Search by Drug Name or NDC
NDC 68682-0023-90 Methazolamide 50 mg/1 Details
Methazolamide 50 mg/1
Methazolamide is a ORAL TABLET in the HUMAN PRESCRIPTION DRUG category. It is labeled and distributed by Oceanside Pharmaceuticals. The primary component is METHAZOLAMIDE.
MedlinePlus Drug Summary
Methazolamide is used to treat glaucoma (a condition in which increased pressure in the eye can lead to gradual loss of vision). Methazolamide is in a class of medications called carbonic anhydrase inhibitors. It works by decreasing the pressure in the eye.
Related Packages: 68682-0023-90Last Updated: 12/01/2022
MedLinePlus Full Drug Details: Methazolamide
Product Information
| NDC | 68682-0023 |
|---|---|
| Product ID | 68682-023_0962cf33-b872-4006-82f8-8610567a07d6 |
| Associated GPIs | 37100030000305 |
| GCN Sequence Number | 008169 |
| GCN Sequence Number Description | methazolamide TABLET 50 MG ORAL |
| HIC3 | R1E |
| HIC3 Description | CARBONIC ANHYDRASE INHIBITORS |
| GCN | 34740 |
| HICL Sequence Number | 003643 |
| HICL Sequence Number Description | METHAZOLAMIDE |
| Brand/Generic | Generic |
| Proprietary Name | Methazolamide |
| Proprietary Name Suffix | n/a |
| Non-Proprietary Name | methazolamide |
| Product Type | HUMAN PRESCRIPTION DRUG |
| Dosage Form | TABLET |
| Route | ORAL |
| Active Ingredient Strength | 50 |
| Active Ingredient Units | mg/1 |
| Substance Name | METHAZOLAMIDE |
| Labeler Name | Oceanside Pharmaceuticals |
| Pharmaceutical Class | n/a |
| DEA Schedule | n/a |
| Marketing Category | ANDA |
| Application Number | ANDA207438 |
| Listing Certified Through | 2024-12-31 |
Package
Package Images

NDC 68682-0023-90 (68682002390)
| NDC Package Code | 68682-023-90 |
|---|---|
| Billing NDC | 68682002390 |
| Package | 90 TABLET in 1 BOTTLE (68682-023-90) |
| Marketing Start Date | 2020-10-02 |
| NDC Exclude Flag | N |
| Pricing Information | N/A |
Standard Product Labeling (SPL)/Prescribing Information SPL 0962cf33-b872-4006-82f8-8610567a07d6 Details
DESCRIPTION
Methazolamide USP, a sulfonamide derivative, is a white or faintly yellow, crystalline powder having a slight odor, soluble in dimethylformamide, slightly soluble in acetone, very slightly soluble in water and in alcohol. The chemical name for methazolamide is: N-[5-(aminosulfonyl)-3-methyl-1,3,4-thiadiazol-2(3H)-ylidene]-acetamide and it has the following structural formula:
Each tablet, for oral administration, contains 25 mg or 50 mg methazolamide USP. In addition, each tablet contains the following inactive ingredients: colloidal silicon dioxide, croscarmellose sodium, dibasic calcium phosphate dihydrate, magnesium stearate, and microcrystalline cellulose.
CLINICAL PHARMACOLOGY
Methazolamide is a potent inhibitor of carbonic anhydrase.
Methazolamide is well absorbed from the gastrointestinal tract. Peak plasma concentrations are observed 1 to 2 hours after dosing. In a multiple-dose, pharmacokinetic study, administration of methazolamide 25 mg bid, 50 mg bid, and 100 mg bid demonstrated a linear relationship between plasma methazolamide levels and methazolamide dose. Peak plasma concentrations (Cmax) for the 25 mg, 50 mg and 100 mg bid regimens were 2.5 mcg/mL, 5.1 mcg/mL, and 10.7 mcg/mL, respectively. The area under the plasma concentration-time curves (AUC) was 1130 mcg.min/mL, 2571 mcg.min/mL, and 5418 mcg.min/mL for the 25 mg, 50 mg, and 100 mg dosage regimens, respectively.
Methazolamide is distributed throughout the body including the plasma, cerebrospinal fluid, aqueous humor of the eye, red blood cells, bile and extra-cellular fluid. The mean apparent volume of distribution (Varea/F) ranges from 17 L to 23 L. Approximately 55% is bound to plasma proteins. The steady-state methazolamide red blood cell:plasma ratio varies with dose and was found to be 27:1, 16:1, and 10:1 following the administration of methazolamide 25 mg bid, 50 mg bid, and 100 mg bid, respectively.
The mean steady-state plasma elimination half-life for methazolamide is approximately 14 hours. At steady-state, approximately 25% of the dose is recovered unchanged in the urine over the dosing interval. Renal clearance accounts for 20% to 25% of the total clearance of drug. After repeated bid-tid dosing, methazolamide accumulates to steady-state concentrations in 7 days.
Methazolamide’s inhibitory action on carbonic anhydrase decreases the secretion of aqueous humor and results in a decrease in intraocular pressure. The onset of the decrease in intraocular pressure generally occurs within 2 to 4 hours, has a peak effect in 6 to 8 hours and a total duration of 10 to 18 hours.
Methazolamide is a sulfonamide derivative; however, it does not have any clinically significant antimicrobial properties. Although methazolamide achieves a high concentration in the cerebrospinal fluid, it is not considered an effective anticonvulsant.
Methazolamide has a weak and transient diuretic effect; therefore, use results in an increase in urinary volume, with excretion of sodium, potassium, and chloride. The drug should not be used as a diuretic. Inhibition of renal bicarbonate reabsorption produces an alkaline urine. Plasma bicarbonate decreases, and a relative, transient metabolic acidosis may occur due to a disequilibrium in carbon dioxide transport in the red cell. Urinary citrate excretion is decreased by approximately 40% after doses of 100 mg every 8 hours. Uric acid output has been shown to decrease 36% in the first 24 hour period.
INDICATIONS AND USAGE
Methazolamide tablets are indicated in the treatment of ocular conditions where lowering intraocular pressure is likely to be of therapeutic benefit, such as chronic open-angle glaucoma, secondary glaucoma, and preoperatively in acute angle-closure glaucoma where lowering the intraocular pressure is desired before surgery.
CONTRAINDICATIONS
Methazolamide tablets therapy is contraindicated in situations in which sodium and/or potassium serum levels are depressed, in cases of marked kidney or liver disease or dysfunction, in adrenal gland failure, and in hyperchloremic acidosis. In patients with cirrhosis, use may precipitate the development of hepatic encephalopathy.
Long-term administration of methazolamide is contraindicated in patients with angle-closure glaucoma, since organic closure of the angle may occur in spite of lowered intraocular pressure.
WARNINGS
Fatalities have occurred, although rarely, due to severe reactions to sulfonamides including Stevens-Johnson syndrome, toxic epidermal necrolysis, fulminant hepatic necrosis, agranulocytosis, aplastic anemia, and other blood dyscrasias. Hypersensitivity reactions may recur when a sulfonamide is readministered, irrespective of the route of administration.
If hypersensitivity or other serious reactions occur, the use of this drug should be discontinued.
Caution is advised for patients receiving high-dose aspirin and methazolamide concomitantly, as anorexia, tachypnea, lethargy, coma, and death have been reported with concomitant use of high-dose aspirin and carbonic anhydrase inhibitors.
PRECAUTIONS
General
Potassium excretion is increased initially upon administration of methazolamide and in patients with cirrhosis or hepatic insufficiency could precipitate a hepatic coma.
In patients with pulmonary obstruction or emphysema, where alveolar ventilation may be impaired, methazolamide should be used with caution because it may precipitate or aggravate acidosis.
Information for patients
Adverse reactions common to all sulfonamide derivatives may occur: anaphylaxis, fever, rash (including erythema multiforme, Stevens-Johnson syndrome, toxic epidermal necrolysis), crystalluria, renal calculus, bone marrow depression, thrombocytopenic purpura, hemolytic anemia, leukopenia, pancytopenia, and agranulocytosis. Precaution is advised for early detection of such reactions, and the drug should be discontinued and appropriate therapy instituted.
Caution is advised for patients receiving high-dose aspirin and methazolamide concomitantly.
Laboratory tests
To monitor for hematologic reactions common to all sulfonamides, it is recommended that a baseline CBC and platelet count be obtained on patients prior to initiating methazolamide therapy and at regular intervals during therapy. If significant changes occur, early discontinuance and institution of appropriate therapy are important. Periodic monitoring of serum electrolytes is also recommended.
Drug interactions
Methazolamide should be used with caution in patients on steroid therapy because of the potential for developing hypokalemia.
Caution is advised for patients receiving high-dose aspirin and methazolamide concomitantly, as anorexia, tachypnea, lethargy, coma and death have been reported with concomitant use of high-dose aspirin and carbonic anhydrase inhibitors (see WARNINGS).
Carcinogenesis, mutagenesis, impairment of fertility
Long-term studies in animals to evaluate the carcinogenic potential of methazolamide and its effect on fertility have not been conducted. Methazolamide was not mutagenic in the Ames bacterial test.
Pregnancy
Teratogenic effects
Pregnancy Category C
Methazolamide has been shown to be teratogenic (skeletal anomalies) in rats when given in doses approximately 40 times the human dose. There are no adequate and well controlled studies in pregnant women. Methazolamide should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
Nursing mothers
It is not known whether this drug is excreted in human milk. Because many drugs are excreted in human milk and because of the potential for serious adverse reactions in nursing infants from methazolamide, a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother.
ADVERSE REACTIONS
Adverse reactions, occurring most often early in therapy, include paresthesias, particularly a “tingling” feeling in the extremities; hearing dysfunction or tinnitus; fatigue; malaise; loss of appetite; taste alteration; gastrointestinal disturbances such as nausea, vomiting, and diarrhea; polyuria; and occasional instances of drowsiness and confusion.
Metabolic acidosis and electrolyte imbalance may occur.
Transient myopia has been reported. This condition invariably subsides upon diminution or discontinuance of the medication.
Other occasional adverse reactions include urticaria, melena, hematuria, glycosuria, hepatic insufficiency, flaccid paralysis, photosensitivity, convulsions, and, rarely, crystalluria and renal calculi. Also see PRECAUTIONS: Information for patients for possible reactions common to sulfonamide derivatives. Fatalities have occurred, although rarely, due to severe reactions to sulfonamides including Stevens-Johnson syndrome, toxic epidermal necrolysis, fulminant hepatic necrosis, agranulocytosis, aplastic anemia, and other blood dyscrasias (see WARNINGS).
To report SUSPECTED ADVERSE REACTIONS, contact Oceanside Pharmaceuticals at 1-800-321-4576 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
OVERDOSAGE
No data are available regarding methazolamide overdosage in humans as no cases of acute poisoning with this drug have been reported. Animal data suggest that even a high dose of methazolamide is nontoxic. No specific antidote is known. Treatment should be symptomatic and supportive.
Electrolyte imbalance, development of an acidotic state, and central nervous system effects might be expected to occur. Serum electrolyte levels (particularly potassium) and blood pH levels should be monitored.
Supportive measures may be required to restore electrolyte and pH balance.
DOSAGE AND ADMINISTRATION
HOW SUPPLIED
Methazolamide Tablets USP 25 mg: White to off-white, circular, biconvex, uncoated tablet, debossed with “25” on one side and plain on the other side.
Bottles of 90 NDC 68682-022-90
Bottles of 100 NDC 68682-022-10
Bottles of 500 NDC 68682-022-50
Bottles of 1,000 NDC 68682-022-01
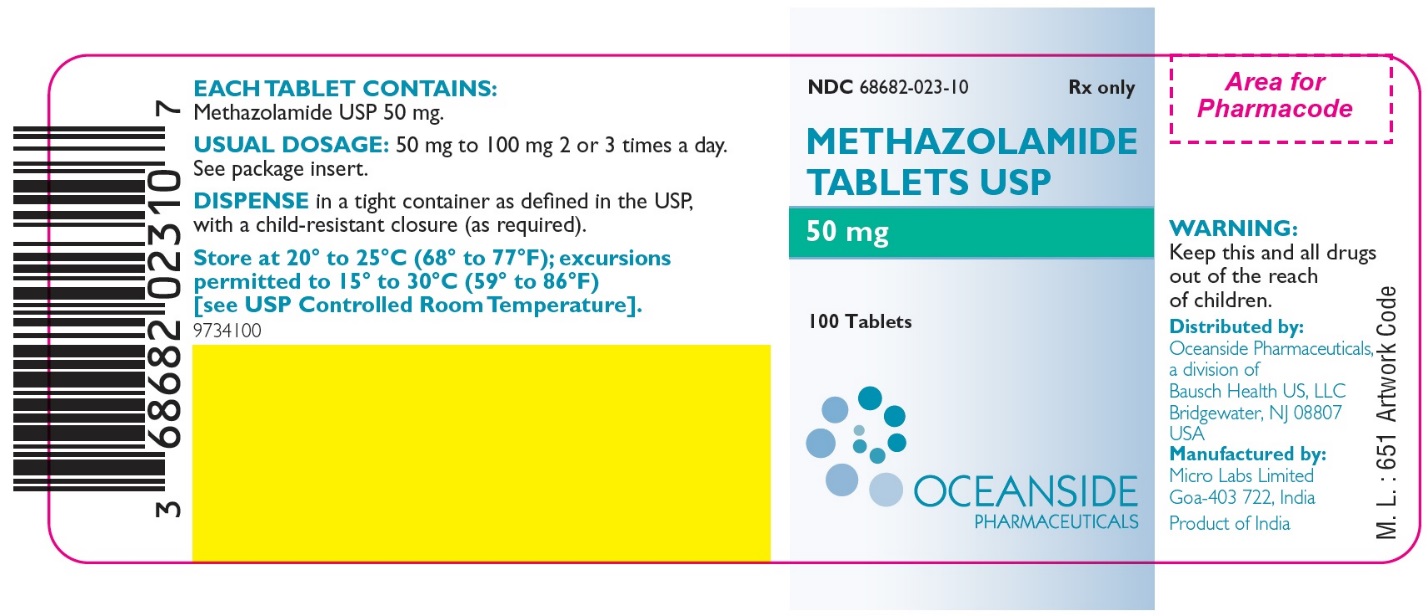
Methazolamide Tablets USP 50 mg: White to off-white, circular, biconvex, uncoated tablet, debossed with “50” on one side and score line on the other side.
Bottles of 90 NDC 68682-023-90
Bottles of 100 NDC 68682-023-10
Bottles of 500 NDC 68682-023-50
Bottles of 1,000 NDC 68682-023-01
Store at 20° to 25°C (68° to 77°F); excursions permitted to 15° to 30°C (59° to 86°F) [see USP Controlled Room Temperature].
Dispense in a tight container as defined in the USP, with a child-resistant closure (as required).
Distributed by:
Oceanside Pharmaceuticals, a division of
Bausch Health US, LLC
Bridgewater, NJ 08807 USA
Manufactured by:
Micro Labs Limited
Goa-403 722, India
© 2020 Bausch Health Companies Inc. or its affiliates
Issued: April 2020
9734301
PACKAGE/LABEL PRINCIPAL DISPLAY PANEL – 25 mg Label
Package/Label Display Panel – Label 50 mg
INGREDIENTS AND APPEARANCE
| METHAZOLAMIDE
methazolamide tablet |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| METHAZOLAMIDE
methazolamide tablet |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Labeler - Oceanside Pharmaceuticals (832011691) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Micro Labs Limited | 915793658 | MANUFACTURE(68682-022, 68682-023) | |
