Search by Drug Name or NDC
NDC 69097-0318-53 Budesonide 0.25 mg/2mL Details
Budesonide 0.25 mg/2mL
Budesonide is a ORAL INHALANT in the HUMAN PRESCRIPTION DRUG category. It is labeled and distributed by Cipla USA Inc.. The primary component is BUDESONIDE.
MedlinePlus Drug Summary
Budesonide is used to prevent difficulty breathing, chest tightness, wheezing, and coughing caused by asthma. Budesonide powder for oral inhalation (Pulmicort Flexhaler) is used in adults and children 6 years of age and older. Budesonide suspension (liquid) for oral inhalation (Pulmicort Respules) is used in children 12 months to 8 years of age. Budesonide belongs to a class of medications called corticosteroids. It works by decreasing swelling and irritation in the airways to allow for easier breathing.
Related Packages: 69097-0318-53Last Updated: 12/01/2022
MedLinePlus Full Drug Details: Budesonide Oral Inhalation
Product Information
| NDC | 69097-0318 |
|---|---|
| Product ID | 69097-318_87894b75-a8c5-4d48-81bd-5ad43d66afc3 |
| Associated GPIs | 44400015001830 |
| GCN Sequence Number | 046525 |
| GCN Sequence Number Description | budesonide AMPUL-NEB 0.25MG/2ML INHALATION |
| HIC3 | B6M |
| HIC3 Description | GLUCOCORTICOIDS, ORALLY INHALED |
| GCN | 17957 |
| HICL Sequence Number | 006545 |
| HICL Sequence Number Description | BUDESONIDE |
| Brand/Generic | Generic |
| Proprietary Name | Budesonide |
| Proprietary Name Suffix | Inhalation Suspension |
| Non-Proprietary Name | Budesonide |
| Product Type | HUMAN PRESCRIPTION DRUG |
| Dosage Form | INHALANT |
| Route | ORAL |
| Active Ingredient Strength | 0.25 |
| Active Ingredient Units | mg/2mL |
| Substance Name | BUDESONIDE |
| Labeler Name | Cipla USA Inc. |
| Pharmaceutical Class | Corticosteroid Hormone Receptor Agonists [MoA], Corticosteroid [EPC] |
| DEA Schedule | n/a |
| Marketing Category | ANDA |
| Application Number | ANDA205710 |
| Listing Certified Through | 2024-12-31 |
Package
Package Images












NDC 69097-0318-53 (69097031853)
| NDC Package Code | 69097-318-53 |
|---|---|
| Billing NDC | 69097031853 |
| Package | 30 POUCH in 1 CARTON (69097-318-53) / 1 AMPULE in 1 POUCH (69097-318-32) / 2 mL in 1 AMPULE |
| Marketing Start Date | 2020-10-15 |
| NDC Exclude Flag | N |
| Pricing Information | |
| Price Per Unit | 1.04496 |
| Pricing Unit | ML |
| Effective Date | 2024-02-21 |
| NDC Description | BUDESONIDE 0.25 MG/2 ML SUSP |
| Pharmacy Type Indicator | C/I |
| OTC | N |
| Explanation Code | 1, 5 |
| Classification for Rate Setting | G |
| As of Date | 2024-02-21 |
Standard Product Labeling (SPL)/Prescribing Information SPL c52c5382-1400-44b9-95be-a30e1cf706da Details
HIGHLIGHTS OF PRESCRIBING INFORMATION
BUDESONIDE inhalation suspension .
Initial U.S. Approval: 2000
INDICATIONS AND USAGE
DOSAGE AND ADMINISTRATION
Recommended dosing based on previous therapy (2). Start with the lowest recommended dose:
- Bronchodilators alone: 0.5 mg once daily or 0.25 mg twice daily
- Inhaled corticosteroids: 0.5 mg once daily or 0.25 mg twice daily up to 0.5 mg twice daily
- Oral corticosteroids: 0.5 mg twice daily or 1 mg once daily
- In symptomatic children not responding to non-steroidal therapy, a starting dose of 0.25 mg once daily may be considered
- If once-daily treatment does not provide adequate control, the total dailydose should be increased and/or administered as a divided dose. Once asthma stability is achieved, titrate the dose downwards.
- For inhalation use via compressed air driven jet nebulizers only (not for use with ultrasonic devices). Not for injection. (2.2)
DOSAGE FORMS AND STRENGTHS
Inhalation suspension: 0.25 mg/2mL, 0.5 mg/2mL, 1 mg/2mL (3)
CONTRAINDICATIONS
WARNINGS AND PRECAUTIONS
- Localized Infections : Candida albicans infection of the mouth and throat may occur. Monitor patients periodically for signs of adverse effects on the oral cavity. Advise patients to rinse the mouth following inhalation. (5.1)
- Deterioration of Disease and Acute Asthma Episodes : Do not use for the relief of acute bronchospasm. (5.2)
- Hypersensitivity Reactions: Anaphylaxis, rash, contact dermatitis, urticaria, angioedema, and bronchospasm have been reported with use of budesonide inhalation suspension. Discontinue budesonide inhalation suspension if such reactions occur (5.3)
- Immunosuppression : Potential worsening of infections (e.g., existing tuberculosis, fungal, bacterial, viral, or parasitic infection; or ocular herpes simplex). Use with caution in patients with these infections. More serious or even fatal course of chickenpox or measles can occur in susceptible patients. (5.4)
- Transferring Patients From Systemic Corticosteroids Therapy : Risk of impaired adrenal function when transferring from oral steroids. Taper patients slowly from systemic corticosteroids if transferring to budesonide inhalation suspension (5.5)
- Hypercorticism and Adrenal Suppression : May occur with very high dosages or at the regular dosage in susceptible individuals. If such changes occur, reduce budesonide inhalation suspension slowly. (5.6)
- Reduction in Bone Mineral Density with Long-Term Administration :
- Monitor patients with major risk factors for decreased bone mineral content. (5.7)
- Effects on Growth : Monitor growth of pediatric patients. (5.8)
- Glaucoma and Cataracts : Close monitoring is warranted. (5.9)
- Paradoxical Bronchospasm : Discontinue budesonide inhalation suspension and institute alternative therapy if paradoxical bronchospasm occurs. (5.10)
- Eosinophilic Conditions and Churg-Strauss Syndrome : Be alert to eosinophilic conditions. (5.11)
ADVERSE REACTIONS
Most common adverse reactions (incidence ≥3%) are respiratory infection, rhinitis, coughing, otitis media, viral infection, moniliasis, gastroenteritis, vomiting, diarrhea, abdominal pain, ear infection, epistaxis, conjunctivitis, rash (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Cipla Ltd 1-866-604-3268 or FDA at 1-800-FDA-1088 or FDA or www.fda.gov/medwatch.
DRUG INTERACTIONS
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 1/2022
Table Of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
1.1 Maintenance Treatment of Asthma
2 DOSAGE AND ADMINISTRATION
2.1 Dosing Recommendations
2.2 Directions for Use
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Local Effects
5.2 Deterioration of Disease and Acute Asthma Episodes
5.3 Hypersensitivity Reactions Including Anaphylaxis
5.4 Immunosuppression
5.5 Transferring Patients from Systemic Corticosteroid Therapy
5.6 Hypercorticism and Adrenal Suppression
5.7 Reduction in Bone Mineral Density
5.8 Effects on Growth
5.9 Glaucoma and Cataracts
5.10 Paradoxical Bronchospasm and Upper Airway Symptoms
5.11 Eosinophilic Conditions and Churg-Strauss Syndrome
5.12 Drug Interactions with Strong Cytochrome P450 3A4 Inhibitors
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Post-marketing Experience
7 DRUG INTERACTIONS
7.1 Inhibitors of Cytochrome P4503A4
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Hepatic Impairment
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
17.1 Administration with a Jet Nebulizer
17.2 Oral Candidiasis
17.3 Not for Acute Symptoms
17.4 Hypersensitivity Including Anaphylaxis
17.5 Immunosuppression
17.6 Hypercorticism and Adrenal Suppression
17.7 Reduction in Bone Mineral Density
17.8 Reduced Growth Velocity
17.9 Ocular Effects
17.10 Use Daily
- *
- Sections or subsections omitted from the full prescribing information are not listed.
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
The recommended starting dose and highest recommended dose of budesonide inhalation suspension, based on prior asthma therapy, are listed in the following table.
| Previous Therapy
| Recommended Starting Dose
| Highest Recommended Dose
|
| Bronchodilators alone
| 0.5 mg total daily dose administered either once daily or twice daily in divided doses | 0.5 mg total daily dose |
| Inhaled Corticosteroids
| 0.5 mg total daily dose administered either once daily or twice daily in divided doses | 1 mg total daily dose |
| Oral Corticosteroids
| 1 mg total daily dose administered either as 0.5 mg twice daily or 1 mg once daily | 1 mg total daily dose |
2.1 Dosing Recommendations
Dosing recommendations based on previous therapy are as follows:
- Bronchodilators alone: 0.5 mg once daily or 0.25 mg twice daily
- Inhaled corticosteroids: 0.5 mg once daily or 0.25 mg twice daily up to 0.5 mg twice daily
- Oral corticosteroids: 0.5 mg twice daily or 1 mg once daily
In symptomatic children not responding to non-steroidal therapy, a starting dose of 0.25 mg once daily may be considered. If once-daily treatment does not provide adequate control, the total daily dose should be increased and/or administered as a divided dose. In all patients, it is desirable to downward-titrate to the lowest effective dose once asthma stability is achieved.
2.2 Directions for Use
Budesonide inhalation suspension should be administered via jet nebulizer connected to an air compressor with an adequate air flow, equipped with a mouthpiece or suitable face mask. Ultrasonic nebulizers are not suitable for the adequate administration of budesonide inhalation suspension and, therefore, are NOT recommended.
The effects of mixing budesonide inhalation suspension with other nebulizable medications have not been adequately assessed. Budesonide inhalation suspension should be administered separately in the nebulizer [see Patient Counseling Information(17.1)].
A Pari-LC-Jet Plus Nebulizer (with face mask or mouthpiece) connected to a Pari Master compressor was used to deliver budesonide inhalation suspension to each patient in 3 U.S. controlled clinical studies. The safety and efficacy of budesonide inhalation suspension delivered by other nebulizers and compressors have not been established.
3 DOSAGE FORMS AND STRENGTHS
Budesonide inhalation suspension is available in three strengths, each containing 2 mL: 0.25 mg/2 mL, 0.5 mg/2 mL, and 1 mg/2 mL. Budesonide inhalation suspension is supplied in sealed aluminum foil envelopes containing one plastic strip of either one single-dose ampule or five single-dose ampules together with patient instructions for use. There are 30 ampules in a carton. Each single-dose ampule contains 2 mL of sterile liquid suspension.
4 CONTRAINDICATIONS
The use of budesonide inhalation suspension is contraindicated in the following conditions:
• Primary treatment of status asthmaticus or other acute episodes of asthma where intensive measures are required.
• Hypersensitivity to budesonide or any of the ingredients of budesonide inhalation suspension [see Warnings and Precautions (5.3), Description (11),Adverse Reactions(6.2)].
5 WARNINGS AND PRECAUTIONS
5.1 Local Effects
In clinical trials with budesonide inhalation suspension, localized infections with Candida albicans occurred in the mouth and pharynx in some patients. The incidences of localized infections of Candida albicans were similar between the placebo and budesonide inhalation suspension treatment groups. If these infections develop, they may require treatment with appropriate local or systemic antifungal therapy and/or discontinuance of treatment with budesonide inhalation suspension. Patients should rinse the mouth after inhalation of budesonide inhalation suspension.
5.2 Deterioration of Disease and Acute Asthma Episodes
Budesonide inhalation suspension is not a bronchodilator and is not indicated for the rapid relief of acute bronchospasm or other acute episodes of asthma.
Patients should be instructed to contact their physician immediately if episodes of asthma not responsive to their usual doses of bronchodilators occur during the course of treatment with budesonide inhalation suspension. During such episodes, patients may require therapy with oral corticosteroids.
5.3 Hypersensitivity Reactions Including Anaphylaxis
Hypersensitivity reactions including anaphylaxis, rash, contact dermatitis, urticaria, angioedema, and bronchospasm have been reported with use of budesonide inhalation suspension. Discontinue budesonide inhalation suspension if such reactions occur [see Contraindications (4)].
5.4 Immunosuppression
Patients who are on drugs that suppress the immune system are more susceptible to infection than healthy individuals. Chicken pox and measles, for example, can have a more serious or even fatal course in susceptible children or adults using corticosteroids. In children or adults who have not had these diseases, or been properly immunized, particular care should be taken to avoid exposure. How the dose, route, and duration of corticosteroid administration affect the risk of developing a disseminated infection is not known. The contribution of the underlying disease and/or prior corticosteroid treatment to the risk is also not known. If exposed to chicken pox, therapy with varicella zoster immune globulin (VZIG) or pooled intravenous immunoglobulin (IVIG), as appropriate, may be indicated. If exposed to measles, prophylaxis with pooled intramuscular immunoglobulin (IG) may be indicated. (See the respective package inserts for complete VZIG and IG prescribing information). If chicken pox develops, treatment with antiviral agents may be considered.
The clinical course of chicken pox or measles infection in patients on inhaled corticosteroids has not been studied. However, a clinical study has examined the immune responsiveness of asthma patients 12 months to 8 years of age who were treated with budesonide inhalation suspension. An open-label non-randomized clinical study examined the immune responsiveness of varicella vaccine in 243 asthma patients 12 months to 8 years of age who were treated with budesonide inhalation suspension 0.25 mg to 1 mg daily (n=151) or noncorticosteroid asthma therapy (n=92) (ie, beta2-agonists, leukotriene receptor antagonists, cromones). The percentage of patients developing a seroprotective antibody titer of ≥5.0 (gpELISA value) in response to the vaccination was similar in patients treated with budesonide inhalation suspension (85%) compared to patients treated with non-corticosteroid asthma therapy (90%). No patient treated with budesonide inhalation suspension developed chicken pox as a result of vaccination.
Inhaled corticosteroids should be used with caution, if at all, in patients with active or quiescent tuberculosis infection of the respiratory tract, untreated systemic fungal, bacterial, viral, or parasitic infections; or ocular herpes simplex.
5.5 Transferring Patients from Systemic Corticosteroid Therapy
Particular care is needed for patients who are transferred from systemically active corticosteroids to inhaled corticosteroids because deaths due to adrenal insufficiency have occurred in asthmatic patients during and after transfer from systemic corticosteroids to less systemically available inhaled corticosteroids. After withdrawal from systemic corticosteroids, a number of months are required for recovery of hypothalamic-pituitary-adrenal (HPA)-axis function.
Patients who have been previously maintained on 20 mg or more per day of prednisone (or its equivalent) may be most susceptible, particularly when their systemic corticosteroids have been almost completely withdrawn.
During this period of HPA-axis suppression, patients may exhibit signs and symptoms of adrenal insufficiency when exposed to trauma, surgery, infection (particularly gastroenteritis) or other conditions associated with severe electrolyte loss. Although budesonide inhalation suspension may provide control of asthma symptoms during these episodes, in recommended doses it supplies less than normal physiological amounts of glucocorticosteroid systemically and does NOT provide the mineralocorticoid activity that is necessary for coping with these emergencies.
During periods of stress or a severe asthma attack, patients who have been withdrawn from systemic corticosteroids should be instructed to resume oral corticosteroids (in large doses) immediately and to contact their physicians for further instructions. These patients should also be instructed to carry a medical identification card indicating that they may need supplementary systemic corticosteroids during periods of stress or a severe asthma attack.
Patients requiring oral corticosteroids should be weaned slowly from systemic corticosteroid use after transferring to budesonide inhalation suspension. Initially, budesonide inhalation suspension should be used concurrently with the patient's usual maintenance dose of systemic corticosteroid. After approximately one week, gradual withdrawal of the systemic corticosteroid may be initiated by reducing the daily or alternate daily dose. Further incremental reductions may be made after an interval of one or two weeks, depending on the response of the patient. Generally, these decrements should not exceed 25% of the prednisone dose or its equivalent. A slow rate of withdrawal is strongly recommended.
Lung function (FEV1 or AM PEF), beta-agonist use, and asthma symptoms should be carefully monitored during withdrawal of oral corticosteroids. In addition to monitoring asthma signs and symptoms, patients should be observed for signs and symptoms of adrenal insufficiency such as fatigue, lassitude, weakness, nausea and vomiting, and hypotension.
Transfer of patients from systemic corticosteroid therapy to budesonide inhalation suspension may unmask allergic or other immunologic conditions previously suppressed by the systemic corticosteroid therapy, e.g., rhinitis, conjunctivitis, eosinophilic conditions, eczema, and arthritis [see Dosage and Administration (2)].
During withdrawal from oral corticosteroids, patients may experience symptoms of systemically active corticosteroid withdrawal (e.g., joint and/or muscular pain, lassitude, depression) despite maintenance or even improvement of respiratory function.
5.6 Hypercorticism and Adrenal Suppression
Budesonide inhalation suspension, will often help control asthma symptoms with less suppression of HPA function than therapeutically equivalent oral doses of prednisone. Since individual sensitivity to effects on cortisol production exists, physicians should consider this information when prescribing budesonide inhalation suspension. Because of the possibility of systemic absorption of inhaled corticosteroids, patients treated with budesonide inhalation suspension should be observed carefully for any evidence of systemic corticosteroid effects. Particular care should be taken in observing patients post-operatively or during periods of stress for evidence of inadequate adrenal response. It is possible that systemic corticosteroid effects such as hypercorticism, and adrenal suppression (including adrenal crisis) may appear in a small number of patients, particularly when budesonide is administered at higher than recommended doses over prolonged periods of time. If such effects occur, the dosage of budesonide inhalation suspension should be reduced slowly, consistent with accepted procedures for tapering of systemic corticosteroids and for management of asthma.
5.7 Reduction in Bone Mineral Density
Decreases in bone mineral density (BMD) have been observed with long-term administration of products containing inhaled corticosteroids. The clinical significance of small changes in BMD with regard to long-term outcomes is unknown. Patients with major risk factors for decreased bone mineral content, such as prolonged immobilization, family history of osteoporosis, poor nutrition, or chronic use of drugs that can reduce bone mass (e.g., anticonvulsants and corticosteroids), should be monitored and treated with established standards of care.
5.8 Effects on Growth
Orally inhaled corticosteroids, including budesonide, may cause a reduction in growth velocity when administered to pediatric patients. Monitor the growth of pediatric patients receiving budesonide inhalation suspension routinely (e.g., via stadiometry). To minimize the systemic effects of orally inhaled corticosteroids, including budesonide inhalation suspension, each patient should be titrated to his/her lowest effective dose [see Use in Specific Populations (8.4)].
5.9 Glaucoma and Cataracts
Glaucoma, increased intraocular pressure, and cataracts have been reported following the long term administration of inhaled corticosteroids, including budesonide. Therefore, close monitoring is warranted in patients with a change in vision or with a history of increased intraocular pressure, glaucoma, and/or cataracts.
5.10 Paradoxical Bronchospasm and Upper Airway Symptoms
As with other inhaled asthma medications, bronchospasm, with an immediate increase in wheezing, may occur after dosing. If acute bronchospasm occurs following dosing with budesonide inhalation suspension, it should be treated immediately with a fast-acting inhaled bronchodilator. Treatment with budesonide inhalation suspension should be discontinued and alternate therapy instituted.
5.11 Eosinophilic Conditions and Churg-Strauss Syndrome
In rare cases, patients on inhaled corticosteroids may present with systemic eosinophilic conditions. Some of these patients have clinical features of vasculitis consistent with Churg- Strauss syndrome, a condition that is often treated with systemic corticosteroids therapy. These events usually, but not always, have been associated with the reduction and/or withdrawal of oral corticosteroid therapy following the introduction of inhaled corticosteroids. Healthcare providers should be alert to eosinophilia, vasculitis rash, worsening pulmonary symptoms, cardiac complications, and/or neuropathy presenting in their patients. A causal relationship between budesonide and these underlying conditions has not been established.
5.12 Drug Interactions with Strong Cytochrome P450 3A4 Inhibitors
Caution should be exercised when considering the coadministration of budesonide inhalation suspension with ketoconazole, and other known strong CYP3A4 inhibitors (e.g., ritonavir, atazanavir, clarithromycin, indinavir, itraconazole, nefazodone, nelfinavir, saquinavir, telithromycin) because adverse effects related to increased systemic exposure to budesonide may occur [see Drug Interactions (7.1), Clinical Pharmacology (12.3)].
6 ADVERSE REACTIONS
Systemic and inhaled corticosteroid use may result in the following:
• Candida albicans Infection [see Warnings and Precautions (5.1)]
• Hypersensitivity Reactions Including Anaphylaxis [see Warnings and Precautions (5.3)]
• Immunosuppression [see Warnings and Precautions (5.4)]
• Hypercorticism and Adrenal Suppression [see Warnings and Precautions (5.6)]
• Reduction in Bone Mineral Density [see Warnings and Precautions (5.7)]
• Growth Effects in Pediatric Patients [see Warnings and Precautions (5.8), Use in Specific Populations (8.4)]
• Glaucoma, Increased Intraocular Pressure and Cataracts [see Warnings and Precautions (5.9)]
• Eosinophilic Conditions and Churg-Strauss Syndrome [see Warnings and Precautions (5.11)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The incidence of common adverse reactions is based on three double-blind, placebo-controlled, randomized U.S. clinical trials in which 945 patients, 12 months to 8 years of age, (98 patients ≥12 months and <2 years of age; 225 patients ≥2 and <4 years of age; and 622 patients ≥4 and ≤8 years of age) were treated with budesonide inhalation suspension (0.25 to 1 mg total daily dose for 12 weeks) or vehicle placebo. The incidence and nature of adverse events reported for budesonide inhalation suspension was comparable to that reported for placebo. The following table shows the incidence of adverse events in U.S. controlled clinical trials, regardless of relationship to treatment, in patients previously receiving bronchodilators and/or inhaled corticosteroids. This population included a total of 605 male and 340 female patients and 78.4% were Caucasian, 13.8% African American, 5.5% Hispanic and 2.3% Other.
| Adverse Events | Vehicle Placebo (n=227) % | BUDESONIDE INHALATION SUSPENSION Total Daily Dose | ||
| 0.25 mg (n=178) % | 0.5 mg (n=223) % | 1 mg (n=317) % |
||
| Respiratory System Disorder
| ||||
| Respiratory Infection | 36 | 34 | 35 | 38 |
| Rhinitis | 9 | 7 | 11 | 12 |
| Coughing | 5 | 5 | 9 | 8 |
| Resistance Mechanism Disorders
| ||||
| Otitis Media | 11 | 12 | 11 | 9 |
| Viral Infection | 3 | 4 | 5 | 3 |
| Moniliasis | 2 | 4 | 3 | 4 |
| Gastrointestinal System Disorders
| ||||
| Gastroenteritis | 4 | 5 | 5 | 5 |
| Vomiting | 3 | 2 | 4 | 4 |
| Diarrhea | 2 | 4 | 4 | 2 |
| Abdominal Pain | 2 | 3 | 2 | 3 |
| Hearing and Vestibular Disorders
| ||||
| Ear Infection | 4 | 2 | 4 | 5 |
| Platelet, Bleeding and Clotting Disorders
| ||||
| Epistaxis | 1 | 2 | 4 | 3 |
| Vision Disorders
| ||||
| Conjunctivitis | 2 | <1 | 4 | 2 |
| Skin and Appendages Disorders
| ||||
| Rash | 3 | <1 | 4 | 2 |
The information below includes all adverse reactions by system organ class with an incidence of 1 to < 3%, in at least one budesonide inhalation suspension treatment group where the incidence was higher with budesonide inhalation suspension than with placebo, regardless of relationship to treatment.
Blood and lymphatic system disorders: cervical lymphadenopathy
Ear and labyrinth disorders: earache
General disorders and administration site conditions: fatigue, flu-like disorder
Immune system disorders: allergic reaction
Infections and infestations: eye infection, herpes simplex, external ear infection, infection
Injury, poisoning and procedural complication: fracture
Metabolism and nutrition disorders: anorexia
Musculoskeletal and connective tissue disorders: myalgia
Nervous system disorders: hyperkinesia
Psychiatric disorders: emotional lability
Respiratory, thoracic, and mediastinal disorders: chest pain, dysphonia, stridor
Skin and subcutaneous tissue disorders: contact dermatitis, eczema, pustular rash, pruritus, purpura
The incidence of reported adverse events was similar between the 447 budesonide inhalation suspension -treated (mean total daily dose 0.5 to 1 mg) and 223 conventional therapy-treated pediatric asthma patients followed for one year in three open-label studies.
6.2 Post-marketing Experience
The following adverse reactions have been reported during post-approval use of budesonide inhalation suspension. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure. Some of these adverse reactions may also have been observed in clinical studies with budesonide inhalation suspension.
Endocrine disorders: symptoms of hypocorticism and hypercorticism [see Warnings and Precautions (5.6)]
Eye disorders: cataracts, glaucoma, increased intraocular pressure [see Warnings and Precautions (5.9)]
General disorders and administration site conditions: fever, pain
Immune system disorders: immediate and delayed hypersensitivity reactions including, anaphylaxis, angioedema, bronchospasm, rash, contact dermatitis, and urticaria [see Contraindications (4), Warnings and Precautions (5.10)]
Infection and Infestation: sinusitis, pharyngitis, bronchitis
Musculoskeletal and connective tissue disorders: avascular necrosis of the femoral head, osteoporosis, growth suppression
Nervous system disorders: headache
Psychiatric disorders: psychiatric symptoms including psychosis, depression, aggressive reactions, irritability, nervousness, restlessness, and anxiety
Respiratory, thoracic, and mediastinal disorders: cough, dysphonia and throat irritation
Skin and subcutaneous tissue disorders: skin bruising, facial skin irritation
Cases of growth suppression have been reported for inhaled corticosteroids including post-marketing reports for budesonide inhalation suspension [see Warnings and Precautions (5.8), Use in Specific Populations (8.4)].
7 DRUG INTERACTIONS
7.1 Inhibitors of Cytochrome P4503A4
The main route of metabolism of corticosteroids, including budesonide, is via cytochrome P450 (CYP) isoenzyme 3A4 (CYP3A4). After oral administration of ketoconazole, a strong inhibitor of CYP3A4, the mean plasma concentration of orally administered budesonide increased. Concomitant administration of a CYP3A4 inhibitor may inhibit the metabolism of, and increase the systemic exposure to, budesonide. Caution should be exercised when considering the coadministration of budesonide inhalation suspension with long-term ketoconazole and other known strong CYP3A4 inhibitors (e.g., ritonavir, atazanavir, clarithromycin, indinavir, itraconazole, nefazodone, nelfinavir, saquinavir, telithromycin) [see Warnings and Precautions (5.12), Clinical Pharmacology (12.3)].
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
There are no adequate well-controlled studies of budesonide inhalation suspension in pregnant women. However, there are published studies on the use of budesonide, the active ingredient in budesonide inhalation suspension, in pregnant women. In animal reproduction studies, budesonide, administered by the subcutaneous route, caused structural abnormalities, was embryocidal, and reduced fetal weights in rats and rabbits at less than the maximum recommended human daily inhalation dose (MRHDID), but these effects were not seen in rats that received inhaled doses approximately 2 times the MRHDID (see Data). Studies of pregnant women have not shown that inhaled budesonide increases the risk of abnormalities when administered during pregnancy. Experience with oral corticosteroids suggests that rodents are more prone to structural abnormalities from corticosteroid exposure than humans.
The estimated background risk of major birth defects and miscarriage of the indicated populations is unknown. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively.
Clinical Considerations
Disease-Associated Maternal and/or Embryo/Fetal risk
In women with poorly or moderately controlled asthma, there is an increased risk of several perinatal adverse outcomes such as preeclampsia in the mother and prematurity, low birth weight, and small for gestational age in the neonate. Pregnant women with asthma should be closely monitored and medication adjusted as necessary to maintain optimal asthma control.
Labor or Delivery
There are no well-controlled human studies that have investigated the effects of budesonide inhalation suspension during labor and delivery.
Data
Human Data
Studies of pregnant women have not shown that inhaled budesonide increases the risk of abnormalities when administered during pregnancy. The results from a large population-based prospective cohort epidemiological study reviewing data from three Swedish registries covering approximately 99% of the pregnancies from 1995-1997 (i.e., Swedish Medical Birth Registry; Registry of Congenital Malformations; Child Cardiology Registry) indicate no increased risk for congenital malformations from the use of inhaled budesonide during early pregnancy. Congenital malformations were studied in 2014 infants born to mothers reporting the use of inhaled budesonide for asthma in early pregnancy (usually 1012 weeks after the last menstrual period), the period when most major organ malformations occur. The rate of recorded congenital malformations was similar compared to the general population rate (3.8% vs. 3.5%, respectively). In addition, after exposure to inhaled budesonide, the number of infants born with orofacial clefts was similar to the expected number in the normal population (4 children vs. 3.3, respectively).
These same data were utilized in a second study bringing the total to 2534 infants whose mothers were exposed to inhaled budesonide. In this study, the rate of congenital malformations among infants whose mothers were exposed to inhaled budesonide during early pregnancy was not different from the rate for all newborn babies during the same period (3.6%).
Animal Data
In a fertility and reproduction study, male rats were subcutaneously dosed for 9 weeks and females for 2 weeks prior to pairing and throughout the mating period. Females were dosed up until weaning of their offspring. Budesonide caused a decrease in prenatal viability and viability in the pups at birth and during lactation, along with a decrease in maternal body-weight gain, at doses 0.2 times the MRHDID (on a mcg/m2basis at maternal subcutaneous doses of 20 mcg/kg/day and above). No such effects were noted at a dose 0.05 times the MRHDID (on a mcg/m2basis at a maternal subcutaneous dose of 5 mcg/kg/day).
In an embryo-fetal development study in pregnant rabbits dosed during the period of organogenesis from gestation days 618, budesonide produced fetal loss, decreased fetal weight, and skeletal abnormalities at doses 0.5 times the MRHDID (on a mcg/m2basis at a maternal subcutaneous dose of 25 mcg/kg/day). In an embryo-fetal development study in pregnant rats dosed during the period of organogenesis from gestation days 6-15, budesonide produced similar adverse fetal effects at doses approximately 5 times the MRHDID (on a mcg/m2basis at a maternal subcutaneous dose of 500 mcg/kg/day). In another embryo-fetal development study in pregnant rats, no structural abnormalities or embryocidal effects were seen at doses approximately 2 times the MRHDID (on a mcg/m2basis at maternal inhalation doses up to 250 mcg/kg/day).
In a peri-and post-natal development study, rats dosed from gestation day 15 to postpartum day 21, budesonide had no effects on delivery, but did have an effect on growth and development of offspring. Offspring survival was reduced and surviving offspring had decreased mean body weights at birth and during lactation at doses less than 0.2 times the MRHDID and higher (on a mcg/m2basis at maternal subcutaneous doses of 20 mcg/kg/day and higher). These findings occurred in the presence of maternal toxicity.
8.2 Lactation
There are no available data on the effects of budesonide inhalation suspension on the breastfed child or on milk production. Budesonide, like other inhaled corticosteroids, is present in human milk [see Data]. The developmental and health benefits of breastfeeding should be considered along with the mother's clinical need for budesonide inhalation suspension and any potential adverse effects on the breastfed infant from budesonide inhalation suspension or from the underlying maternal condition.
Data
Human data with budesonide delivered via dry powder inhaler indicates that the total daily oral dose of budesonide available in breast milk to the infant is approximately 0.3% to 1% of the dose inhaled by the mother [see Clinical Pharmacology (12.3)].
8.4 Pediatric Use
Safety and effectiveness in children six months to 12 months of age has been evaluated but not established. Safety and effectiveness in children 12 months to 8 years of age have been established [see Clinical Pharmacology (12.2), Adverse Reactions, (6.1)].
A 12-week study in 141 pediatric patients 6 to 12 months of age with mild to moderate asthma or recurrent/persistent wheezing was conducted. All patients were randomized to receive either 0.5 mg or 1 mg of budesonide inhalation suspension or placebo once daily. Adrenal-axis function was assessed with an ACTH stimulation test at the beginning and end of the study, and mean changes from baseline in this variable did not indicate adrenal suppression in patients who received budesonide inhalation suspension versus placebo. However, on an individual basis, 7 patients in this study (6 in the budesonide inhalation suspension treatment arms and 1 in the placebo arm) experienced a shift from having a normal baseline stimulated cortisol level to having a subnormal level at Week 12 [see Clinical Pharmacology (12.2)]. Pneumonia was observed more frequently in patients treated with budesonide inhalation suspension than in patients treated with placebo, (N = 2, 1, and 0) in the budesonide inhalation suspension 0.5 mg, 1 mg, and placebo groups, respectively.
A dose dependent effect on growth was also noted in this 12-week trial. Infants in the placebo arm experienced an average growth of 3.7 cm over 12 weeks compared with 3.5 cm and 3.1 cm in the budesonide inhalation suspension 0.5 mg and 1 mg arms respectively. This corresponds to estimated mean (95% CI) reductions in 12-week growth velocity between placebo and budesonide inhalation suspension 0.5 mg of 0.2 cm (-0.6 to 1.0) and between placebo and budesonide inhalation suspension 1 mg of 0.6 cm (-0.2 to 1.4). These findings support that the use of budesonide inhalation suspension in infants 6 to 12 months of age may result in systemic effects and are consistent with findings of growth suppression in other studies with inhaled corticosteroids.
Controlled clinical studies have shown that inhaled corticosteroids may cause a reduction in growth velocity in pediatric patients. In these studies, the mean reduction in growth velocity was approximately one centimeter per year (range 0.3 to 1.8 cm per year) and appears to be related to dose and duration of exposure. This effect has been observed in the absence of laboratory evidence of hypothalamic-pituitary-adrenal (HPA)-axis suppression, suggesting that growth velocity is a more sensitive indicator of systemic corticosteroid exposure in pediatric patients than some commonly used tests of HPA-axis function. The long-term effects of this reduction in growth velocity associated with orally inhaled corticosteroids, including the impact on final adult height, are unknown. The potential for "catch up" growth following discontinuation of treatment with orally inhaled corticosteroids has not been adequately studied.
In a study of asthmatic children 5-12 years of age, those treated with budesonide administered via a dry powder inhaler 200 mcg twice daily (n=311) had a 1.1-centimeter reduction in growth compared with those receiving placebo (n=418) at the end of one year; the difference between these two treatment groups did not increase further over three years of additional treatment. By the end of four years, children treated with the budesonide dry powder inhaler and children treated with placebo had similar growth velocities. Conclusions drawn from this study may be confounded by the unequal use of corticosteroids in the treatment groups and inclusion of data from patients attaining puberty during the course of the study.
The growth of pediatric patients receiving inhaled corticosteroids, including budesonide inhalation suspension, should be monitored routinely (e.g., via stadiometry). The potential growth effects of prolonged treatment should be weighed against clinical benefits obtained and the risks and benefits associated with alternative therapies. To minimize the systemic effects of inhaled corticosteroids, including budesonide inhalation suspension, each patient should be titrated to his/her lowest effective dose [see Dosage and Administration (2), Warnings and Precautions (5.8)].
8.5 Geriatric Use
Of the 215 patients in 3 clinical trials of budesonide inhalation suspension in adult patients, 65 (30%) were 65 years of age or older, while 22 (10%) were 75 years of age or older. No overall differences in safety were observed between these patients and younger patients, and other reported clinical or medical surveillance experience has not identified differences in responses between the elderly and younger patients.
8.6 Hepatic Impairment
Formal pharmacokinetic studies using budesonide inhalation suspension have not been conducted in patients with hepatic impairment. However, since budesonide is predominantly cleared by hepatic metabolism, impairment of liver function may lead to accumulation of budesonide in plasma. Therefore, patients with hepatic disease should be closely monitored.
10 OVERDOSAGE
The potential for acute toxic effects following overdose of budesonide inhalation suspension is low. If inhaled corticosteroids are used at excessive doses for prolonged periods, systemic corticosteroid effects such as hypercorticism or growth suppression may occur [see Warnings and Precautions (5.6)].
11 DESCRIPTION
Budesonide, the active component of budesonide inhalation suspension, is a corticosteroid designated chemically as (RS)-11β, 16α, 17, 21-tetrahydroxypregna-1, 4-diene-3, 20-dione cyclic 16, 17-acetal with butyraldehyde. Budesonide is provided as a mixture of two epimers (22R and 22S). The empirical formula of budesonide is C25H34O6 and its molecular weight is 430.5. Its structural formula is:

Budesonide is a white to off-white, tasteless, odorless powder that is practically insoluble in water and in heptane, sparingly soluble in ethanol, and freely soluble in chloroform. Its partition coefficient between octanol and water at pH 7.4 is 1.6 x 103.
budesonide inhalation suspension is a sterile suspension for inhalation via jet nebulizer and contains the active ingredient budesonide (micronized), and the inactive ingredients disodium edetate, sodium chloride, sodium citrate dihydrate, citric acid monohydrate, polysorbate 80, and Water for Injection. Three dose strengths are available in single-dose ampules (ampules): 0.25 mg, 0.5 mg, and 1 mg per 2 mL ampule. For budesonide inhalation suspension, like all other nebulized treatments, the amount delivered to the lungs will depend on patient factors, the jet nebulizer utilized, and compressor performance. Using the Pari-LC-Jet Plus Nebulizer/Pari Master compressor system, under in vitro conditions, the mean delivered dose at the mouthpiece (% nominal dose) was approximately 17% at a mean flow rate of 5.5 L/min. The mean nebulization time was 5 minutes or less. budesonide inhalation suspension should be administered from jet nebulizers at adequate flow rates, via face masks or mouthpieces [see Dosage and Administration (2)].
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Budesonide is an anti-inflammatory corticosteroid that exhibits potent glucocorticoid activity and weak mineralocorticoid activity. In standard in vitro and animal models, budesonide has approximately a 200-fold higher affinity for the glucocorticoid receptor and a 1000-fold higher topical anti-inflammatory potency than cortisol (rat croton oil ear edema assay). As a measure of systemic activity, budesonide is 40 times more potent than cortisol when administered subcutaneously and 25 times more potent when administered orally in the rat thymus involution assay. The clinical significance of these findings is unknown.
The activity of budesonide inhalation suspension is due to the parent drug, budesonide. In glucocorticoid receptor affinity studies, the 22R form was two times as active as the 22S epimer. In vitro studies indicated that the two forms of budesonide do not interconvert.
The precise mechanism of corticosteroid actions on inflammation in asthma is not well known. Inflammation is an important component in the pathogenesis of asthma. Corticosteroids have been shown to have a wide range of inhibitory activities against multiple cell types (e.g., mast cells, eosinophils, neutrophils, macrophages, and lymphocytes) and mediators (e.g., histamine, eicosanoids, leukotrienes, and cytokines) involved in allergic- and non-allergic-mediated inflammation. The anti-inflammatory actions of corticosteroids may contribute to their efficacy in asthma.
Studies in asthmatic patients have shown a favorable ratio between topical anti-inflammatory activities and systemic corticosteroid effects over a wide dose range of inhaled budesonide in a variety of formulations and delivery systems including an inhalation-driven, multi-dose dry powder inhaler and the inhalation suspension for nebulization. This is explained by a combination of a relatively high local anti-inflammatory effect, extensive first pass hepatic degradation of orally absorbed drug (85-95%) and the low potency of metabolites (see below).
12.2 Pharmacodynamics
The therapeutic effects of conventional doses of orally inhaled budesonide are largely explained by its direct local action on the respiratory tract. To confirm that systemic absorption is not a significant factor in the clinical efficacy of inhaled budesonide, a clinical study in adult patients with asthma was performed comparing 400 mcg budesonide administered via a pressurized metered dose inhaler with a tube spacer to 1400 mcg of oral budesonide and placebo. The study demonstrated the efficacy of inhaled budesonide but not orally administered budesonide, even though systemic budesonide exposure was comparable for both treatments, indicating that the inhaled treatment is working locally in the lung. Thus, the therapeutic effect of conventional doses of orally inhaled budesonide are largely explained by its direct action on the respiratory tract.
Improvement in the control of asthma symptoms following inhalation of budesonide inhalation suspension can occur within 2-8 days of beginning treatment, although maximum benefit may not be achieved for 4-6 weeks.
Budesonide administered via a dry powder inhaler has been shown in various challenge models (including histamine, methacholine, sodium metabisulfite, and adenosine monophosphate) to decrease bronchial hyperresponsiveness in asthmatic patients. The clinical relevance of these models is not certain.
Pre-treatment with budesonide administered as 1600 mcg daily (800 mcg twice daily) via a dry powder inhaler for 2 weeks reduced the acute (early-phase reaction) and delayed (late-phase reaction) decrease in FEV1 following inhaled allergen challenge.
HPA Axis Effects
The effects of budesonide inhalation suspension on the hypothalamic-pituitary-adrenal (HPA) axis were studied in three, 12-week, double-blind, placebo-controlled studies in 293 pediatric patients, 6 months to 8 years of age, with persistent asthma. For most patients, the ability to increase cortisol production in response to stress, as assessed by the short cosyntropin (ACTH) stimulation test, remained intact with budesonide inhalation suspension treatment at recommended doses. In the subgroup of children age 6 months to 2 years (n=21) receiving a total daily dose of budesonide inhalation suspension equivalent to 0.25 mg (n=5), 0.5 mg (n=5), 1 mg (n=8), or placebo (n=3), the mean change from baseline in ACTH-stimulated cortisol levels showed a decline in peak stimulated cortisol at 12 weeks compared to an increase in the placebo group. These mean differences were not statistically significant compared to placebo. Another 12-week study in 141 pediatric patients 6 to 12 months of age with mild to moderate asthma or recurrent/persistent wheezing was conducted. All patients were randomized to receive either 0.5 mg or 1 mg of budesonide inhalation suspension or placebo once daily. A total of 28, 17, and 31 patients in the budesonide inhalation suspension 0.5 mg, 1 mg, and placebo arms respectively, had an evaluation of serum cortisol levels post-ACTH stimulation both at baseline and at the end of the study. The mean change from baseline to Week 12 ACTH-stimulated minus basal plasma cortisol levels did not indicate adrenal suppression in patients treated with budesonide inhalation suspension versus placebo. However, 7 patients in this study (4 of whom received budesonide inhalation suspension 0.5 mg, 2 of whom received budesonide inhalation suspension 1 mg and 1 of whom received placebo) showed a shift from normal baseline stimulated cortisol level (≥500 nmol/L) to a subnormal level (<500 nmol/L) at Week 12. In 4 of these patients receiving budesonide inhalation suspension, the cortisol values were near the cutoff value of 500 nmol/L.
The effects of budesonide inhalation suspension at doses of 0.5 mg twice daily, and 1 mg and 2 mg twice daily (2 times and 4 times the highest recommended total daily dose, respectively) on 24-hour urinary cortisol excretion were studied in 18 patients between 6 to 15 years of age with persistent asthma in a cross-over study design (4 weeks of treatment per dose level). There was a dose-related decrease in urinary cortisol excretion at 2 and 4 times the recommended daily dose. The two higher doses of budesonide inhalation suspension (1 and 2 mg twice daily) showed statistically significantly reduced (43-52%) urinary cortisol excretion compared to the run-in period. The highest recommended dose of budesonide inhalation suspension, 1 mg total daily dose, did not show statistically significantly reduced urinary cortisol excretion compared to the run-in period.
budesonide inhalation suspension, like other inhaled corticosteroid products, may impact the HPA axis, especially in susceptible individuals, in younger children, and in patients given high doses for prolonged periods [see Warnings and Precautions (5.5)].
12.3 Pharmacokinetics
In asthmatic children 4-6 years of age, the total absolute bioavailability (ie, lung + oral) following administration of budesonide inhalation suspension via jet nebulizer was approximately 6% of the labeled dose.
In children, a peak plasma concentration of 2.6 nmol/L was obtained approximately 20 minutes after nebulization of a 1 mg dose. Systemic exposure, as measured by AUC and Cmax, is similar for young children and adults after inhalation of the same dose of budesonide inhalation suspension.
Distribution:
In asthmatic children 4-6 years of age, the volume of distribution at steady-state of budesonide was 3 L/kg, approximately the same as in healthy adults. Budesonide is 85-90% bound to plasma proteins, the degree of binding being constant over the concentration range (1-100 nmol/L) achieved with, and exceeding, recommended doses. Budesonide showed little or no binding to corticosteroid-binding globulin. Budesonide rapidly equilibrated with red blood cells in a concentration independent manner with a blood/plasma ratio of about 0.8.
Metabolism:
In vitro studies with human liver homogenates have shown that budesonide is rapidly and extensively metabolized. Two major metabolites formed via cytochrome P450 (CYP) isoenzyme 3A4 (CYP3A4) catalyzed biotransformation have been isolated and identified as 16α-hydroxyprednisolone and 6β-hydroxybudesonide. The corticosteroid activity of each of these two metabolites is less than 1% of that of the parent compound. No qualitative difference between the in vitro and in vivo metabolic patterns has been detected. Negligible metabolic inactivation was observed in human lung and serum preparations.
Excretion/Elimination:
Budesonide is primarily cleared by the liver. Budesonide is excreted in urine and feces in the form of metabolites. In adults, approximately 60% of an intravenous radiolabeled dose was recovered in the urine. No unchanged budesonide was detected in the urine.
In asthmatic children 4-6 years of age, the terminal half-life of budesonide after nebulization is 2.3 hours, and the systemic clearance is 0.5 L/min, which is approximately 50% greater than in healthy adults after adjustment for differences in weight.
Special Populations:
No differences in pharmacokinetics due to race, gender, or age have been identified.
Hepatic Insufficiency:
Reduced liver function may affect the elimination of corticosteroids. The pharmacokinetics of budesonide were affected by compromised liver function as evidenced by a doubled systemic availability after oral ingestion. The intravenous pharmacokinetics of budesonide were, however, similar in cirrhotic patients and in healthy adults.
Nursing Mothers:
The disposition of budesonide when delivered by inhalation from a dry powder inhaler at doses of 200 or 400 mcg twice daily for at least 3 months was studied in eight lactating women with asthma from 1 to 6 months postpartum. Systemic exposure to budesonide in these women appears to be comparable to that in non-lactating women with asthma from other studies. Breast milk obtained over eight hours post-dose revealed that the maximum concentration of budesonide for the 400 and 800 mcg doses was 0.39 and 0.78 nmol/L, respectively, and occurred within 45 minutes after dosing. The estimated oral daily dose of budesonide from breast milk to the infant is approximately 0.007 and 0.014 mcg/kg/day for the two dose regimens used in this study, which represents approximately 0.3% to 1% of the dose inhaled by the mother. Budesonide levels in plasma samples obtained from five infants at about 90 minutes after breast-feeding (and about 140 minutes after drug administration to the mother) were below quantifiable levels (<0.02 nmol/L in four infants and <0.04 nmol/L in one infant) [see Use in Specific Populations (8.3)].
Drug-Drug Interactions
Inhibitors of cytochrome P450 enzymes
Ketoconazole: Ketoconazole, a strong inhibitor of cytochrome P450 (CYP) isoenzyme 3A4 (CYP3A4), the main metabolic enzyme for corticosteroids, increased plasma levels of orally ingested budesonide [see Warnings and Precautions (5.12),Drug Interactions (7.1)].
Cimetidine: At recommended doses, cimetidine, a nonspecific inhibitor of CYP enzymes, had a slight but clinically insignificant effect on the pharmacokinetics of oral budesonide.
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
In a two-year study in Sprague-Dawley rats, budesonide caused a statistically significant increase in the incidence of gliomas in male rats at an oral dose of 50 mcg/kg (approximately 0.5 and 0.1 times, respectively, the MRHDID in adults and children 12 months to 8 years of age on a mcg/m2 basis). No tumorigenicity was seen in male rats at oral doses up to 25 mcg/kg (approximately 0.2 and 0.04 times, respectively,MRHDID in adults and children 12 months to 8 years of age on a mcg/m2 basis) and in female rats at oral doses up to 50 mcg/kg (approximately 0.5 and 0.1 times, respectively, the MRHDID in adults and children 12 months to 8 years of age on a mcg/m2 basis). In two additional two-year studies in male Fischer and Sprague-Dawley rats, budesonide caused no gliomas at an oral dose of 50 mcg/kg (approximately 0.5 and 0.1 times, respectively, the MRHDID in adults and children 12 months to 8 years of age on a mcg/m2 basis). However, in the male Sprague-Dawley rats, budesonide caused a statistically significant increase in the incidence of hepatocellular tumors at an oral dose of 50 mcg/kg (approximately 0.5 and 0.1 times, respectively, the MRHDID in adults and children 12 months to 8 years of age on a mcg/m2 basis). The concurrent reference corticosteroids (prednisolone and triamcinolone acetonide) in these two studies showed similar findings.
In a 91-week study in mice, budesonide caused no treatment-related carcinogenicity at oral doses up to 200 mcg/kg (approximately equivalent to and 0.1 times, respectively, the MRHDID in adults and children 12 months to 8 years of age on a mcg/m2basis).
Budesonide was not mutagenic or clastogenic in six different test systems: Ames Salmonella/microsome plate test, mouse micronucleus test, mouse lymphoma test, chromosome aberration test in human lymphocytes, sex-linked recessive lethal test in Drosophila melanogaster, and DNA repair analysis in rat hepatocyte culture.
Fertility and reproductive performance were unaffected in rats at subcutaneous doses up to 80 mcg/kg approximately equivalent to the MRHDID in adults on a mcg/m2basis. However, it caused a decrease in prenatal viability and viability in the pups at birth and during lactation, along with a decrease in maternal body-weight gain, at subcutaneous doses of 20 mcg/kg and above approximately 0.2 times than the MRHDID in adults on a mcg/m2basis. No such effects were noted at 5 mcg/kg (approximately 0.05 times the MRHDID in adults on a mcg/m2basis).
14 CLINICAL STUDIES
Three double-blind, placebo-controlled, parallel group, randomized U.S. clinical trials of 12-weeks duration each were conducted in 1018 pediatric patients, 6 months to 8 years of age, 657 males and 361 females (798 Caucasians, 140 Blacks, 56 Hispanics, 3 Asians, 21 Others) with persistent asthma of varying disease duration (2 to 107 months) and severity. Doses of 0.25 mg, 0.5 mg, and 1 mg administered either once or twice daily were compared to placebo to provide information about appropriate dosing to cover a range of asthma severity. A Pari-LC-Jet Plus Nebulizer (with a face mask or mouthpiece) connected to a Pari Master compressor was used to deliver budesonide inhalation suspension to patients in the 3 U.S. controlled clinical trials. The co-primary endpoints were nighttime and daytime asthma symptom scores (0-3 scale). Improvements were addressed in terms of the primary efficacy variables of changes from baseline to the double-blind treatment period in nighttime and daytime asthma symptom scores (scale 0-3) as recorded in the patient diaries. Baseline was defined as the mean of the last seven days prior to randomization. The double-blind treatment period was defined as the mean over 12 week treatment period. Each of the five doses discussed below were studied in one or two, but not all three of the U.S. studies.
Results of the 3 controlled clinical trials for recommended dosages of budesonide inhalation suspension (0.25 mg to 0.5 mg once or twice daily, or 1 mg once daily, up to a total daily dose of 1 mg) in 946 patients, 12 months to 8 years of age, are presented below. Statistically significant decreases in nighttime and daytime symptom scores of asthma were observed at budesonide inhalation suspension doses of 0.25 mg once daily (one study), 0.25 mg twice daily, and 0.5 mg twice daily compared to placebo. Use of budesonide inhalation suspension resulted in statistically significant decreases in either nighttime or daytime symptom scores, but not both, at doses of 1 mg once daily, and 0.5 mg once daily (one study). Symptom reduction in response to budesonide inhalation suspension occurred across gender and age. Statistically significant reductions in the need for bronchodilator therapy were also observed at all the doses of budesonide inhalation suspension studied.
Improvements in lung function were associated with budesonide inhalation suspension in the subgroup of patients capable of performing lung function testing. Statistically significant increases were seen in FEV1 [budesonide inhalation suspension 0.5 mg once daily and 1 mg once daily (one study); 0.5 mg twice daily] and morning PEF [budesonide inhalation suspension 1 mg once daily (one study); 0.25 mg twice daily; 0.5 mg twice daily] compared to placebo.
A numerical reduction in nighttime and daytime symptom scores (0-3 scale) of asthma was observed within 2-8 days, although maximum benefit was not achieved for 4-6 weeks after starting treatment. The reduction in nighttime and daytime asthma symptom scores was maintained throughout the 12 weeks of the double-blind trials.
Patients Not Receiving Inhaled Corticosteroid Therapy
The efficacy of budesonide inhalation suspension at doses of 0.25 mg, 0.5 mg, and 1 mg once daily was evaluated in 344 pediatric patients, 12 months to 8 years of age, with mild to moderate persistent asthma (mean baseline nighttime asthma symptom scores of the treatment groups ranged from 1.07 to 1.34) who were not well controlled by bronchodilators alone. The changes from baseline to Weeks 0-12 in nighttime asthma symptom scores are shown in Figure 1. Nighttime asthma symptom scores showed statistically significant decreases in the patients treated with budesonide inhalation suspension compared to placebo. Similar decreases were also observed for daytime asthma symptom scores.
Changes from baseline to the double-blind phase for the budesonide treatment groups compared to placebo were made using analysis of variance techniques. The model included terms for the respective changes from baseline as the dependent variable and terms for treatment, center and treatment by center interaction as exploratory variables (see Figures 1-3).
Figure 1: A 12-Week Trial in Pediatric Patients Not on Inhaled Corticosteroid Therapy Prior to Study Entry. Nighttime Asthma Change from Baseline

p-value – 0.25 mg: 0.001, 0.5mg: 0.010, 1.0 mg: 0.009
Patients Previously Maintained on Inhaled Corticosteroids
The efficacy of budesonide inhalation suspension at doses of 0.25 mg and 0.5 mg twice daily was evaluated in 133 pediatric asthma patients, 4 to 8 years of age, previously maintained on inhaled corticosteroids (mean FEV1 79.5% predicted; mean baseline nighttime asthma symptom scores of the treatment groups ranged from 1.04 to 1.18; mean baseline dose of beclomethasone dipropionate of 265 mcg/day, ranging between 42 to 1008 mcg/day; mean baseline dose of triamcinolone acetonide of 572 mcg/day, ranging between 200 to 1200 mcg/day). The changes from baseline to Weeks 0-12 in nighttime asthma symptom scores are shown in Figure 2. Nighttime asthma symptom scores showed statistically significant decreases in patients treated with budesonide inhalation suspension compared to placebo. Similar decreases were also observed for daytime asthma symptom scores.
Statistically significant increases in FEV1 compared to placebo were observed with budesonide inhalation suspension at a dose of 0.5 mg twice daily and in morning PEF for both doses (0.25 mg and 0.5 mg twice daily).
Figure 2: A 12-Week Trial in Pediatric Patients Previously Maintained on Inhaled Corticosteroid Therapy Prior to Study Entry. Nighttime Asthma Change from Baseline
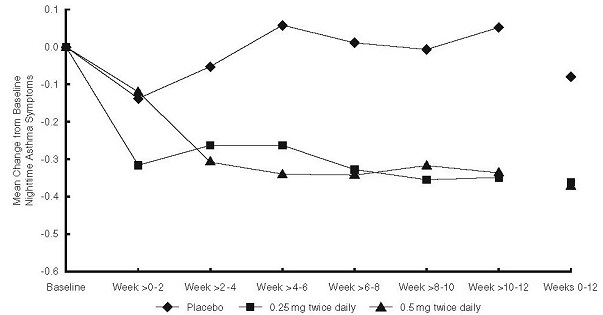
p-values: 0.25 mg: 0.022, 0.5 mg: 0.021
Patients Receiving Once-Daily or Twice-Daily Dosing
The efficacy of budesonide inhalation suspension at doses of 0.25 mg once daily, 0.25 mg twice daily, 0.5 mg twice daily, and 1 mg once daily, was evaluated in 469 pediatric patients 12 months to 8 years of age (mean baseline nighttime asthma symptom scores of the treatment groups ranged from 1.13 to 1.31). Approximately 70% were not previously receiving inhaled corticosteroids. The changes from baseline to Weeks 0-12 in nighttime asthma symptom scores are shown in Figure 3. budesonide inhalation suspension at doses of 0.25 mg and 0.5 mg twice daily, and 1 mg once daily, demonstrated statistically significant decreases in nighttime asthma symptom scores compared to placebo. Similar decreases were also observed for daytime asthma symptom scores.
budesonide inhalation suspension at a dose of 0.5 mg twice daily resulted in statistically significant increases compared to placebo in FEV1, and at doses of 0.25 mg and 0.5 mg twice daily and 1 mg once daily statistically significant increases in morning PEF.
The evidence supports the efficacy of the same nominal dose of budesonide inhalation suspension administered on either a once-daily or twice-daily schedule. However, when all measures are considered together, the evidence is stronger for twice-daily dosing [see Dosage and Administration (2)].
Figure 3: A 12-Week Trial in Pediatric Patients Either Maintained on Bronchodilators Alone or Inhaled Corticosteroid Therapy Prior to Study Entry. Nighttime Asthma Change from Baseline

p-values: 0.25 mg qd: 0.121, 0.25 mg bid: <0.001, 0.5 mg bid: 0.003, 1.0 mg qd: 0.005
16 HOW SUPPLIED/STORAGE AND HANDLING
Budesonide inhalation suspension is supplied in sealed aluminum foil envelope containing one plastic strip of either one single-dose ampule or five single-dose ampules together with patient instructions for use. There are 30 ampules in a carton. Each single-dose ampule contains 2 mL of sterile liquid suspension
| 1 ampule in foil envelope |
||
| Budesonide Inhalation Suspension, 0.25 mg/2 mL | 1 ampule in foil envelope 30 ampules per carton | 69097-318-32 69097-318-53 |
| Budesonide Inhalation Suspension, 0.5 mg/2 mL | 1 ampule in foil envelope 30 ampules per carton | 69097-319-32 69097-319-53 |
| Budesonide Inhalation Suspension, 1 mg/2 mL | 1 ampule in foil envelope 30 ampules per carton | 69097-321-32 69097-321-53 |
| 5 ampules in foil envelope |
||
| Budesonide Inhalation Suspension, 0.25 mg/2 mL | 5 ampules in foil envelope 30 ampules per carton | 69097-318-86 69097-318-87 |
| Budesonide Inhalation Suspension, 0.5 mg/2 mL | 5 ampules in foil envelope 30 ampules per carton | 69097-319-86 69097-319-87 |
| Budesonide Inhalation Suspension, 1 mg/2 mL | 5 ampules in foil envelope 30 ampules per carton | 69097-321-86 69097-321-87 |
Budesonide inhalation suspension should be stored upright at controlled room temperature 20 to 25°C (68 to 77°F) [see USP], and protected from light. When an envelope has been opened, the shelf life of the unused ampules is 2 weeks when protected. After opening the aluminum foil envelope, the unused ampules should be returned to the aluminum foil envelope to protect them from light. Any opened ampule must be used promptly. Gently shake the ampule using a circular motion before use. Keep out of reach of children. Do not freeze.
17 PATIENT COUNSELING INFORMATION
17.1 Administration with a Jet Nebulizer
Patients should be advised that budesonide inhalation suspension should be administered with a jet nebulizer connected to a compressor with an adequate air flow, equipped with a mouthpiece or suitable face mask. Ultrasonic nebulizers are not suitable for the adequate administration of budesonide inhalation suspension and, therefore, are not recommended. The effects of mixing budesonide inhalation suspension with other nebulizable medications have not been adequately assessed. budesonide inhalation suspension should be administered separately in the nebulizer [see Dosage and Administration (2)].
17.2 Oral Candidiasis
Patients should be advised that localized infections with Candida albicans occurred in the mouth and pharynx in some patients. If oropharyngeal candidiasis develops, it should be treated with appropriate local or systemic (i.e. oral) antifungal therapy while still continuing therapy with budesonide inhalation suspension , but at times therapy with budesonide inhalation suspension may need to be temporarily interrupted under close medical supervision. Rinsing the mouth after inhalation is advised [see Warnings and Precautions (5.1)].
17.3 Not for Acute Symptoms
budesonide inhalation suspension is not meant to relieve acute asthma symptoms and extra doses should not be used for that purpose. Acute symptoms should be treated with an inhaled, short-acting beta2-agonist such as albuterol. (The healthcare professional should provide that patient with such medication and instruct the patient in how it should be used.) Patients should be instructed to notify their healthcare professional immediately if they experience any of the following:
- Decreasing effectiveness of inhaled, short-acting beta2-agonists
- Need for more inhalations than usual of inhaled, short- acting beta2-agonists
- Significant decrease in lung function as outlined by the physician
Patients should not stop therapy with budesonide inhalation suspension without physician/provider guidance since symptoms may recur after discontinuation [see Warnings and Precautions (5.2)].
17.4 Hypersensitivity Including Anaphylaxis
Hypersensitivity reactions including anaphylaxis, rash, contact dermatitis, urticaria, angioedema, and bronchospasm have been reported with use of budesonide inhalation suspension. Discontinue budesonide inhalation suspension if such reactions occur [see Contraindications (4); Warning and Precautions (5.3)].
17.5 Immunosuppression
Patients who are on immunosuppressant doses of corticosteroids should be warned to avoid exposure to chickenpox or measles and, if exposed, to consult their physician without delay. If exposure to such a person occurs, and the child has not had chicken pox or been properly vaccinated, a physician should be consulted without delay. Patients should be informed of potential worsening of existing tuberculosis, fungal, bacterial, viral, or parasitic infections, or ocular herpes simplex [see Warnings and Precautions (5.4)].
17.6 Hypercorticism and Adrenal Suppression
Patients should be advised that budesonide inhalation suspension may cause systemic corticosteroid effects of hypercorticism and adrenal suppression. Additionally, patients should be instructed that deaths due to adrenal insufficiency have occurred during and after transfer from systemic corticosteroids. Patients should taper slowly from systemic corticosteroids if transferring to budesonide inhalation suspension [see Warnings and Precautions (5.6)].
17.7 Reduction in Bone Mineral Density
Patients who are at an increased risk for decreased BMD should be advised that the use of corticosteroids may pose an additional risk [see Warnings and Precautions (5.7)] .
17.8 Reduced Growth Velocity
Patients should be informed that orally inhaled corticosteroids, including budesonide inhalation suspension, may cause a reduction in growth velocity when administered to pediatric patients. Healthcare professionals should closely follow the growth of children and adolescents taking corticosteroids by any route [see Warnings and Precautions (5.8)].
17.9 Ocular Effects
Long-term use of inhaled corticosteroids may increase the risk of some eye problems (cataracts or glaucoma); regular eye examinations should be considered [see Warnings and Precautions (5.9)].
17.10 Use Daily
Patients should be advised to use budesonide inhalation suspension at regular intervals once or twice a day, since its effectiveness depends on regular use. Maximum benefit may not be achieved for 4 to 6 weeks or longer after starting treatment. If symptoms do not improve in that time frame or if the condition worsens, patients should be instructed to contact their healthcare professional.
See accompanying Patient Information and Instructions for Use.
Manufactured By:
Cipla Ltd.
Indore SEZ, Pithampur, India
Manufactured for:
Cipla USA, Inc.
10 Independence Boulevard,
Suite 300, Warren, NJ 07059
Revised: 6/2021
SPL UNCLASSIFIED SECTION
Patient Information and Instructions for Use
BUDESONIDE inhalation suspension (bew DEH so nide)
2 mL ampules containing 0.25 mg, 0.5 mg, or 1 mg
Do not swallow.
Only use budesonide inhalation suspension with a jet nebulizer machine that is connected to an air compressor.
Do not use with an ultrasonic nebulizer.
Read the Patient Information that comes with budesonide inhalation suspension before your child starts using it and each time you get a refill. There may be new information. This information does not take the place of talking with your healthcare provider about your child's medical condition or treatment. If you have any questions about budesonide inhalation suspension, ask your healthcare provider or pharmacist.
What is BUDESONIDE inhalation suspension?
Budesonide inhalation suspension is an inhaled corticosteroid medicine. Budesonide inhalation suspension is a long-term maintenance medicine used to control and prevent asthma symptoms in children ages 12 months to 8 years.
Inhaled corticosteroids help to decrease inflammation in the lungs. Inflammation in the lungs can lead to asthma symptoms. Budesonide inhalation suspension helps reduce swelling and inflammation in the lungs, and helps keep the airways open to reduce asthma symptoms.
Budesonide inhalation suspension does not treat the sudden symptoms (wheezing, cough, shortness of breath, and chest pain or tightness) of an asthma attack. Always have a short-acting beta2-agonist medicine (rescue inhaler) with you to treat sudden symptoms. If your child does not have an inhaled, short-acting bronchodilator, ask your healthcare provider to have one prescribed for your child.
It is not known if budesonide inhalation suspension is safe or effective in children younger than 12 months or older than 8 years.
Who should not use budesonide inhalation suspension?
Do not use budesonide inhalation suspension:
• to treat sudden symptoms of asthma
• if your child is allergic to budesonide or any of the ingredients in budesonide inhalation suspension. See the end of this leaflet for a complete list of ingredients in budesonide inhalation suspension.
What should I tell my healthcare provider before using budesonide inhalation suspension?
Before your child uses budesonide inhalation suspension, tell your healthcare provider if your child:
• has an allergy. See the section "Who should not use budesonide inhalation suspension? There is a complete list of ingredients in budesonide inhalation suspension at the end of this leaflet.
• has or recently had chicken pox or measles, or has recently been near anyone with chicken pox or measles.
• has or had tuberculosis of the respiratory tract.
• has certain kinds of infections that have not been treated, including:
◦ fungal infections
◦ bacterial infections
◦ viral infections
◦ parasitic infections
◦ herpes simplex infection of the eye (ocular herpes simplex)
Budesonide inhalation suspension may not be right for children who have had any of these types of infections.
• has decreased bone mineral density (bone strength). Your child is at risk for decreased bone mineral density if he or she:
◦ is inactive for a long period of time
◦ has a family history of osteoporosis
◦ does not eat well (poor nutrition)
◦ takes bone thinning medicines (such as anticonvulsant medicines or corticosteroids) for a long time.
• has an eye problem such as increased pressure in the eye, glaucoma or cataracts.
• has liver problems
• is planning to have surgery.
• has any other medical conditions
• is pregnant or plans to become pregnant. It is not known if budesonide inhalation suspension will harm your unborn baby.
• is breast-feeding or plans to breast-feed. budesonide inhalation suspension can pass into breast milk. You and your healthcare provider should decide if you will use budesonide inhalation suspension or breast-feed.
Tell your healthcare provider about all the medicine your child takes, including prescription and non-prescription medicines, vitamins, and herbal supplements.
Using budesonide inhalation suspension with certain other medicines may affect each other causing side effects. Especially tell your healthcare provider if your child takes:
• corticosteroids
• anti-seizure medicine (anticonvulsants)
• medicines that suppress the immune system (immunosuppressant)
• ketoconazole (Nizoral)
• certain medicines that can affect how your liver breaks down medicine
Ask your healthcare provider or pharmacist for a list of these medicines, if you are not sure.
Know the medicines your child takes. Keep a list of them and show it to your healthcare provider and pharmacist when your child gets a new medicine.
How should I use budesonide inhalation suspension?
• Use budesonide inhalation suspension exactly as prescribed by your healthcare provider. Your child must use budesonide inhalation suspension regularly for it to work.
• budesonide inhalation suspension comes in three strengths. Your healthcare provider has prescribed the strength that is best for your child.
• Do not stop using budesonide inhalation suspension, and do not change your child's dose of budesonide inhalation suspension without talking to your healthcare provider.
• budesonide inhalation suspension is for inhaled use only. Use budesonide inhalation suspension with a jet nebulizer connected to an air compressor set up with a mouthpiece or face mask. Do not use an ultrasonic nebulizer to give budesonide inhalation suspension.
• Do not mix budesonide inhalation suspension with other nebulizer medicines. If your child uses another medicine by inhalation to treat asthma, talk with your healthcare provider for instructions on when to use the other medicine.
• If your child misses a dose, just give the next regularly scheduled dose when it is due. Do not use budesonide inhalation suspension more often than has been prescribed.
• Improvement in the control of asthma symptoms with budesonide inhalation suspension can occur within 2-8 days. It may take up to 4-6 weeks before maximum improvement is seen.
• Make sure your child always has a short-acting beta2-agonist medicine with him or her. Your child should use the short-acting beta2-agonist medicine for breathing problems between doses of budesonide inhalation suspension or if a sudden asthma attack happens. Call your healthcare provider right away if:
◦ the short-acting rescue medicine does not work as well for relieving asthma symptoms.
◦ your child needs to use the short-acting rescue medicines more often than usual.
◦ your child's breathing problems worsen with budesonide inhalation suspension
• Rinse your child's mouth with water and have him or her spit the water out after each budesonide inhalation suspension treatment. Do not swallow the water. This will lessen the chance of getting a fungal infection (thrush) in the mouth.
• If your child has used long-term corticosteroids and the dose is now being lowered or stopped, a warning card should be carried stating that your child may need corticosteroids during times of stress or during an asthma attack that does not get better with bronchodilator medicines.
• Your healthcare provider may check your child's blood, breathing and do eye exams while using budesonide inhalation suspension.
• Read the Patient Information and Instructions for Use at the end of this leaflet for detailed instructions about how to use budesonide inhalation suspension.
What are the possible side effects of budesonide inhalation suspension?
Budesonide inhalation suspension may cause serious side effects including:
• Thrush (candida), a fungal infection in your mouth and throat. Tell your healthcare provider if your child has any redness or white colored patches in the mouth or throat.
• Worsening of asthma or sudden asthma attacks.
• Allergic reactions. Tell your healthcare provider or get medical help right away if your child has:
◦ skin rash, redness or swelling
◦ severe itching
◦ swelling of the face, mouth and tongue
◦ trouble breathing or swallowing
◦ chest pain
◦ anxiety (feeling of doom)
• Immune system effects and a higher chance of infections. Your child is more likely to get infections when taking medicines that weaken the immune system. Symptoms of infection may include: fever, pain, aches, chills, feeling tired, nausea and vomiting. Tell your healthcare provider about any signs of infection while your child uses budesonide inhalation suspension.
• Adrenal insufficiency. Adrenal insufficiency is a condition in which the adrenal glands do not make enough steroid hormones. Symptoms of adrenal insufficiency include tiredness, weakness, nausea and vomiting, and low blood pressure.
• Decrease in bone mineral density (bone strength). Your healthcare provider may want to check your child for this during treatment with budesonide inhalation suspension.
• Slowed or delayed growth problems. Your child's healthcare provider may want to monitor your child's growth while using budesonide inhalation suspension.
• Eye problems, including glaucoma and cataracts. Your child's healthcare provider may suggest eye exams while using budesonide inhalation suspension.
• Increased wheezing right after taking budesonide inhalation suspension. Always have a fast-acting inhaled bronchodilator medicine with you to treat sudden wheezing.
Call your healthcare provider or get medical help right away if your child has any of the serious side effects listed above.
The most common side effects of budesonide inhalation suspension include:
• respiratory infections. Symptoms may include stuffy nose, sore nose and throat.
• runny nose
• cough
• viral infections
• viral irritation and inflammation of the stomach and intestine (gastroenteritis). Gastroenteritis symptoms may include: stomach area pain, diarrhea, nausea and vomiting, and loss of appetite.
• ear infections
• nosebleed
• pink eye (conjunctivitis)
• rash
Tell your healthcare provider if your child has any side effect that bothers him or her or that does not go away.
For more information, ask your healthcare provider or pharmacist.
Call your healthcare provider for medical advice about side effects. You may report side effects to Cipla Ltd. at 1-866-604-3268 or the FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
How should I store budesonide inhalation suspension?
• Store budesonide inhalation suspension in an upright position between 68°F to 77°F (20°C to 25°C).
• Keep budesonide inhalation suspension in the aluminium foil envelope to protect from light until ready to use.
• After a budesonide inhalation suspension ampule is opened it should be used right away.
• Budesonide inhalation suspension ampules can be stored for 2 weeks after opening the protective aluminium foil envelope.
• Throw away Budesonide inhalation suspension ampules if not used within 2 weeks of opening the protective aluminium foil envelope.
• Do not refrigerate or freeze.
Keep budesonide inhalation suspension and all medicines out of the reach of children.
General Information about budesonide inhalation suspension
Medicines are sometimes prescribed for conditions other than those listed in a Patient Information leaflet. Do not use budesonide inhalation suspension for a condition for which it was not prescribed. Do not give budesonide inhalation suspension to other people, even if they have the same symptoms that you have. It may harm them.
This Patient Information leaflet summarizes the most important information about budesonide inhalation suspension. If you would like more information, talk with your healthcare provider. You can ask your pharmacist or healthcare provider for information about budesonide inhalation suspension that is written for health professionals.
What are the ingredients in Budesonide inhalation suspension?
Active ingredient: budesonide
Inactive ingredients: disodium edetate, sodium chloride, sodium citrate dihydrate, citric acid monohydrate, polysorbate 80 and water for injection.
Patient Instructions for Use
Important: Budesonide inhalation suspension is only for use with a jet nebulizer machine. Make sure you know how to use your jet nebulizer machine before your child uses budesonide inhalation suspension.
Budesonide inhalation suspension is a liquid that is turned into a mist by a nebulizer and inhaled into the lungs.
The face mask should be properly adjusted to optimize delivery and to avoid exposing the eyes to the nebulized medication. Corticosteroid effects on the skin can be avoided if the face is washed after the use of a face mask.
| 1 ampule in foil envelope | 5 ampules in foil envelope |
1. Budesonide inhalation suspension come in a sealed protective aluminum foil envelop.
 | 1. Budesonide inhalation suspension come in a sealed protective aluminum foil envelope.
Figure 1(b)  |
2. Gently shake the budesonide inhalation suspension ampule using a circular motion as shown in Figure 2.
Figure 2

3. Hold the budesonide inhalation suspension ampule upright without squeezing the ampule and open by twisting off the top as shown in Figure 3.
Figure 3

4. Place the open end of the budesonide inhalation suspension ampule into the nebulizer cup (reservoir) and slowly squeeze all of the medicine from the ampule into the nebulizer medicine cup as shown in Figure 4. Throw away the empty ampule.
Figure 4

5. Use your jet nebulizer as directed.
Manufactured by:
Cipla Ltd.
Indore SEZ, Pithampur, India
Manufactured for:
Cipla USA, Inc.
10 Independence Boulevard,
Suite 300, Warren, NJ 07059
Revised: 6/2021
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
NDC 69097-318-32 Rx Only
Budesonide
Inhalation
Suspension
0.25 mg/2mL
FOR INHALATION ONLY.
Each single-dose ampule
delivers 2 mL of a sterile
suspension containing 0.25 mg
of micronized budesonide plus
citric acid monohydrate,
disodium edetate, polysorbate
80, sodium chloride, sodium
citrate dihydrate and water for
injection.
Usual Dosage: See package
insert. Shake gently using a
circular motion prior to use.
Once an ampule is opened,
use the contents immediately.
For use only in a jet nebulizer.
Do NOT use in an ultrasonic
nebulizer.
Store at 20°C to 25°C
(68°F to 77°F) [See USP
Controlled Room
Temperature]. Do not freeze.
Store unopened ampules in
the foil envelope placed
upright in the carton.
Protect from light.
1 envelope x one 2 mL
single-dose ampule
Cipla
Use as directed by a physician.
Follow the Patient Instructions for proper
use of budesonide inhalation suspension.
Keep out of reach of children.

NDC 69097-318-53 Rx ONLY
Budesonide
Inhalation
Suspension
0.25mg/2 mL
FOR INHALATION ONLY.
↑ STORE UPRIGHT ↑
30 x 2 mL single dose ampules, each in a foil pouch
Cipla

NDC 69097-319-32 Rx Only
Budesonide
Inhalation
Suspension
0.5 mg/2mL
FOR INHALATION ONLY.
Each single-dose ampule
delivers 2 mL of a sterile
suspension containing 0.5 mg
of micronized budesonide plus
citric acid monohydrate,
disodium edetate, polysorbate
80, sodium chloride, sodium
citrate dihydrate and water for
injection.
Usual Dosage: See package
insert. Shake gently using a
circular motion prior to use.
Once an ampule is opened,
use the contents immediately.
For use only in a jet nebulizer.
Do NOT use in an ultrasonic
nebulizer.
Store at 20°C to 25°C
(68°F to 77°F) [See USP
Controlled Room
Temperature]. Do not freeze.
Store unopened ampules in
the foil envelope placed
upright in the carton.
Protect from light.
1 envelope x one 2 mL
single-dose ampule
Cipla
0.5 mg/2 mL
Use as directed by a physician.
Follow the Patient Instructions for proper
use of budesonide inhalation suspension.
Keep out of reach of children.

NDC 69097-319-53 Rx Only
Budesonide
Inhalation Suspension
0.5 mg/2mL
FOR INHALATION ONLY.
↑ STORE UPRIGHT ↑
30 x 2 mL single dose ampules, each in a foil pouch.
Cipla

NDC 69097-321-32 Rx Only
Budesonide
Inhalation
Suspension
1 mg/2mL
FOR INHALATION ONLY.
Each single-dose ampule
delivers 2 mL of a sterile
suspension containing 1 mg
of micronized budesonide plus
citric acid monohydrate,
disodium edetate, polysorbate
80, sodium chloride, sodium
citrate dihydrate and water for
injection.
Usual Dosage: See package
insert. Shake gently using a
circular motion prior to use.
Once an ampule is opened,
use the contents immediately.
For use only in a jet nebulizer.
Do NOT use in an ultrasonic
nebulizer.
Store at 20°C to 25°C
(68°F to 77°F) [See USP
Controlled Room
Temperature]. Do not freeze.
Store unopened ampules in
the foil envelope placed
upright in the carton.
Protect from light.
1 envelope x one 2 mL
single-dose ampule
Cipla
0.5 mg/2 mL
Use as directed by a physician.
Follow the Patient Instructions for proper
use of budesonide inhalation suspension.
Keep out of reach of children.

NDC 69097-321-53 Rx Only
Budesonide
Inhalation Suspension
1 mg/2mL
FOR INHALATION ONLY.
↑ STORE UPRIGHT ↑
30 x 2 mL single dose ampules, each in a foil pouch.
Cipla

Budesonide
Inhalation
Suspension
0.25 mg/2 mL
FOR INHALATION ONLY.
1 envelope x five 2 mL
Single-dose ampules
Cipla

Budesonide
Inhalation
Suspension
0.25 mg/2 mL
FOR INHALATION ONLY.
↑ STORE UPRIGHT↑
30 single-dose
ampules per carton
Cipla

Budesonide
Inhalation
Suspension
0.5 mg/2 mL
FOR INHALATION ONLY.
1 envelope x five 2 mL
Single-dose ampules
Cipla

Budesonide
Inhalation
Suspension
0.5 mg/2 mL
FOR INHALATION ONLY.
↑ STORE UPRIGHT ↑
30 single-dose
ampules per carton
Cipla

Budesonide
Inhalation
Suspension
1 mg/2 mL
FOR INHALATION ONLY.
1 envelope x five 2 mL
Single-dose ampules
Cipla

Budesonide
Inhalation
Suspension
1 mg/2 mL
FOR INHALATION ONLY.
↑ STORE UPRIGHT ↑
30 single-dose
ampules per carton
Cipla

INGREDIENTS AND APPEARANCE
| BUDESONIDE
INHALATION SUSPENSION
budesonide inhalant |
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
| BUDESONIDE
INHALATION SUSPENSION
budesonide inhalant |
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
| BUDESONIDE
INHALATION SUSPENSION
budesonide inhalant |
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
| Labeler - Cipla USA Inc. (078719707) |
| Registrant - Cipla USA Inc. (078719707) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Cipla Ltd. Goa | 650072015 | MANUFACTURE(69097-318, 69097-319, 69097-321) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Cipla Ltd. Indore | 918596409 | MANUFACTURE(69097-318, 69097-319, 69097-321) | |