Search by Drug Name or NDC
NDC 69345-0200-01 Altitude Medical Hand Sanitizer 69.8 mL/100mL Details
Altitude Medical Hand Sanitizer 69.8 mL/100mL
Altitude Medical Hand Sanitizer is a TOPICAL GEL in the HUMAN OTC DRUG category. It is labeled and distributed by Altitude Medical. The primary component is ALCOHOL.
Product Information
| NDC | 69345-0200 |
|---|---|
| Product ID | 69345-200_83ad0fce-61bd-425f-ab57-02cab0c2a3bc |
| Associated GPIs | |
| GCN Sequence Number | n/a |
| GCN Sequence Number Description | n/a |
| HIC3 | n/a |
| HIC3 Description | n/a |
| GCN | n/a |
| HICL Sequence Number | n/a |
| HICL Sequence Number Description | n/a |
| Brand/Generic | n/a |
| Proprietary Name | Altitude Medical Hand Sanitizer |
| Proprietary Name Suffix | n/a |
| Non-Proprietary Name | Ethyl alcohol |
| Product Type | HUMAN OTC DRUG |
| Dosage Form | GEL |
| Route | TOPICAL |
| Active Ingredient Strength | 69.8 |
| Active Ingredient Units | mL/100mL |
| Substance Name | ALCOHOL |
| Labeler Name | Altitude Medical |
| Pharmaceutical Class | n/a |
| DEA Schedule | n/a |
| Marketing Category | OTC MONOGRAPH NOT FINAL |
| Application Number | part333E |
| Listing Certified Through | 2024-12-31 |
Package
Package Images
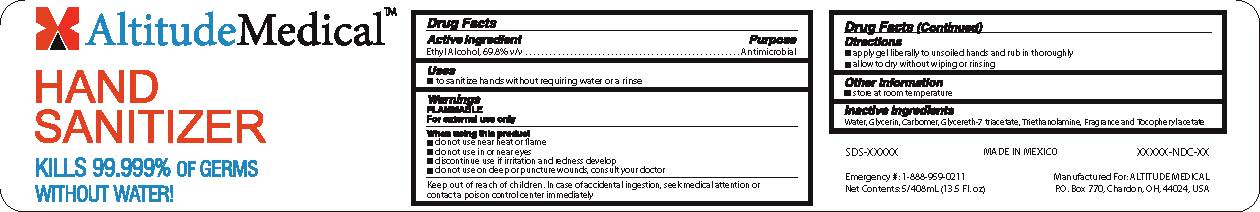




NDC 69345-0200-01 (69345020001)
| NDC Package Code | 69345-200-01 |
|---|---|
| Billing NDC | 69345020001 |
| Package | 5 BOTTLE in 1 BOX (69345-200-01) / 408 mL in 1 BOTTLE |
| Marketing Start Date | 2016-12-07 |
| NDC Exclude Flag | N |
| Pricing Information | N/A |
Standard Product Labeling (SPL)/Prescribing Information SPL b5621324-eff8-4af7-88d1-2250863093ca Details
Warnings
Directions
Inactive ingredients
PRINCIPAL DISPLAY PANEL
PRINCIPAL DISPLAY PANEL
PRINCIPAL DISPLAY PANEL
INGREDIENTS AND APPEARANCE
| ALTITUDE MEDICAL HAND SANITIZER
ethyl alcohol gel |
|||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||
| Labeler - Altitude Medical (963401380) |
| Registrant - CYAN Labs S.A. de C.V. (812754130) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| CYAN Labs S.A. de C.V. | 812754130 | manufacture(69345-200) , label(69345-200) , pack(69345-200) | |
Revised: 10/2018
Document Id: 83ad0fce-61bd-425f-ab57-02cab0c2a3bc
Set id: b5621324-eff8-4af7-88d1-2250863093ca
Version: 3
Effective Time: 20181018