 2.5, 5, and 10 mg of amlodipine for oral administration. In addition to the active ingredient, amlodipine besylate, each tablet contains the following inactive ingredients: microcrystalline cellulose, dibasic calcium phosphate anhydrous, sodium starch glycolate, colloidal silicon dioxide and magnesium stearate.
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Amlodipine is a dihydropyridine calcium antagonist (calcium ion antagonist or slow-channel blocker) that inhibits the transmembrane influx of calcium ions into vascular smooth muscle and cardiac muscle. Experimental data suggest that amlodipine binds to both dihydropyridine and nondihydropyridine binding sites. The contractile processes of cardiac muscle and vascular smooth muscle are dependent upon the movement of extracellular calcium ions into these cells through specific ion channels. Amlodipine inhibits calcium ion influx across cell membranes selectively, with a greater effect on vascular smooth muscle cells than on cardiac muscle cells. Negative inotropic effects can be detected in vitro but such effects have not been seen in intact animals at therapeutic doses. Serum calcium concentration is not affected by amlodipine. Within the physiologic pH range, amlodipine is an ionized compound (pKa=8.6), and its kinetic interaction with the calcium channel receptor is characterized by a gradual rate of association and dissociation with the receptor binding site, resulting in a gradual onset of effect.
Amlodipine is a peripheral arterial vasodilator that acts directly on vascular smooth muscle to cause a reduction in peripheral vascular resistance and reduction in blood pressure.
The precise mechanisms by which amlodipine relieves angina have not been fully delineated, but are thought to include the following:
Exertional Angina: In patients with exertional angina, amlodipine reduces the total peripheral resistance (afterload) against which the heart works and reduces the rate pressure product, and thus myocardial oxygen demand, at any given level of exercise.
Vasospastic Angina: Amlodipine has been demonstrated to block constriction and restore blood flow in coronary arteries and arterioles in response to calcium, potassium epinephrine, serotonin, and thromboxane A2 analog in experimental animal models and in human coronary vessels in vitro. This inhibition of coronary spasm is responsible for the effectiveness of amlodipine in vasospastic (Prinzmetal's or variant) angina.
12.2 Pharmacodynamics
Hemodynamics: Following administration of therapeutic doses to patients with hypertension, amlodipine produces vasodilation resulting in a reduction of supine and standing blood pressures. These decreases in blood pressure are not accompanied by a significant change in heart rate or plasma catecholamine levels with chronic dosing. Although the acute intravenous administration of amlodipine decreases arterial blood pressure and increases heart rate in hemodynamic studies of patients with chronic stable angina, chronic oral administration of amlodipine in clinical trials did not lead to clinically significant changes in heart rate or blood pressures in normotensive patients with angina.
With chronic once daily oral administration, antihypertensive effectiveness is maintained for at least 24 hours. Plasma concentrations correlate with effect in both young and elderly patients. The magnitude of reduction in blood pressure with amlodipine is also correlated with the height of pretreatment elevation; thus, individuals with moderate hypertension (diastolic pressure 105 to 114 mmHg) had about a 50% greater response than patients with mild hypertension (diastolic pressure 90 to 104 mmHg). Normotensive subjects experienced no clinically significant change in blood pressures (+1/–2 mmHg).
In hypertensive patients with normal renal function, therapeutic doses of amlodipine resulted in a decrease in renal vascular resistance and an increase in glomerular filtration rate and effective renal plasma flow without change in filtration fraction or proteinuria.
As with other calcium channel blockers, hemodynamic measurements of cardiac function at rest and during exercise (or pacing) in patients with normal ventricular function treated with amlodipine have generally demonstrated a small increase in cardiac index without significant influence on dP/dt or on left ventricular end diastolic pressure or volume. In hemodynamic studies, amlodipine has not been associated with a negative inotropic effect when administered in the therapeutic dose range to intact animals and man, even when coadministered with beta-blockers to man. Similar findings, however, have been observed in normal or well-compensated patients with heart failure with agents possessing significant negative inotropic effects.
Electrophysiologic Effects: Amlodipine does not change sinoatrial nodal function or atrioventricular conduction in intact animals or man. In patients with chronic stable angina, intravenous administration of 10 mg did not significantly alter A-H and H-V conduction and sinus node recovery time after pacing. Similar results were obtained in patients receiving amlodipine and concomitant beta-blockers. In clinical studies in which amlodipine was administered in combination with beta-blockers to patients with either hypertension or angina, no adverse effects on electrocardiographic parameters were observed. In clinical trials with angina patients alone, amlodipine therapy did not alter electrocardiographic intervals or produce higher degrees of AV blocks.
Drug interactions
Sildenafil: When amlodipine and sildenafil were used in combination, each agent independently exerted its own blood pressure lowering effect [see Drug Interactions (7.1)].
12.3 Pharmacokinetics
After oral administration of therapeutic doses of amlodipine, absorption produces peak plasma concentrations between 6 and 12 hours. Absolute bioavailability has been estimated to be between 64 and 90%. The bioavailability of amlodipine is not altered by the presence of food.
Amlodipine is extensively (about 90%) converted to inactive metabolites via hepatic metabolism with 10% of the parent compound and 60% of the metabolites excreted in the urine. Ex vivo studies have shown that approximately 93% of the circulating drug is bound to plasma proteins in hypertensive patients. Elimination from the plasma is biphasic with a terminal elimination half-life of about 30 to 50 hours. Steady-state plasma levels of amlodipine are reached after 7 to 8 days of consecutive daily dosing.
The pharmacokinetics of amlodipine are not significantly influenced by renal impairment. Patients with renal failure may therefore receive the usual initial dose.
Elderly patients and patients with hepatic insufficiency have decreased clearance of amlodipine with a resulting increase in AUC of approximately 40 to 60%, and a lower initial dose may be required. A similar increase in AUC was observed in patients with moderate to severe heart failure.
Drug interactions
In vitro data indicate that amlodipine has no effect on the human plasma protein binding of digoxin, phenytoin, warfarin, and indomethacin.
Impact of other drugs on amlodipine
Co-administered cimetidine, magnesium-and aluminum hydroxide antacids, sildenafil, and grapefruit juice have no impact on the exposure to amlodipine.
CYP3A inhibitors: Co-administration of a 180 mg daily dose of diltiazem with 5 mg amlodipine in elderly hypertensive patients resulted in a 60% increase in amlodipine systemic exposure. Erythromycin co-administration in healthy volunteers did not significantly change amlodipine systemic exposure. However, strong inhibitors of CYP3A (e.g., itraconazole, clarithromycin) may increase the plasma concentrations of amlodipine to a greater extent [see Drug Interactions (7.1)].
Impact of amlodipine on other drugs
Amlodipine is a weak inhibitor of CYP3Aand may increase exposure to CYP3Asubstrates.
Co-administered amlodipine does not affect the exposure to atorvastatin, digoxin, ethanol and the warfarin prothrombin response time.
Simvastatin: Co-administration of multiple doses of 10 mg of amlodipine with 80 mg simvastatin resulted in a 77% increase in exposure to simvastatin compared to simvastatin alone [see Drug Interactions (7.2)].
Cyclosporine: A prospective study in renal transplant patients (N=11) showed on an average of 40% increase in trough cyclosporine levels when concomitantly treated with amlodipine [see Drug Interactions (7.2)].
Tacrolimus: A prospective study in healthy Chinese volunteers (N=9) with CYP3A5 expressers showed a 2.5-to 4-fold increase in tacrolimus exposure when concomitantly administered with amlodipine compared to tacrolimus alone. This finding was not observed in CYP3A5 non-expressers (N= 6). However, a 3-fold increase in plasma exposure to tacrolimus in a renal transplant patient (CYP3A5 non-expresser) upon initiation of amlodipine for the treatment of post-transplant hypertension resulting in reduction of tacrolimus dose has been reported. Irrespective of the CYP3A5 genotype status, the possibility of an interaction cannot be excluded with these drugs [see Drug Interactions (7.2)].
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Rats and mice treated with amlodipine maleate in the diet for up to two years, at concentrations calculated to provide daily dosage levels of 0.5, 1.25, and 2.5 amlodipine mg/kg/day, showed no evidence of a carcinogenic effect of the drug. For the mouse, the highest dose was, on a mg/m2 basis, similar to the maximum recommended human dose of 10 mg amlodipine/day.3 For the rat, the highest dose was, on a mg/m2 basis, about twice the maximum recommended human dose.3
Mutagenicity studies conducted with amlodipine maleate revealed no drug related effects at either the gene or chromosome level.
There was no effect on the fertility of rats treated orally with amlodipine maleate (males for 64 days and females for 14 days prior to mating) at doses up to 10 mg amlodipine/kg/day (8 times the maximum recommended human dose3 of 10 mg/day on a mg/m2 basis).
3 Based on patient weight of 50 kg
14 CLINICAL STUDIES
14.1 Effects in Hypertension
Adult Patients
The antihypertensive efficacy of amlodipine has been demonstrated in a total of 15 double-blind, placebo-controlled, randomized studies involving 800 patients on amlodipine and 538 on placebo. Once daily administration produced statistically significant placebo-corrected reductions in supine and standing blood pressures at 24 hours postdose, averaging about 12/6 mmHg in the standing position and 13/7 mmHg in the supine position in patients with mild to moderate hypertension. Maintenance of the blood pressure effect over the 24-hour dosing interval was observed, with little difference in peak and trough effect. Tolerance was not demonstrated in patients studied for up to 1 year. The 3 parallel, fixed dose, dose response studies showed that the reduction in supine and standing blood pressures was dose-related within the recommended dosing range. Effects on diastolic pressure were similar in young and older patients. The effect on systolic pressure was greater in older patients, perhaps because of greater baseline systolic pressure. Effects were similar in black patients and in white patients.
Pediatric Patients
Two hundred sixty-eight hypertensive patients aged 6 to 17 years were randomized first to amlodipine 2.5 or 5 mg once daily for 4 weeks and then randomized again to the same dose or to placebo for another 4 weeks. Patients receiving 2.5 mg or 5 mg at the end of 8 weeks had significantly lower systolic blood pressure than those secondarily randomized to placebo. The magnitude of the treatment effect is difficult to interpret, but it is probably less than 5 mmHg systolic on the 5 mg dose and 3.3 mmHg systolic on the 2.5 mg dose. Adverse events were similar to those seen in adults.
14.2 Effects in Chronic Stable Angina
The effectiveness of 5 to 10 mg/day of amlodipine in exercise-induced angina has been evaluated in 8 placebo-controlled, double-blind clinical trials of up to 6 weeks duration involving 1038 patients (684 amlodipine, 354 placebo) with chronic stable angina. In 5 of the 8 studies, significant increases in exercise time (bicycle or treadmill) were seen with the 10 mg dose. Increases in symptom-limited exercise time averaged 12.8% (63 sec) for Amlodipine 10 mg, and averaged 7.9% (38 sec) for Amlodipine 5 mg. Amlodipine 10 mg also increased time to 1 mm ST segment deviation in several studies and decreased angina attack rate. The sustained efficacy of amlodipine in angina patients has been demonstrated over long-term dosing. In patients with angina, there were no clinically significant reductions in blood pressures (4/1 mmHg) or changes in heart rate (+0.3 bpm).
14.3 Effects in Vasospastic Angina
In a double-blind, placebo-controlled clinical trial of 4 weeks duration in 50 patients, amlodipine therapy decreased attacks by approximately 4/week compared with a placebo decrease of approximately 1/week (p<0.01). Two of 23 amlodipine and 7 of 27 placebo patients discontinued from the study due to lack of clinical improvement.
14.4 Effects in Documented Coronary Artery Disease
In PREVENT, 825 patients with angiographically documented coronary artery disease were randomized to amlodipine besylate tablets (5 to 10 mg once daily) or placebo and followed for 3 years. Although the study did not show significance on the primary objective of change in coronary luminal diameter as assessed by quantitative coronary angiography, the data suggested a favorable outcome with respect to fewer hospitalizations for angina and revascularization procedures in patients with CAD.
CAMELOT enrolled 1318 patients with CAD recently documented by angiography, without left main coronary disease and without heart failure or an ejection fraction <40%. Patients (76% males, 89% Caucasian, 93% enrolled at U.S. sites, 89% with a history of angina, 52% without PCI, 4% with PCI and no stent, and 44% with a stent) were randomized to double-blind treatment with either amlodipine besylate tablets (5 to 10 mg once daily) or placebo in addition to standard care that included aspirin (89%), statins (83%), beta-blockers (74%), nitroglycerin (50%), anti-coagulants (40%), and diuretics (32%), but excluded other calcium channel blockers. The mean duration of follow-up was 19 months. The primary endpoint was the time to first occurrence of one of the following events: hospitalization for angina pectoris, coronary revascularization, myocardial infarction, cardiovascular death, resuscitated cardiac arrest, hospitalization for heart failure, stroke/TIA, or peripheral vascular disease. A total of 110 (16.6%) and 151 (23.1%) first events occurred in the amlodipine besylate tablets and placebo groups, respectively, for a hazard ratio of 0.691 (95% CI: 0.540 to 0.884, p = 0.003). The primary endpoint is summarized in Figure 1 below. The outcome of this study was largely derived from the prevention of hospitalizations for angina and the prevention of revascularization procedures (see Table 1). Effects in various subgroups are shown in Figure 2.
In an angiographic substudy (n=274) conducted within CAMELOT, there was no significant difference between amlodipine and placebo on the change of atheroma volume in the coronary artery as assessed by intravascular ultrasound.
Figure 1 -Kaplan-Meier Analysis of Composite Clinical Outcomes for Amlodipine versus Placebo
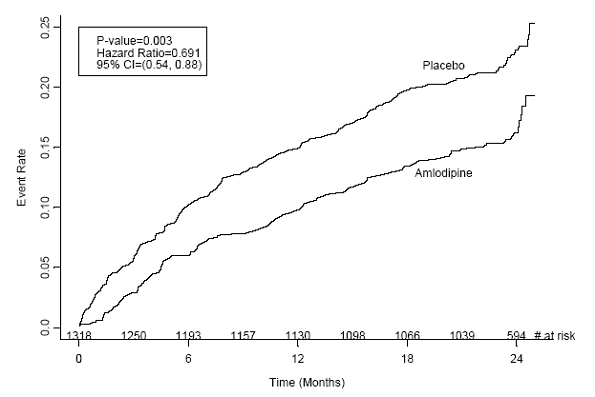
Figure 2 – Effects on Primary Endpoint of Amlodipine versus Placebo across Sub-Groups

Table 1 below summarizes the significant composite endpoint and clinical outcomes from the composites of the primary endpoint. The other components of the primary endpoint including cardiovascular death, resuscitated cardiac arrest, myocardial infarction, hospitalization for heart failure, stroke/TIA, or peripheral vascular disease did not demonstrate a significant difference between amlodipine and placebo.
| Clinical Outcomes N (%) | Amlodipine (N=663) | Placebo (N=655) | Risk Reduction (p-value) |
|---|---|---|---|
| Composite CV Endpoint | 110 (16.6) | 151 (23.1) | 31% (0.003) |
| Hospitalization for Angina* | 51 (7.7) | 84 (12.8) | 42% (0.002) |
| Coronary Revascularization* | 78 (11.8) | 103 (15.7) | 27% (0.033) |
| *Total patients with these events |
|||
14.5 Studies in Patients with Heart Failure
Amlodipine has been compared to placebo in four 8 to 12 week studies of patients with NYHA Class II/III heart failure, involving a total of 697 patients. In these studies, there was no evidence of worsened heart failure based on measures of exercise tolerance, NYHA classification, symptoms, or left ventricular ejection fraction. In a long-term (follow-up at least 6 months, mean 13.8 months) placebo-controlled mortality/morbidity study of amlodipine 5 to 10 mg in 1153 patients with NYHA Classes III (n=931) or IV (n=222) heart failure on stable doses of diuretics, digoxin, and ACE inhibitors, amlodipine had no effect on the primary endpoint of the study which was the combined endpoint of all-cause mortality and cardiac morbidity (as defined by life-threatening arrhythmia, acute myocardial infarction, or hospitalization for worsened heart failure), or on NYHA classification, or symptoms of heart failure. Total combined all-cause mortality and cardiac morbidity events were 222/571 (39%) for patients on amlodipine and 246/583 (42%) for patients on placebo; the cardiac morbid events represented about 25% of the endpoints in the study.
Another study (PRAISE-2) randomized patients with NYHA Class III (80%) or IV (20%) heart failure without clinical symptoms or objective evidence of underlying ischemic disease, on stable doses of ACE inhibitors (99%), digitalis (99%), and diuretics (99%), to placebo (n=827) or amlodipine (n=827) and followed them for a mean of 33 months. There was no statistically significant difference between amlodipine and placebo in the primary endpoint of all-cause mortality (95% confidence limits from 8% reduction to 29% increase on amlodipine). With amlodipine there were more reports of pulmonary edema.
16 HOW SUPPLIED/STORAGE AND HANDLING
2.5 mg Tablets
Amlodipine besylate tablets, USP 2.5 mg (amlodipine besylate equivalent to 2.5 mg of amlodipine per tablet) are supplied as white to off white, round, flat-faced, beveled edge tablets '211' debossed on one side and plain on the other side and supplied as follows:
NDC 67877-197-90 Bottle of 90
NDC 67877-197-05 Bottle of 500
NDC 67877-197-10 Bottle of 1000
NDC 67877-197-38 Carton of 100 tablets (10x10 unit-dose)
5 mg Tablets
Amlodipine besylate tablets, USP 5 mg (amlodipine besylate equivalent to 5 mg of amlodipine per tablet) are white to off white, round, flat-faced, beveled edge tablets '210' debossed on one side and plain on the other side and supplied as follows:
NDC 67877-198-90 Bottle of 90
NDC 67877-198-05 Bottle of 500
NDC 67877-198-10 Bottle of 1000
NDC 67877-198-38 Carton of 100 tablets (10x10 unit-dose)
10 mg Tablets
Amlodipine besylate tablets, USP 10 mg (amlodipine besylate equivalent to 10 mg of amlodipine per tablet) are white to off white, round, flat-faced, beveled edge tablets '209' debossed on one side and plain on the other side and supplied as follows:
NDC 67877-199-90 Bottle of 90
NDC 67877-199-05 Bottle of 500
NDC 67877-199-10 Bottle of 1000
NDC 67877-199-38 Carton of 100 tablets (10x10 unit-dose)
Storage
Store at 20° to 25°C (68° to 77°F), excursions permitted to 15° to 30°C (59° to 86°F) (see USP Controlled Room Temperature), and dispense in tight, light resistant container (USP).
Manufactured by:
Alkem Laboratories Ltd.,
INDIA.
Distributed by:
Ascend Laboratories, LLC
Parsippany, NJ 07054
Revised Date: October, 2021
PATIENT PACKAGE INSERT
AMLODIPINE BESYLATE TABLETS, USP 2.5 mg, 5 mg, and 10 mg
Read this information carefully before you start taking amlodipine besylate tablets, USP and each time you refill your prescription. There may be new information. This information does not replace talking with your doctor. If you have any questions about amlodipine besylate tablets, USP, ask your doctor. Your doctor will know if amlodipine besylate tablets, USP is right for you.
What is amlodipine besylate?
Amlodipine besylate is a type of medicine known as a calcium channel blocker (CCB). It is used to treat high blood pressure (hypertension) and a type of chest pain called angina. It can be used by itself or with other medicines to treat these conditions.
High Blood Pressure (hypertension)
High blood pressure comes from blood pushing too hard against your blood vessels. Amlodipine besylate relaxes your blood vessels, which lets your blood flow more easily and helps lower your blood pressure. Drugs that lower blood pressure lower your risk of having a stroke or heart attack.
Angina
Angina is a pain or discomfort that keeps coming back when part of your heart does not get enough blood. Angina feels like a pressing or squeezing pain, usually in your chest under the breastbone. Sometimes you can feel it in your shoulders, arms, neck, jaws, or back. Amlodipine besylate can relieve this pain.
Who should not use amlodipine besylate?
Do not use amlodipine besylate if you are allergic to amlodipine (the active ingredient in amlodipine besylate tablets), or to the inactive ingredients. Your doctor or pharmacist can give you a list of these ingredients.
What should I tell my doctor before taking amlodipine besylate?
Tell your doctor about any prescription and non-prescription medicines you are taking, including natural or herbal remedies.
Tell your doctor if you:
- ever had heart disease
- ever had liver problems
- are pregnant, or plan to become pregnant. Your doctor will decide if amlodipine besylate is the best treatment for you.
- are breast-feeding. Amlodipine passes into your milk.
How should I take amlodipine besylate tablets, USP?
- Take amlodipine besylate tablets, USP once a day, with or without food.
- It may be easier to take your dose if you do it at the same time every day, such as with breakfast or dinner, or at bedtime. Do not take more than one dose of amlodipine besylate at a time.
- If you miss a dose, take it as soon as you remember. Do not take amlodipine besylate if it has been more than 12 hours since you missed your last dose. Wait and take the next dose at your regular time.
- Other medicines: You can use nitroglycerin and amlodipine besylate together. If you take nitroglycerin for angina, don't stop taking it while you are taking amlodipine besylate.
- While you are taking amlodipine besylate, do not stop taking your other prescription medicines, including any other blood pressure medicines, without talking to your doctor.
- If you took too much amlodipine besylate, call your doctor or Poison Control Center, or go to the nearest hospital emergency room right away.
What should I avoid while taking amlodipine besylate?
- Do not start any new prescription or non-prescription medicines or supplements, unless you check with your doctor first.
What are the possible side effects of amlodipine besylate?
Amlodipine besylate may cause the following side effects. Most side effects are mild or moderate:
- swelling of your legs or ankles
- tiredness, extreme sleepiness
- stomach pain, nausea
- dizziness
- flushing (hot or warm feeling in your face)
- arrhythmia (irregular heartbeat)
- heart palpitations (very fast heartbeat)
- muscle rigidity, tremor and/or abnormal muscle movement
It is rare, but when you first start taking amlodipine besylate or increase your dose, you may have a heart attack or your angina may get worse. If that happens, call your doctor right away or go directly to a hospital emergency room.
Tell your doctor if you are concerned about any side effects you experience. These are not all the possible side effects of amlodipine besylate. For a complete list, ask your doctor or pharmacist.
How do I store amlodipine besylate tablets, USP?
Keep amlodipine besylate tablets, USP away from children. Store amlodipine besylate tablets, USP at 20° to 25°C (68° to 77°F), excursions permitted to 15° to 30°C (59° to 86°F) (see USP Controlled Room Temperature). Keep amlodipine besylate tablets, USP out of the light. Do not store in the bathroom. Keep amlodipine besylate tablets, USP in a dry place.
General advice about amlodipine besylate tablets, USP
Sometimes, doctors will prescribe a medicine for a condition that is not written in the patient information leaflets. Only use amlodipine besylate tablets, USP the way your doctor told you to. Do not give amlodipine besylate tablets, USP to other people, even if they have the same symptoms you have. It may harm them.
You can ask your pharmacist or doctor for information about amlodipine besylate tablets, USP.
Manufactured by:
Alkem Labloratories Ltd.,
INDIA.
Distributed by:
Ascend Laboratories, LLC
Parsippany, NJ 07054
Revised Date: October, 2021
PT1453-07
INGREDIENTS AND APPEARANCE
| AMLODIPINE BESYLATE
amlodipine besylate tablet |
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
| AMLODIPINE BESYLATE
amlodipine besylate tablet |
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
| AMLODIPINE BESYLATE
amlodipine besylate tablet |
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
| Labeler - Ascend Laboratories, LLC (141250469) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Alkem Laboratories Limited | 677605851 | MANUFACTURE(67877-197, 67877-198, 67877-199) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Alkem Laboratories Limited | 915628612 | MANUFACTURE(67877-197, 67877-198, 67877-199) | |