Search by Drug Name or NDC
NDC 70512-0100-30 Clotrimazole Cream 1% 10 mg/g Details
Clotrimazole Cream 1% 10 mg/g
Clotrimazole Cream 1% is a TOPICAL CREAM in the HUMAN OTC DRUG category. It is labeled and distributed by SOLA Pharmaceuticals. The primary component is CLOTRIMAZOLE.
MedlinePlus Drug Summary
Topical clotrimazole is used to treat tinea corporis (ringworm; fungal skin infection that causes a red scaly rash on different parts of the body), tinea cruris (jock itch; fungal infection of the skin in the groin or buttocks), and tinea pedis (athlete's foot; fungal infection of the skin on the feet and between the toes). Clotrimazole is in a class of antifungal medications called imidazoles. It works by stopping the growth of fungi that cause infection.
Related Packages: 70512-0100-30Last Updated: 12/01/2022
MedLinePlus Full Drug Details: Clotrimazole Topical
Product Information
| NDC | 70512-0100 |
|---|---|
| Product ID | 70512-100_e314d3ba-c23e-39c0-e053-2995a90a7ca9 |
| Associated GPIs | 90154020003705 |
| GCN Sequence Number | 007361 |
| GCN Sequence Number Description | clotrimazole CREAM (G) 1 % TOPICAL |
| HIC3 | Q5F |
| HIC3 Description | TOPICAL ANTIFUNGALS |
| GCN | 30370 |
| HICL Sequence Number | 004137 |
| HICL Sequence Number Description | CLOTRIMAZOLE |
| Brand/Generic | Generic |
| Proprietary Name | Clotrimazole Cream 1% |
| Proprietary Name Suffix | n/a |
| Non-Proprietary Name | Clotrimazole Cream 1% |
| Product Type | HUMAN OTC DRUG |
| Dosage Form | CREAM |
| Route | TOPICAL |
| Active Ingredient Strength | 10 |
| Active Ingredient Units | mg/g |
| Substance Name | CLOTRIMAZOLE |
| Labeler Name | SOLA Pharmaceuticals |
| Pharmaceutical Class | Azole Antifungal [EPC], Azoles [CS] |
| DEA Schedule | n/a |
| Marketing Category | OTC MONOGRAPH FINAL |
| Application Number | part333C |
| Listing Certified Through | 2023-12-31 |
Package
Package Images
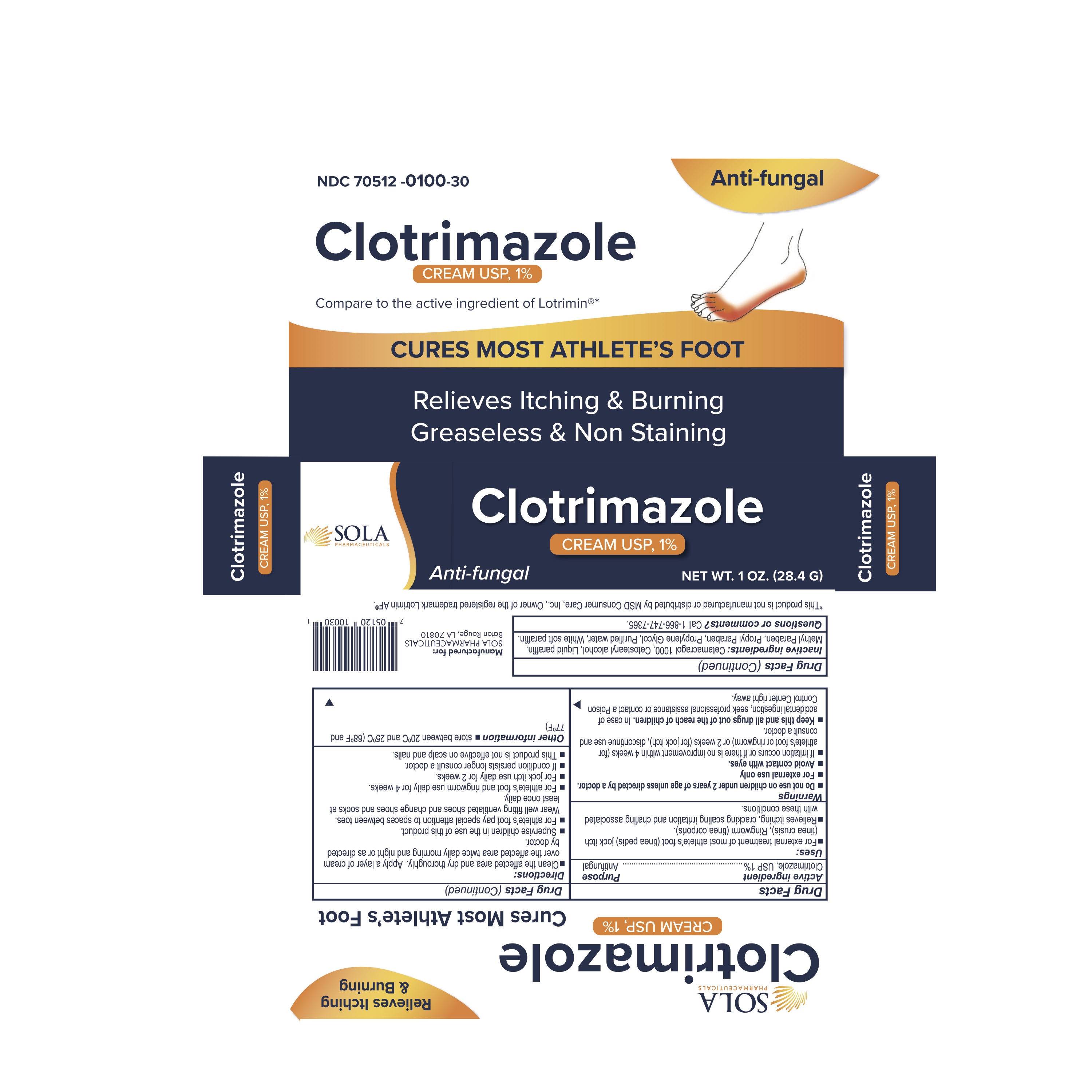



NDC 70512-0100-30 (70512010030)
| NDC Package Code | 70512-100-30 |
|---|---|
| Billing NDC | 70512010030 |
| Package | 1 TUBE in 1 CARTON (70512-100-30) / 30 g in 1 TUBE |
| Marketing Start Date | 2020-04-14 |
| NDC Exclude Flag | N |
| Pricing Information | |
| Price Per Unit | 0.09678 |
| Pricing Unit | GM |
| Effective Date | 2024-02-21 |
| NDC Description | CLOTRIMAZOLE 1% TOPICAL CREAM |
| Pharmacy Type Indicator | C/I |
| OTC | Y |
| Explanation Code | 1, 5 |
| Classification for Rate Setting | G |
| As of Date | 2024-02-21 |
Standard Product Labeling (SPL)/Prescribing Information SPL a3424c66-68ee-0631-e053-2995a90a3e7a Details
Usage
Warnings
Do not use on children under 2 years of age unless directed by a doctor.
For external use only.
Avoid contact with eyes.
If irritation occurs or if there is no improvement within 4 weeks (for athlete's foot or ringworm) or 2 weeks (for jock itch), discontinue use and consult a doctor.
Keep this and all drugs out of the reach of children. In case of accidental ingestion, seek professional assistance or contact a Poison Control Center right away.
SPL UNCLASSIFIED SECTION
Directions
- Clean the affected area and dry thoroughly. Apply a layer of cream over the affected area twice daily morning and night or as directed by doctor.
- Supervise children in the use of this product.
- For athlete's foot pay special attention to the spaces between toes. Wear well fitting ventilated shoes and change shoes and socks at least once daily.
- For athlete's foot and ringworm use daily for 4 weeks.
- For jock itch use daily for 2 weeks.
- If condition persist longer consult a doctor.
- This product is not effective on scalp or nails.
Inactive Ingredients
Other Information
Package Display Panel
INGREDIENTS AND APPEARANCE
| CLOTRIMAZOLE CREAM 1%
clotrimazole cream 1% cream |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Labeler - SOLA Pharmaceuticals (080121345) |