Search by Drug Name or NDC
NDC 70954-0017-40 Thiothixene 10 mg/1 Details
Thiothixene 10 mg/1
Thiothixene is a ORAL CAPSULE in the HUMAN PRESCRIPTION DRUG category. It is labeled and distributed by Novitium Pharma LLC. The primary component is THIOTHIXENE.
MedlinePlus Drug Summary
Thiothixene is used to treat the symptoms of schizophrenia (a mental illness that causes disturbed or unusual thinking, loss of interest in life, and strong or inappropriate emotions). Thiothixene is in a group of medications called conventional antipsychotics. It works by decreasing abnormal excitement in the brain.
Related Packages: 70954-0017-40Last Updated: 12/01/2022
MedLinePlus Full Drug Details: Thiothixene
Product Information
| NDC | 70954-0017 |
|---|---|
| Product ID | 70954-017_358d888a-c9fe-47ff-b2fb-1322de14eabc |
| Associated GPIs | 59300020100120 |
| GCN Sequence Number | 003996 |
| GCN Sequence Number Description | thiothixene CAPSULE 10 MG ORAL |
| HIC3 | H7P |
| HIC3 Description | ANTIPSYCHOTICS,DOPAMINE ANTAGONISTS, THIOXANTHENES |
| GCN | 15691 |
| HICL Sequence Number | 001668 |
| HICL Sequence Number Description | THIOTHIXENE |
| Brand/Generic | Generic |
| Proprietary Name | Thiothixene |
| Proprietary Name Suffix | n/a |
| Non-Proprietary Name | Thiothixene |
| Product Type | HUMAN PRESCRIPTION DRUG |
| Dosage Form | CAPSULE |
| Route | ORAL |
| Active Ingredient Strength | 10 |
| Active Ingredient Units | mg/1 |
| Substance Name | THIOTHIXENE |
| Labeler Name | Novitium Pharma LLC |
| Pharmaceutical Class | Typical Antipsychotic [EPC] |
| DEA Schedule | n/a |
| Marketing Category | ANDA |
| Application Number | ANDA211642 |
| Listing Certified Through | 2024-12-31 |
Package
Package Images














NDC 70954-0017-40 (70954001740)
| NDC Package Code | 70954-017-40 |
|---|---|
| Billing NDC | 70954001740 |
| Package | 100 BLISTER PACK in 1 CARTON (70954-017-40) / 1 CAPSULE in 1 BLISTER PACK (70954-017-30) |
| Marketing Start Date | 2019-04-05 |
| NDC Exclude Flag | N |
| Pricing Information | N/A |
Standard Product Labeling (SPL)/Prescribing Information SPL b067b400-66cd-422d-a631-9ce0c935d2b2 Details
WARNING
WARNING
Increased Mortality in Elderly Patients with Dementia-Related Psychosis: Elderly patients with dementia-related psychosis treated with antipsychotic drugs are at an increased risk of death. Analyses of seventeen placebo-controlled trials (modal duration of 10 weeks), largely in patients taking atypical antipsychotic drugs, revealed a risk of death in drug-treated patients of between 1.6 to 1.7 times the risk of death in placebo-treated patients. Over the course of a typical 10-week controlled trial, the rate of death in drug-treated patients was about 4.5%, compared to a rate of about 2.6% in the placebo group. Although the causes of death were varied, most of the deaths appeared to be either cardiovascular (e.g., heart failure, sudden death) or infectious (e.g., pneumonia) in nature. Observational studies suggest that, similar to atypical antipsychotic drugs, treatment with conventional antipsychotic drugs may increase mortality. The extent to which the findings of increased mortality in observational studies may be attributed to the antipsychotic drug as opposed to some characteristic(s) of the patients is not clear. Thiothixene is not approved for the treatment of patients with dementia-related psychosis (see WARNINGS).
DESCRIPTION
Thiothixene is a thioxanthene derivative. Specifically, it is the cis isomer of N,N-dimethyl-9-[3-(4- methyl-1-piperazinyl)propylidene]thioxanthene-2-sulfonamide. It may be represented by the following structural formula
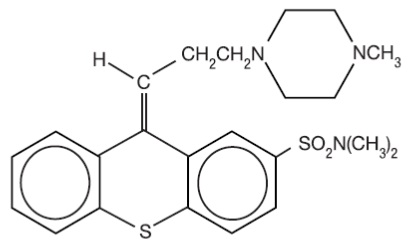
The thioxanthenes differ from the phenothiazines by the replacement of nitrogen in the central ring with a carbon-linked side chain fixed in space in a rigid structural configuration. An N,N-dimethyl sulfonamide functional group is bonded to the thioxanthene nucleus.
Each capsule contains 1 mg, 2 mg, 5 mg or 10 mg of thiothixene, USP and the following inactive ingredients: colloidal silicon dioxide, croscarmellose sodium, lactose monohydrate, magnesium stearate, microcrystalline cellulose, pregelatinized starch and sodium lauryl sulfate. Each of the empty gelatin capsules contains FD&C Blue No. 1, FD&C Red No. 40, FD&C Yellow No. 6, gelatin, sodium lauryl sulfate and titanium dioxide. In addition, the 1 mg empty gelatin capsules contain D&C Red No. 28 and the 2 mg empty gelatin capsules contain D&C Yellow No. 10.
The imprinting ink contains the following: ammonia solution, black iron oxide, potassium hydroxide, propylene glycol and shellac.
ACTION
INDICATIONS & USAGE
CONTRAINDICATIONS
Thiothixene capsules are contraindicated in patients with circulatory collapse, comatose states, central nervous system depression due to any cause, and blood dyscrasias. Thiothixene is contraindicated in individuals who have shown hypersensitivity to the drug. It is not known whether there is a cross sensitivity between the thioxanthenes and the phenothiazine derivatives, but this possibility should be considered.
WARNINGS
Increased Mortality in Elderly Patients with Dementia-Related Psychosis
Elderly patients with dementia-related psychosis treated with antipsychotic drugs are at an increased risk of death. Thiothixene is not approved for the treatment of patients with dementia-related psychosis (see BOXED WARNING).
Tardive Dyskinesia
Tardive dyskinesia, a syndrome consisting of potentially irreversible, involuntary, dyskinetic movements may develop in patients treated with antipsychotic drugs, including thiothixene (1). Although the prevalence of the syndrome appears to be highest among the elderly, especially elderly women, it is impossible to rely upon prevalence estimates to predict, at the inception of antipsychotic treatment, which patients are likely to develop the syndrome. Whether antipsychotic drug products differ in their potential to cause tardive dyskinesia is unknown.
Both the risk of developing the syndrome and the likelihood that it will become irreversible are believed to increase as the duration of treatment and the total cumulative dose of antipsychotic drugs administered to the patient increase. However, the syndrome can develop, although much less commonly, after relatively brief treatment periods at low doses.
There is no known treatment for established cases of tardive dyskinesia, although the syndrome may remit, partially or completely, if antipsychotic treatment is withdrawn. Antipsychotic treatment, itself, however, may suppress (or partially suppress) the signs and symptoms of the syndrome and thereby may possibly mask the underlying disease process. The effect that symptomatic suppression has upon the long-term course of the syndrome is unknown.
Given these considerations, antipsychotics should be prescribed in a manner that is most likely to minimize the occurrence of tardive dyskinesia. Chronic antipsychotic treatment should generally be reserved for patients who suffer from a chronic illness that, 1) is known to respond to antipsychotic drugs, and 2) for whom alternative, equally effective, but potentially less harmful treatments are not available or appropriate. In patients who do require chronic treatment, the smallest dose and the shortest duration of treatment producing a satisfactory clinical response should be sought. The need for continued treatment should be reassessed periodically.
If signs and symptoms of tardive dyskinesia appear in a patient on antipsychotics, drug discontinuation should be considered. However, some patients may require treatment despite the presence of the syndrome.
(For further information about the description of tardive dyskinesia and its clinical detection, please refer to Information for Patients in the PRECAUTIONS section, and to the ADVERSE REACTIONS section.)
Neuroleptic Malignant Syndrome (NMS)
A potentially fatal symptom complex sometimes referred to as Neuroleptic Malignant Syndrome (NMS) has been reported in association with antipsychotic drugs, including thiothixene2. Clinical manifestations of NMS are hyperpyrexia, muscle rigidity, altered mental status and evidence of autonomic instability (irregular pulse or blood pressure, tachycardia, diaphoresis, and cardiac dysrhythmias).
The diagnostic evaluation of patients with this syndrome is complicated. In arriving at a diagnosis, it is important to identify cases where the clinical presentation includes both serious medical illness (e.g., pneumonia, systemic infection, etc.) and untreated or inadequately treated extrapyramidal signs and symptoms (EPS). Other important considerations in the differential diagnosis include central anticholinergic toxicity, heat stroke, drug fever and primary central nervous system (CNS) pathology.
The management of NMS should include 1) immediate discontinuation of antipsychotic drugs and other drugs not essential to concurrent therapy, 2) intensive symptomatic treatment and medical monitoring, and treatment of any concomitant serious medical problems for which specific treatments are available. There is no general agreement about specific pharmacological treatment regimens for uncomplicated NMS.
If a patient requires antipsychotic drug treatment after recovery from NMS, the potential reintroduction of drug therapy should be carefully considered. The patient should be carefully monitored, since recurrences of NMS have been reported.
Pregnancy
Safe use of thiothixene during pregnancy has not been established. Therefore, this drug should be given to pregnant patients only when, in the judgment of the physician, the expected benefits from the treatment exceed the possible risks to mother and fetus.
Non-teratogenic Effects
Neonates exposed to antipsychotic drugs, during the third trimester of pregnancy are at risk for extrapyramidal and/or withdrawal symptoms following delivery. There have been reports of agitation, hypertonia, hypotonia, tremor, somnolence, respiratory distress and feeding disorder in these neonates. These complications have varied in severity; while in some cases symptoms have been self-limited, in other cases neonates have required intensive care unit support and prolonged hospitalization.
Thiothixene Capsules should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
Animal reproduction studies and clinical experience to date have not demonstrated any teratogenic effects.
In the animal reproduction studies with thiothixene, there was some decrease in conception rate and litter size, and an increase in resorption rate in rats and rabbits. Similar findings have been reported with other psychotropic agents. After repeated oral administration of thiothixene to rats (5 to 15 mg/kg/day), rabbits (3 to 50 mg/kg/day), and monkeys (1 to 3 mg/kg/day) before and during gestation, no teratogenic effects were seen.
Usage in Children
The use of thiothixene in children under 12 years of age is not recommended because safe conditions for its use have not been established.
As is true with many CNS drugs, thiothixene may impair the mental and/or physical abilities required for the performance of potentially hazardous tasks such as driving a car or operating machinery, especially during the first few days of therapy. Therefore, the patient should be cautioned accordingly.
As in the case of other CNS-acting drugs, patients receiving thiothixene should be cautioned about the possible additive effects (which may include hypotension) with CNS depressants and with alcohol.
PRECAUTIONS
GENERAL PRECAUTIONS
An antiemetic effect was observed in animal studies with thiothixene; since this effect may also occur in man, it is possible that thiothixene may mask signs of over-dosage of toxic drugs and may obscure conditions such as intestinal obstruction and brain tumor.
In consideration of the known capability of thiothixene and certain other psychotropic drugs to precipitate convulsions, extreme caution should be used in patients with a history of convulsive disorders or those in a state of alcohol withdrawal, since it may lower the convulsive threshold. Although thiothixene potentiates the actions of the barbiturates, the dosage of the anticonvulsant therapy should not be reduced when thiothixene is administered concurrently.
Though exhibiting rather weak anticholinergic properties, thiothixene should be used with caution in patients who might be exposed to extreme heat or who are receiving atropine or related drugs. Use with caution in patients with cardiovascular disease.
Caution as well as careful adjustment of the dosages is indicated when thiothixene is used in conjunction with other CNS depressants.
Also, careful observation should be made for pigmentary retinopathy and lenticular pigmentation (fine lenticular pigmentation has been noted in a small number of patients treated with thiothixene for prolonged periods). Blood dyscrasias (agranulocytosis, pancytopenia, thrombocytopenic purpura), and liver damage (jaundice, biliary stasis), have been reported with related drugs.
Antipsychotic drugs, including thiothixene3, elevate prolactin levels; the elevation persists during chronic administration. Tissue culture experiments indicate that approximately one-third of human breast cancers are prolactin dependent in vitro, a factor of potential importance if the prescription of these drugs is contemplated in a patient with a previously detected breast cancer. Although disturbances such as galactorrhea, amenorrhea, gynecomastia, and impotence have been reported, the clinical significance of elevated serum prolactin levels is unknown for most patients. An increase in mammary neoplasms has been found in rodents after chronic administration of antipsychotic drugs. Neither clinical studies nor epidemiologic studies conducted to date, however, have shown an association between chronic administration of these drugs and mammary tumorigenesis; the available evidence is considered too limited to be conclusive at this time.
Leukopenia, Neutropenia and Agranulocytosis
Class Effect
In clinical trial and/or post-marketing experience, events of leukopenia/neutropenia and agranulocytosis have been reported temporally related to antipsychotic agents
.
Possible risk factors for leukopenia/neutropenia include preexisting low white blood cell count (WBC) and history of drug induced leukopenia/neutropenia. Patients with a history of a clinically significant low WBC or drug induced leukopenia/neutropenia should have their complete blood count (CBC) monitored frequently during the first few months of therapy and discontinuation of thiothixene should be considered at the first sign of a clinically significant decline in WBC in the absence of other causative factors.
Patients with clinically significant neutropenia should be carefully monitored for fever or other symptoms or signs of infection and treated promptly if such symptoms or signs occur. Patients with severe neutropenia (absolute neutrophil count < 1000/mm3) should discontinue thiothixene and have their WBC followed until recovery.
INFORMATION FOR PATIENTS
Given the likelihood that some patients exposed chronically to antipsychotics will develop tardive dyskinesia, it is advised that all patients in whom chronic use is contemplated be given, if possible, full information about this risk. The decision to inform patients and/or their guardians must obviously take into account the clinical circumstances and the competency of the patient to understand the information provided.
DRUG INTERACTIONS
Hepatic microsomal enzyme inducing agents, such as carbamazepine, were found to significantly increase the clearance of thiothixene. Patients receiving these drugs should be observed for signs of reduced thiothixene effectiveness4,5.
Due to a possible additive effect with hypotensive agents, patients receiving these drugs should be observed closely for signs of excessive hypotension when thiothixene is added to their drug regimen6.
ADVERSE REACTIONS
ADVERSE REACTIONS
NOTE: Not all of the following adverse reactions have been reported with thiothixene. However, since thiothixene has certain chemical and pharmacologic similarities to the phenothiazines, all of the known side effects and toxicity associated with phenothiazine therapy should be borne in mind when thiothixene is used.
Cardiovascular Effects
Tachycardia, hypotension, light-headedness, and syncope. In the event hypotension occurs, epinephrine should not be used as a pressor agent since a paradoxical further lowering of blood pressure may result. Nonspecific EKG changes have been observed in some patients receiving thiothixene. These changes are usually reversible and frequently disappear on continued thiothixene therapy. The incidence of these changes is lower than that observed with some phenothiazines. The clinical significance of these changes is not known.
CNS Effects
Drowsiness, usually mild, may occur although it usually subsides with continuation of thiothixene therapy. The incidence of sedation appears similar to that of the piperazine group of phenothiazines but less than that of certain aliphatic phenothiazines. Restlessness, agitation and insomnia have been noted with thiothixene. Seizures and paradoxical exacerbation of psychotic symptoms have occurred with thiothixene infrequently.
Hyperreflexia has been reported in infants delivered from mothers having received structurally related drugs.
In addition, phenothiazine derivatives have been associated with cerebral edema and cerebrospinal fluid abnormalities.
Extrapyramidal Symptoms
Extrapyramidal symptoms, such as pseudoparkinsonism, akathisia and dystonia have been reported (see Adverse Reactions: Dystonia: Class Effect). Management of these extrapyramidal symptoms depends upon the type and severity. Rapid relief of acute symptoms may require the use of an injectable antiparkinson agent. More slowly emerging symptoms may be managed by reducing the dosage of thiothixene and/or administering an oral antiparkinson agent.
Dystonia
Class Effect
Symptoms of dystonia, prolonged abnormal contractions of muscle groups, may occur in susceptible individuals during the first few days of treatment. Dystonic symptoms include: spasm of the neck muscles, sometimes progressing to tightness of the throat, swallowing difficulty, difficulty breathing, and/or protrusion of the tongue. While these symptoms can occur at low doses, they occur more frequently and with greater severity with high potency and at higher doses of first generation antipsychotic drugs. An elevated risk of acute dystonia is observed in males and younger age groups.
Persistent Tardive Dyskinesia
As with all antipsychotic agents, tardive dyskinesia may appear in some patients on long-term therapy with thiothixene, or may occur after drug therapy has been discontinued. The syndrome is characterized by rhythmical involuntary movements of the tongue, face, mouth or jaw (e.g., protrusion of tongue, puffing of cheeks, puckering of mouth, chewing movements). Sometimes these may be accompanied by involuntary movements of extremities.
Since early detection of tardive dyskinesia is important, patients should be monitored on an ongoing basis. It has been reported that fine vermicular movement of the tongue may be an early sign of the syndrome. If this or any other presentation of the syndrome is observed, the clinician should consider possible discontinuation of antipsychotic medication. (See WARNINGS.)
Hepatic Effects
Elevations of serum transaminase and alkaline phosphatase, usually transient, have been infrequently observed in some patients. No clinically confirmed cases of jaundice attributable to thiothixene have been reported.
Hematologic Effects
As is true with certain other psychotropic drugs, leukopenia and leucocytosis, which are usually transient, can occur occasionally with thiothixene. Other antipsychotic drugs have been associated with agranulocytosis, eosinophilia, hemolytic anemia, thrombocytopenia and pancytopenia.
Allergic Reactions
Rash, pruritus, urticaria, photosensitivity and rare cases of anaphylaxis have been reported with thiothixene. Undue exposure to sunlight should be avoided. Although not experienced with thiothixene, exfoliative dermatitis and contact dermatitis (in nursing personnel) have been reported with certain phenothiazines.
Endocrine/Reproductive
Hyperprolactinemia3, lactation, menstrual irregularities, moderate breast enlargement and amenorrhea have occurred in a small percentage of females receiving thiothixene. If persistent, this may necessitate a reduction in dosage or the discontinuation of therapy. Phenothiazines have been associated with false positive pregnancy tests, gynecomastia, hypoglycemia, hyperglycemia and glycosuria.
Autonomic Effects
Dry mouth, blurred vision, nasal congestion, constipation, increased sweating, increased salivation and impotence have occurred infrequently with thiothixene therapy. Phenothiazines have been associated with miosis, mydriasis, and adynamic ileus.
Other Adverse Reactions
Hyperpyrexia, anorexia, nausea, vomiting, diarrhea, increase in appetite and weight, weakness or fatigue, polydipsia, and peripheral edema.
Although not reported with thiothixene, evidence indicates there is a relationship between phenothiazine therapy and the occurrence of a systemic lupus erythematosus-like syndrome.
Neuroleptic Malignant Syndrome (NMS)
Please refer to the text regarding NMS in the WARNINGS section.
NOTE: Sudden deaths have occasionally been reported in patients who have received certain phenothiazine derivatives. In some cases, the cause of death was apparently cardiac arrest or asphyxia due to failure of the cough reflex. In others, the cause could not be determined nor could it be established that death was due to phenothiazine administration.
DOSAGE & ADMINISTRATION
Dosage of thiothixene capsules should be individually adjusted depending on the chronicity and severity of the symptoms of schizophrenia. In general, small doses should be used initially and gradually increased to the optimal effective level, based on patient response.
Some patients have been successfully maintained on once a day thiothixene capsule therapy.
The use of thiothixene capsules in children under 12 years of age is not recommended because safe conditions for its use have not been established.
In milder conditions, an initial dose of 2 mg three times daily is recommended. If indicated, a subsequent increase to 15 mg/day total daily dose is often effective.
In more severe conditions, an initial dose of 5 mg twice daily is recommended.
The usual optimal dose is 20 mg to 30 mg daily. If indicated, an increase to 60 mg/day total daily dose is often effective. Exceeding a total daily dose of 60 mg rarely increases the beneficial response.
OVERDOSAGE
Manifestations include muscular twitching, drowsiness and dizziness. Symptoms of gross overdosage may include CNS depression, rigidity, weakness, torticollis, tremor, salivation, dysphagia, hypotension, disturbances of gait, or coma.
Treatment
Essentially symptomatic and supportive. Early gastric lavage is helpful. Keep patient under careful observation and maintain an open airway, since involvement of the extrapyramidal system may produce dysphagia and respiratory difficulty in severe overdosage. If hypotension occurs, the standard measures for managing circulatory shock should be used (I.V. fluids and/or vasoconstrictors).
If a vasoconstrictor is needed, norepinephrine and phenylephrine are the most suitable drugs. Other pressor agents, including epinephrine, are not recommended, since phenothiazine derivatives may reverse the usual pressor action of these agents and cause further lowering of blood pressure.
If CNS depression is marked, symptomatic treatment is indicated. Extrapyramidal symptoms may be treated with antiparkinson drugs.
There are no data on the use of peritoneal or hemodialysis, but they are known to be of little value in phenothiazine intoxication.
HOW SUPPLIED
Thiothixene Capsules, USP are available containing 1 mg, 2 mg, 5 mg or 10 mg of thiothixene, USP.
Thiothixene, USP 1 mg capsule is a size 4 hard gelatin capsule with a caramel opaque cap and a blue opaque body filled with white to off-white powder. The capsule is imprinted “014” on the cap and “novitium” on the body in black ink. They are available as below:
NDC 70954-014-10 Bottle of 100 capsules
NDC 70954-014-20 Bottle of 1000 capsules
Thiothixene, USP 2 mg capsule is a size 4 hard gelatin capsule with a caramel opaque cap and a yellow opaque body filled with white to off-white powder. The capsule is imprinted “015” on the cap and “novitium” on the body in black ink. They are available as below:
NDC 70954-015-10 Bottle of 100 capsules
NDC 70954-015-20 Bottle of 1000 capsules
NDC 70954-015-40 Unit dose blister packages of 100 (10 cards of 10 capsules each)
Thiothixene, USP 5 mg capsule is a size 4 hard gelatin capsule with a caramel opaque cap and a white opaque body filled with white to off-white powder. The capsule is imprinted “016” on the cap and “novitium” on the body in black ink. They are available as below:
NDC 70954-016-10 Bottle of 100 capsules
NDC 70954-016-20 Bottle of 1000 capsules
NDC 70954-016-40 Unit dose blister packages of 100 (10 cards of 10 capsules each)
Thiothixene, USP 10 mg capsule is a size 4 hard gelatin capsule with a caramel opaque cap and a peach opaque body filled with white to off-white powder. The capsule is imprinted “017” on the cap and “novitium” on the body in black ink. They are available as below:
NDC 70954-017-10 Bottle of 100 capsules
NDC 70954-017-20 Bottle of 1000 capsules
NDC 70954-017-40 Unit dose blister packages of 100 (10 cards of 10 capsules each)
Store at 20° to 25°C (68° to 77°F). [See USP Controlled Room Temperature.] Protect from light.
Preserve in tight, light-resistant container as defined in the USP.
REFERENCES
- Worldwide Labeling Safety Report: Dyskinesia and Dyskinesia Tardive and Thiothixene, (16Apr02).
- Worldwide Labeling Safety Report: Neuroleptic Malignant Syndrome and Thiothixene, (16Apr02).
- Worldwide Labeling Safety Report: Hyperprolactinemia and Thiothixene, (16Apr02).
- Ereshefsky L, Saklad SR, Watanabe MD, et al. Thiothixene Pharmacokinetic Interactions: A Study of Hepatic Enzyme Inducers, Clearance Inhibitors, and Demographic Variables. Journal of Clinical Psychopharmacology, 11(5):296–301, (1991).
- Worldwide Labeling Safety Report: Drug Interaction and Thiothixene, (09May02).
- McEvoy GK, Miller JL, Snow EK, et al. AHFS Drug Information. American Society of Health- System Pharmacists, Inc., p. 2334-2336, (2002).
- Worldwide Labeling Safety Report: Menstrual Disorder and Thiothixene, (16Apr02).
Manufactured by:
Novitium Pharma LLC
70 Lake Drive, East Windsor
New Jersey 08520
Issued: 08/2020
LB4233-01
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
Thiothixene Capsules, USP 1 mg 100's counts

Thiothixene Capsules, USP 1 mg 1000's counts

Thiothixene Capsules, USP 2 mg 100's counts

Thiothixene Capsules, USP 2 mg 1000's counts

Thiothixene Capsules, USP 2 mg Unit dose blister packages of 100 (10 cards of 10 capsules each)


Thiothixene Capsules, USP 5 mg 100's counts

Thiothixene Capsules, USP 5 mg 1000's counts

Thiothixene Capsules, USP 5 mg Unit dose blister packages of 100 (10 cards of 10 capsules each)


Thiothixene Capsules, USP 10 mg 100's counts

Thiothixene Capsules, USP 10 mg 1000's counts

Thiothixene Capsules, USP 10 mg Unit dose blister packages of 100 (10 cards of 10 capsules each)


INGREDIENTS AND APPEARANCE
| THIOTHIXENE
thiothixene capsule |
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
| THIOTHIXENE
thiothixene capsule |
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
| THIOTHIXENE
thiothixene capsule |
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
| THIOTHIXENE
thiothixene capsule |
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
| Labeler - Novitium Pharma LLC (080301870) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Novitium Pharma LLC | 080301870 | MANUFACTURE(70954-014, 70954-015, 70954-016, 70954-017) , PACK(70954-014, 70954-015, 70954-016, 70954-017) | |