Search by Drug Name or NDC
NDC 71335-0435-05 Diphenhydramine Hydrochloride 25 mg/1 Details
Diphenhydramine Hydrochloride 25 mg/1
Diphenhydramine Hydrochloride is a ORAL CAPSULE in the HUMAN OTC DRUG category. It is labeled and distributed by Bryant Ranch Prepack. The primary component is DIPHENHYDRAMINE HYDROCHLORIDE.
MedlinePlus Drug Summary
Diphenhydramine is used to relieve red, irritated, itchy, watery eyes; sneezing; and runny nose caused by hay fever, allergies, or the common cold. Diphenhydramine is also used to relieve cough caused by minor throat or airway irritation. Diphenhydramine is also used to prevent and treat motion sickness, and to treat insomnia (difficulty falling asleep or staying asleep). Diphenhydramine is also used to control abnormal movements in people who have early stage parkinsonian syndrome (a disorder of the nervous system that causes difficulties with movement, muscle control, and balance) or who are experiencing movement problems as a side effect of a medication. Diphenhydramine will relieve the symptoms of these conditions but will not treat the cause of the symptoms or speed recovery. Diphenhydramine should not be used to cause sleepiness in children. Diphenhydramine is in a class of medications called antihistamines. It works by blocking the action of histamine, a substance in the body that causes allergic symptoms.
Related Packages: 71335-0435-05Last Updated: 12/01/2022
MedLinePlus Full Drug Details: Diphenhydramine
Product Information
| NDC | 71335-0435 |
|---|---|
| Product ID | 71335-0435_b5a7fa2e-d908-49b7-8441-72a692a02614 |
| Associated GPIs | 41200030100105 |
| GCN Sequence Number | n/a |
| GCN Sequence Number Description | n/a |
| HIC3 | n/a |
| HIC3 Description | n/a |
| GCN | n/a |
| HICL Sequence Number | n/a |
| HICL Sequence Number Description | n/a |
| Brand/Generic | n/a |
| Proprietary Name | Diphenhydramine Hydrochloride |
| Proprietary Name Suffix | n/a |
| Non-Proprietary Name | Diphenhydramine Hydrochloride |
| Product Type | HUMAN OTC DRUG |
| Dosage Form | CAPSULE |
| Route | ORAL |
| Active Ingredient Strength | 25 |
| Active Ingredient Units | mg/1 |
| Substance Name | DIPHENHYDRAMINE HYDROCHLORIDE |
| Labeler Name | Bryant Ranch Prepack |
| Pharmaceutical Class | Histamine H1 Receptor Antagonists [MoA], Histamine-1 Receptor Antagonist [EPC] |
| DEA Schedule | n/a |
| Marketing Category | OTC MONOGRAPH FINAL |
| Application Number | part341 |
| Listing Certified Through | 2023-12-31 |
Package
Package Images
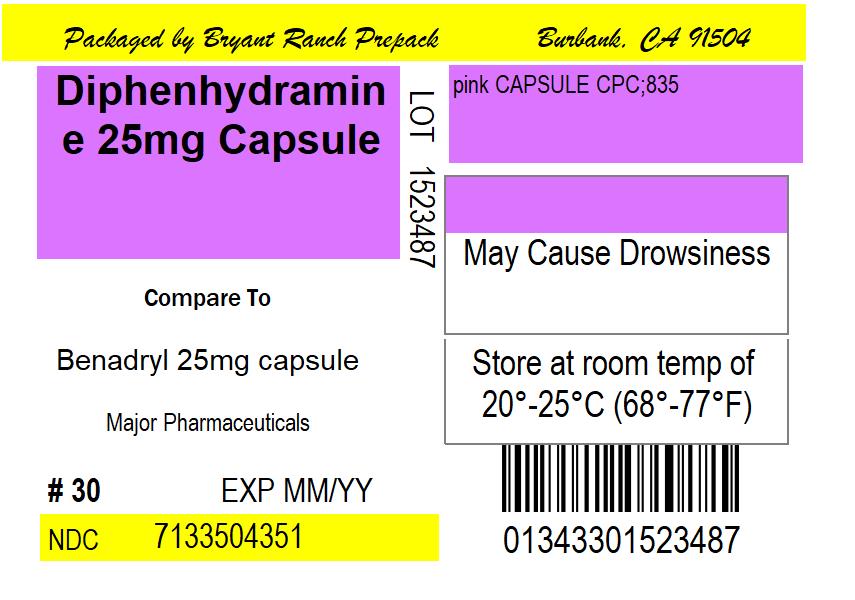
NDC 71335-0435-05 (71335043505)
| NDC Package Code | 71335-0435-5 |
|---|---|
| Billing NDC | 71335043505 |
| Package | 15 CAPSULE in 1 BOTTLE (71335-0435-5) |
| Marketing Start Date | 2019-01-28 |
| NDC Exclude Flag | N |
| Pricing Information | N/A |
Standard Product Labeling (SPL)/Prescribing Information SPL b5a7fa2e-d908-49b7-8441-72a692a02614 Details
Use
25 MG
- Temporarily relieves these symptoms due to hay fever or other upper respiratory allergies:
- runny nose
- sneezing
- itchy, watery eyes
- itchy throat and nose
- Temporarily relieves these symptoms due to the common cold:
- runny nose
- sneezing
50 MG
- Temporarily relieves these symptoms due to hay fever or other upper respiratory allergies and common cold
- sneezing
- runny nose
- itchy, watery eyes
- itchy throat and nose
WARNINGS
Ask a doctor before use if you have
When using this product
KEEP OUT OF REACH OF CHILDREN
Directions
- Take every 4-6 hours
- Do not take more than 6 doses in 24 hours
25 MG
| adults and children 12 years of age and over | 1 to 2 capsules |
| children 6 years to under 12 years of age | 1 capsule |
| children under 6 years of age | do not use this product in children under 6 years of age |
50 MG
| adults and children 12 years of age and over | 1 capsule |
| children 6 years to under 12 years of age | Ask a doctor, the proper dosage strength is not available in this package** |
**Do not attempt to break capsules. The proper dosage strength and dosing information for children under 12 years of age is available on the 25 mg package.
Other Information
HOW SUPPLIED
NDC: 71335-0435-1: 30 Tablets in a BOTTLE
NDC: 71335-0435-2: 20 Tablets in a BOTTLE
NDC: 71335-0435-3: 42 Tablets in a BOTTLE
NDC: 71335-0435-4: 24 Tablets in a BOTTLE
NDC: 71335-0435-5: 15 Tablets in a BOTTLE
NDC: 71335-0435-6: 60 Tablets in a BOTTLE
NDC: 71335-0435-7: 10 Tablets in a BOTTLE
NDC: 71335-0435-8: 6 Tablets in a BOTTLE
NDC: 71335-0435-9: 90 Tablets in a BOTTLE
NDC: 71335-0435-0: 100 Tablets in a BOTTLE
INGREDIENTS AND APPEARANCE
| DIPHENHYDRAMINE HYDROCHLORIDE
diphenhydramine hydrochloride capsule |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Labeler - Bryant Ranch Prepack (171714327) |
| Registrant - Bryant Ranch Prepack (171714327) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Bryant Ranch Prepack | 171714327 | REPACK(71335-0435) , RELABEL(71335-0435) | |