Search by Drug Name or NDC
NDC 70369-0005-02 Calcium Carbonate 1250 mg/1 Details
Calcium Carbonate 1250 mg/1
Calcium Carbonate is a ORAL TABLET in the HUMAN OTC DRUG category. It is labeled and distributed by CitraGen Pharmaceuticals, Inc.. The primary component is CALCIUM CARBONATE.
MedlinePlus Drug Summary
Calcium carbonate is a dietary supplement used when the amount of calcium taken in the diet is not enough. Calcium is needed by the body for healthy bones, muscles, nervous system, and heart. Calcium carbonate also is used as an antacid to relieve heartburn, acid indigestion, and upset stomach. It is available with or without a prescription. This medication is sometimes prescribed for other uses; ask your doctor or pharmacist for more information.
Related Packages: 70369-0005-02Last Updated: 12/01/2022
MedLinePlus Full Drug Details: Calcium Carbonate
Product Information
| NDC | 70369-0005 |
|---|---|
| Product ID | 70369-005_b7c052ea-a1aa-043e-e053-2995a90acebf |
| Associated GPIs | |
| GCN Sequence Number | n/a |
| GCN Sequence Number Description | n/a |
| HIC3 | n/a |
| HIC3 Description | n/a |
| GCN | n/a |
| HICL Sequence Number | n/a |
| HICL Sequence Number Description | n/a |
| Brand/Generic | n/a |
| Proprietary Name | Calcium Carbonate |
| Proprietary Name Suffix | 1250 mg |
| Non-Proprietary Name | Calcium Carbonate |
| Product Type | HUMAN OTC DRUG |
| Dosage Form | TABLET |
| Route | ORAL |
| Active Ingredient Strength | 1250 |
| Active Ingredient Units | mg/1 |
| Substance Name | CALCIUM CARBONATE |
| Labeler Name | CitraGen Pharmaceuticals, Inc. |
| Pharmaceutical Class | Blood Coagulation Factor [EPC], Calcium [CS], Cations, Divalent [CS], Increased Coagulation Factor Activity [PE] |
| DEA Schedule | n/a |
| Marketing Category | OTC MONOGRAPH FINAL |
| Application Number | part331 |
| Listing Certified Through | 2024-12-31 |
Package
Package Images
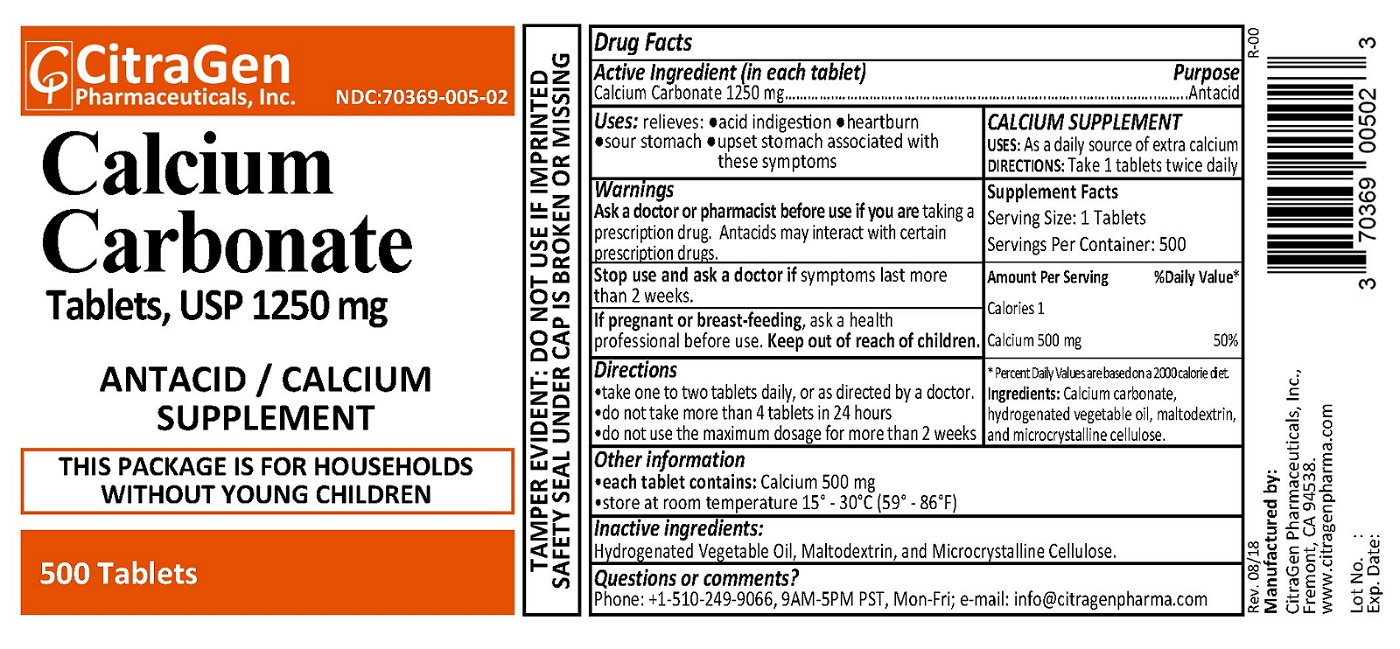

NDC 70369-0005-02 (70369000502)
| NDC Package Code | 70369-005-02 |
|---|---|
| Billing NDC | 70369000502 |
| Package | 500 TABLET in 1 BOTTLE (70369-005-02) |
| Marketing Start Date | 2018-09-26 |
| NDC Exclude Flag | N |
| Pricing Information | N/A |
Standard Product Labeling (SPL)/Prescribing Information SPL 766b3f3f-8bd1-281c-e053-2991aa0af54b Details
Warnings
Directions
Other information
Questions or comments?
SPL UNCLASSIFIED SECTION
PRINCIPAL DISPLAY PANEL
PRINCIPAL DISPLAY PANEL
INGREDIENTS AND APPEARANCE
| CALCIUM CARBONATE
1250 MG
calcium carbonate tablet |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - CitraGen Pharmaceuticals, Inc. (024949457) |
| Registrant - CitraGen Pharmaceuticals, Inc. (024949457) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| CitraGen Pharmaceuticals, Inc. | 024949457 | manufacture(70369-005) | |
Revised: 12/2020
Document Id: b7c052ea-a1aa-043e-e053-2995a90acebf
Set id: 766b3f3f-8bd1-281c-e053-2991aa0af54b
Version: 3
Effective Time: 20201231