Search by Drug Name or NDC
NDC 70000-0433-02 Leader Stomach Relief 262 mg/1 Details
Leader Stomach Relief 262 mg/1
Leader Stomach Relief is a ORAL TABLET, CHEWABLE in the HUMAN OTC DRUG category. It is labeled and distributed by CARDINAL HEALTH 110, LLC. DBA LEADER. The primary component is BISMUTH SUBSALICYLATE.
MedlinePlus Drug Summary
Bismuth subsalicylate is used to treat diarrhea, heartburn, and upset stomach in adults and children 12 years of age and older. Bismuth subsalicylate is in a class of medications called antidiarrheal agents. It works by decreasing the flow of fluids and electrolytes into the bowel, reduces inflammation within the intestine, and may kill the organisms that can cause diarrhea.
Related Packages: 70000-0433-02Last Updated: 12/01/2022
MedLinePlus Full Drug Details: Bismuth Subsalicylate
Product Information
| NDC | 70000-0433 |
|---|---|
| Product ID | 70000-0433_9df0c4ba-19dd-4c07-a1c1-9e9d376bf0f1 |
| Associated GPIs | 47300010000507 |
| GCN Sequence Number | 002860 |
| GCN Sequence Number Description | bismuth subsalicylate TAB CHEW 262 MG ORAL |
| HIC3 | D6D |
| HIC3 Description | ANTIDIARRHEALS |
| GCN | 08420 |
| HICL Sequence Number | 001246 |
| HICL Sequence Number Description | BISMUTH SUBSALICYLATE |
| Brand/Generic | Generic |
| Proprietary Name | Leader Stomach Relief |
| Proprietary Name Suffix | n/a |
| Non-Proprietary Name | Bismuth subsalicylate |
| Product Type | HUMAN OTC DRUG |
| Dosage Form | TABLET, CHEWABLE |
| Route | ORAL |
| Active Ingredient Strength | 262 |
| Active Ingredient Units | mg/1 |
| Substance Name | BISMUTH SUBSALICYLATE |
| Labeler Name | CARDINAL HEALTH 110, LLC. DBA LEADER |
| Pharmaceutical Class | n/a |
| DEA Schedule | n/a |
| Marketing Category | OTC MONOGRAPH FINAL |
| Application Number | part335 |
| Listing Certified Through | 2023-12-31 |
Package
Package Images
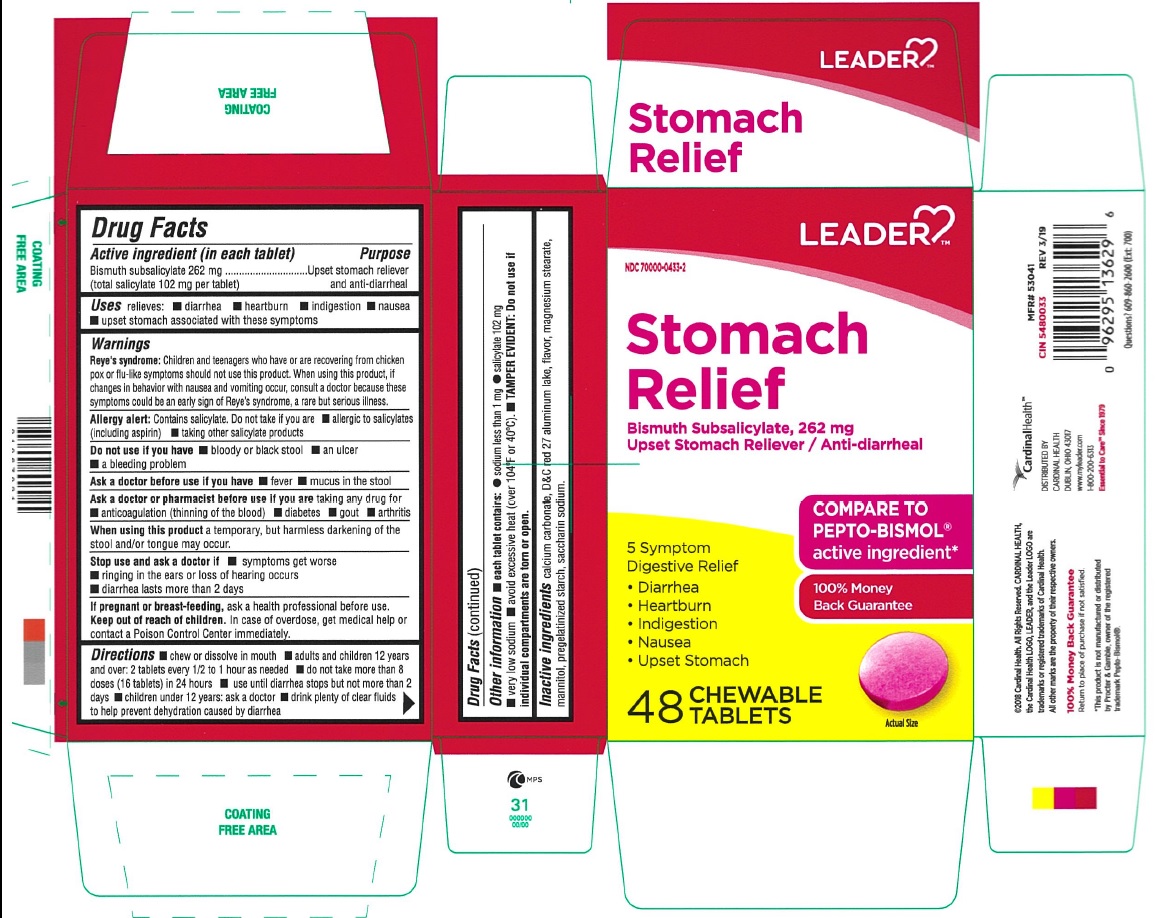
NDC 70000-0433-02 (70000043302)
| NDC Package Code | 70000-0433-2 |
|---|---|
| Billing NDC | 70000043302 |
| Package | 8 BLISTER PACK in 1 BOX (70000-0433-2) / 6 TABLET, CHEWABLE in 1 BLISTER PACK |
| Marketing Start Date | 2019-06-06 |
| NDC Exclude Flag | N |
| Pricing Information | |
| Price Per Unit | 0.07904 |
| Pricing Unit | EA |
| Effective Date | 2022-07-20 |
| NDC Description | STOMACH RELIEF 262 MG CHEW TAB |
| Pharmacy Type Indicator | C/I |
| OTC | Y |
| Explanation Code | 1 |
| Classification for Rate Setting | G |
| As of Date | 2022-08-03 |
Standard Product Labeling (SPL)/Prescribing Information SPL 91fbcda6-a068-4ed0-9502-caf395580ad9 Details
WARNINGS
Reye's Syndrome: Children and teenagers who have or are recovering from chicken pox or flu-like symptoms should not use this product. When using this product, if changes in behaviour with nausea and vomiting occur, consult a doctor because these symptoms could be an early sign of Reye's Syndrome, a rare but serious illness.
Allergy alert: Contains salicylate. Do not take if you are
- allergic to salicylates (including aspirin)
- taking other salicylate products
ASK A DOCTOR OR PHARMACIST BEFORE USE IF YOU ARE
STOP USE AND ASK DOCTOR IF
KEEP OUT OF REACH OF CHILDREN
DIRECTIONS
- chew or dissolve in mouth
- adults and children 12 years and over: 2 tablets every 1/2 to 1 hour as needed
- do not take more than 8 doses (16 tablets) in 24 hours
- use until diarrhea stops but not more than 2 days
- children under 12 years: ask a doctor
- drink plenty of fluids to help prevent dehydration which may accompany diarrhea
OTHER INFORMATION
INACTIVE INGREDIENTS
PRINCIPAL DISPLAY PANEL
INGREDIENTS AND APPEARANCE
| LEADER STOMACH RELIEF
bismuth subsalicylate tablet, chewable |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Labeler - CARDINAL HEALTH 110, LLC. DBA LEADER (063997360) |