Search by Drug Name or NDC
NDC 71335-0876-02 Donepezil Hydrochloride 5 mg/1 Details
Donepezil Hydrochloride 5 mg/1
Donepezil Hydrochloride is a ORAL TABLET, FILM COATED in the HUMAN PRESCRIPTION DRUG category. It is labeled and distributed by Bryant Ranch Prepack. The primary component is DONEPEZIL HYDROCHLORIDE.
MedlinePlus Drug Summary
Donepezil is used to treat dementia (a brain disorder that affects the ability to remember, think clearly, communicate, and perform daily activities and may cause changes in mood and personality) in people who have Alzheimer's disease (AD; a brain disease that slowly destroys the memory and the ability to think, learn, communicate and handle daily activities). Donepezil is in a class of medications called cholinesterase inhibitors. It improves mental function (such as memory, attention, the ability to interact with others, speak, think clearly, and perform regular daily activities) by increasing the amount of a certain naturally occurring substance in the brain. Donepezil may improve the ability to think and remember or slow the loss of these abilities in people who have AD. However, donepezil will not cure AD or prevent the loss of mental abilities at some time in the future.
Related Packages: 71335-0876-02Last Updated: 12/01/2022
MedLinePlus Full Drug Details: Donepezil
Product Information
| NDC | 71335-0876 |
|---|---|
| Product ID | 71335-0876_3ac29f6f-0993-416f-8352-e6c0a5ef3e55 |
| Associated GPIs | |
| GCN Sequence Number | n/a |
| GCN Sequence Number Description | n/a |
| HIC3 | n/a |
| HIC3 Description | n/a |
| GCN | n/a |
| HICL Sequence Number | n/a |
| HICL Sequence Number Description | n/a |
| Brand/Generic | n/a |
| Proprietary Name | Donepezil Hydrochloride |
| Proprietary Name Suffix | n/a |
| Non-Proprietary Name | Donepezil Hydrochloride |
| Product Type | HUMAN PRESCRIPTION DRUG |
| Dosage Form | TABLET, FILM COATED |
| Route | ORAL |
| Active Ingredient Strength | 5 |
| Active Ingredient Units | mg/1 |
| Substance Name | DONEPEZIL HYDROCHLORIDE |
| Labeler Name | Bryant Ranch Prepack |
| Pharmaceutical Class | Cholinesterase Inhibitor [EPC], Cholinesterase Inhibitors [MoA] |
| DEA Schedule | n/a |
| Marketing Category | ANDA |
| Application Number | ANDA201724 |
| Listing Certified Through | 2023-12-31 |
Package
Package Images

NDC 71335-0876-02 (71335087602)
| NDC Package Code | 71335-0876-2 |
|---|---|
| Billing NDC | 71335087602 |
| Package | 30 TABLET, FILM COATED in 1 BOTTLE (71335-0876-2) |
| Marketing Start Date | 2018-10-23 |
| NDC Exclude Flag | N |
| Pricing Information | N/A |
Standard Product Labeling (SPL)/Prescribing Information SPL 3ac29f6f-0993-416f-8352-e6c0a5ef3e55 Details
HIGHLIGHTS OF PRESCRIBING INFORMATION
DONEPEZIL HYDROCHLORIDE tablets, for oral use
Initial U.S. Approval: 1996
INDICATIONS AND USAGE
Donepezil hydrochlorideis an acetylcholinesterase inhibitor indicated for the treatment of dementia of the Alzheimer’s type. Efficacy has been demonstrated in patients with mild, moderate, and severe Alzheimer’s Disease (1) (1)
DOSAGE AND ADMINISTRATION
•Mild to Moderate Alzheimer’s Disease - 5 mg or 10 mg once daily (2.1)
•Moderate to Severe Alzheimer’s Disease - 10 mg to 23 mg once daily (2.2) (2)
DOSAGE FORMS AND STRENGTHS
- Tablets: 5 mg and 10 mg (3)
CONTRAINDICATIONS
- Known hypersensitivity to donepezil hydrochloride or to piperidine derivatives (4)
WARNINGS AND PRECAUTIONS
• Cholinesterase inhibitors are likely to exaggerate succinylcholine-type muscle relaxation during anesthesia (5.1).
• Cholinesterase inhibitors may have vagotonic effects on the sinoatrial and atrioventricular nodes manifesting as bradycardia or heart block (5.2).
• Donepezil hydrochloride can cause vomiting. Patients should be observed closely at initiation of treatment and after dose increases (5.3).
• Patients should be monitored closely for symptoms of active or occult gastrointestinal (GI) bleeding, especially those at increased risk for developing ulcers (5.4).
• The use of donepezil hydrochloride in a dose of 23 mg once daily is associated with weight loss (5.5)
• Cholinomimetics may cause bladder outflow obstructions (5.6).
• Cholinomimetics are believed to have some potential to cause generalized convulsions (5.7).
• Cholinesterase inhibitors should be prescribed with care to patients with a history of asthma or obstructive pulmonary disease (5.8). (5)
ADVERSE REACTIONS
Most common adverse reactions in clinical studies of donepezil hydrochloride are nausea, diarrhea, insomnia, vomiting, muscle cramps, fatigue, and anorexia (6.1).
To report SUSPECTED ADVERSE REACTIONS, contact Alembic Pharmaceuticals Limited at 1-866-210-9797 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch (6)
DRUG INTERACTIONS
- Cholinesterase inhibitors have the potential to interfere with the activity of anticholinergic medications (7.2).
- A synergistic effect may be expected with concomitant administration of succinylcholine, similar neuromuscular blocking agents, or cholinergic agonists (7.3).
USE IN SPECIFIC POPULATIONS
Pregnancy: Based on animal data, may cause fetal harm (8.1). (8)
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 12/2021
Table Of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1. INDICATIONS AND USAGE
2. DOSAGE AND ADMINISTRATION
2.1. Dosing in Mild to Moderate Alzheimer's Disease
2.2. Dosing in Moderate to Severe Alzheimer's Disease
2.3. Administration Information
3. DOSAGE FORMS AND STRENGTHS
4. CONTRAINDICATIONS
5. WARNINGS AND PRECAUTIONS
5.1. Anesthesia
5.2. Cardiovascular Conditions
5.3. Nausea and Vomiting
5.4. Peptic Ulcer Disease and GI Bleeding
5.5. Weight Loss
5.6. Genitourinary Conditions
5.7. Neurological Conditions: Seizures
5.8. Pulmonary Conditions
6. ADVERSE REACTIONS
6.1. Clinical Trials Experience
6.2. Postmarketing Experience
7. DRUG INTERACTIONS
7.1. Use with Anticholinergics
7.2. Use with Cholinomimetics and Other Cholinesterase Inhibitors
8. USE IN SPECIFIC POPULATIONS
8.1. Pregnancy
8.2 Lactation
8.4. Pediatric Use
8.5. Geriatric Use
8.6. Lower Weight Individuals
10. OVERDOSAGE
11. DESCRIPTION
12. CLINICAL PHARMACOLOGY
12.1. Mechanism of Action
12.3 Pharmacokinetics
13. NONCLINICAL TOXICOLOGY
13.1. Carcinogenesis, Mutagenesis, Impairment of Fertility
13.2. Animal Toxicology and/or Pharmacology
14. CLINICAL STUDIES
14.1. Mild to Moderate Alzheimer's Disease
14.2. Moderate to Severe Alzheimer's Disease
16. HOW SUPPLIED/STORAGE AND HANDLING
17. PATIENT COUNSELING INFORMATION
- *
- Sections or subsections omitted from the full prescribing information are not listed.
1. INDICATIONS AND USAGE
2. DOSAGE AND ADMINISTRATION
2.1. Dosing in Mild to Moderate Alzheimer's Disease
The recommended starting dosage of donepezil hydrochloride is 5 mg administered once per day in the evening, just prior to retiring. The maximum recommended dosage of donepezil hydrochloride in patients with mild to moderate Alzheimer’s disease is 10 mg per day. A dose of 10 mg should not be administered until patients have been on a daily dose of 5 mg for 4 to 6 weeks.
2.2. Dosing in Moderate to Severe Alzheimer's Disease
The recommended starting dosage of donepezil hydrochloride is 5 mg administered once per day in the evening, just prior to retiring. The maximum recommended dosage of donepezil hydrochloride tablets in patients with moderate to severe Alzheimer’s disease is 23 mg per day. A dose of 10 mg should not be administered until patients have been on a daily dose of 5 mg for 4 to 6 weeks. A dose of 23 mg per day should not be administered until patients have been on a daily dose of 10 mg for at least 3 months.
3. DOSAGE FORMS AND STRENGTHS
Donepezil hydrochloride tablets, USP are supplied as film-coated, round tablets containing 5 mg or 10 mg of donepezil hydrochloride USP.
The 5 mg tablets are white to off white. The tablet is debossedwith 'L160' on one side and plain on other side.
The 10 mg tablets are yellow. The tablet is debossedwith 'L161' on one side and plain on other side.
4. CONTRAINDICATIONS
5. WARNINGS AND PRECAUTIONS
5.1. Anesthesia
Donepezil hydrochloride, as a cholinesterase inhibitor, is likely to exaggerate succinylcholine-type muscle relaxation during anesthesia.
5.2. Cardiovascular Conditions
Because of their pharmacological action, cholinesterase inhibitors may have vagotonic effects on the sinoatrial and atrioventricular nodes. This effect may manifest as bradycardia or heart block in patients both with and without known underlying cardiac conduction abnormalities. Syncopal episodes have been reported in association with the use of donepezil hydrochloride.
5.3. Nausea and Vomiting
Donepezil hydrochloride, as a predictable consequence of its pharmacological properties, has been shown to produce diarrhea, nausea, and vomiting. These effects, when they occur, appear more frequently with the 10 mg/day dose than with the 5 mg/day dose, and more frequently with the 23 mg dose than with the 10 mg dose. Specifically, in a controlled trial that compared a dose of 23 mg/day to 10 mg/day in patients who had been treated with donepezil 10 mg/day for at least three months, the incidence of nausea in the 23 mg group was markedly greater than in the patients who continued on 10 mg/day (11.8% vs. 3.4%, respectively), and the incidence of vomiting in the 23 mg group was markedly greater than in the 10 mg group (9.2% vs. 2.5%, respectively). The percent of patients who discontinued treatment due to vomiting in the 23 mg group was markedly higher than in the 10 mg group (2.9% vs. 0.4%, respectively).
Although in most cases, these effects have been mild and transient, sometimes lasting one to three weeks, and have resolved during continued use of donepezil hydrochloride, patients should be observed closely at the initiation of treatment and after dose increases.
5.4. Peptic Ulcer Disease and GI Bleeding
Through their primary action, cholinesterase inhibitors may be expected to increase gastric acid secretion due to increased cholinergic activity. Therefore, patients should be monitored closely for symptoms of active or occult gastrointestinal bleeding, especially those at increased risk for developing ulcers, e.g., those with a history of ulcer disease or those receiving concurrent nonsteroidal anti-inflammatory drugs (NSAIDs). Clinical studies of donepezil hydrochloridein a dose of 5 mg/day to 10 mg/day have shown no increase, relative to placebo, in the incidence of either peptic ulcer disease or gastrointestinal bleeding. Results of a controlled clinical study with 23 mg/day showed an increase, relative to 10 mg/day, in the incidence of peptic ulcer disease (0.4% vs. 0.2%) and gastrointestinal bleeding from any site (1.1% vs. 0.6%).
5.5. Weight Loss
Weight loss was reported as an adverse reaction in 4.7% of patients assigned to donepezil hydrochloride in a dose of 23 mg/day compared to 2.5% of patients assigned to 10 mg/day. Compared to their baseline weights, 8.4% of patients taking 23 mg/day were found to have a weight decrease of ≥7% by the end of the study, while 4.9% of patients taking 10 mg/day were found to have weight loss of ≥7% at the end of the study.
5.6. Genitourinary Conditions
Although not observed in clinical trials of donepezil hydrochloride, cholinomimetics may cause bladder outflow obstruction.
6. ADVERSE REACTIONS
The following serious adverse reactions are described below and elsewhere in the labeling:
- Cardiovascular Conditions [see Warnings and Precautions (5.2)]
- Nausea and Vomiting [see Warnings and Precautions (5.3)]
- Peptic Ulcer Disease and GI Bleeding [see Warnings and Precautions (5.4)]
- Weight Loss [see Warnings and Precautions (5.5)]
- Genitourinary Conditions [see Warnings and Precautions (5.6)]
- Neurological Conditions: Seizures [see Warnings and Precautions (5.7)]
- Pulmonary Conditions [see Warnings and Precautions (5.8)]
6.1. Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Donepezil hydrochloride has been administered to over 1,700 individuals during clinical trials worldwide. Approximately 1,200 of these patients have been treated for at least 3 months and more than 1,000 patients have been treated for at least 6 months. Controlled and uncontrolled trials in the United States included approximately 900 patients. In regards to the highest dose of 10 mg/day, this population includes 650 patients treated for 3 months, 475 patients treated for 6 months, and 116 patients treated for over 1 year. The range of patient exposure is from 1 to 1,214 days.
Mild to Moderate Alzheimer’s Disease
Adverse Reactions Leading to Discontinuation
The rates of discontinuation from controlled clinical trials of donepezil hydrochloride due to adverse reactions for the donepezil hydrochloride 5 mg/day treatment groups were comparable to those of placebo treatment groups at approximately 5%. The rate of discontinuation of patients who received 7-day escalations from 5 mg/day to 10 mg/day, was higher at 13%.
The most common adverse events leading to discontinuation, defined as those occurring in at least 2% of patients and at twice or more the incidence seen in placebo patients, are shown in Table 1.
| Table 1. Most Common Adverse Reactions Leading to Discontinuation Group in Patients with Mild to Moderate Alzheimer’s Disease | |||
| Adverse Reaction
| Placebo
(n=355) % | 5 mg/day
Donepezil Hydrochloride (n=350) % | 10 mg/day
Donepezil Hydrochloride (n=315) % |
| Nausea | 1 | 1 | 3 |
| Diarrhea | 0 | <1 | 3 |
| Vomiting | <1 | <1 | 2 |
Most Common Adverse Reactions
The most common adverse reactions, defined as those occurring at a frequency of at least 5% in patients receiving 10 mg/day and twice the placebo rate, are largely predicted by donepezil hydrochloride’s cholinomimetic effects. These include nausea, diarrhea, insomnia, vomiting, muscle cramp, fatigue and anorexia. These adverse reactions were often transient, resolving during continued donepezil hydrochloride treatment without the need for dose modification.
There is evidence to suggest that the frequency of these common adverse reactions may be affected by the rate of titration. An open-label study was conducted with 269 patients who received placebo in the 15 and 30-week studies. These patients were titrated to a dose of 10 mg/day over a 6-week period. The rates of common adverse reactions were lower than those seen in patients titrated to 10 mg/day over one week in the controlled clinical trials and were comparable to those seen in patients on 5 mg/day.
See Table 2 for a comparison of the most common adverse reactions following one and six week titration regimens.
| Table 2. Comparison of Rates of Adverse Reactions in Mild to Moderate Patients Titrated to 10 mg/day over 1 and 6 Weeks
|
||||
|
| No titration
| One week titration
| Six week titration
|
|
| Adverse Reaction
| Placebo (n=315)
% | 5 mg/day (n=311)
% | 10 mg/day
(n=315) % | 10 mg/day
(n=269) % |
| Nausea | 6 | 5 | 19 | 6 |
| Diarrhea | 5 | 8 | 15 | 9 |
| Insomnia | 6 | 6 | 14 | 6 |
| Fatigue | 3 | 4 | 8 | 3 |
| Vomiting | 3 | 3 | 8 | 5 |
| Muscle cramps | 2 | 6 | 8 | 3 |
| Anorexia | 2 | 3 | 7 | 3 |
Table 3 lists adverse reactions that occurred in at least 2% of patients in pooled placebo-controlled trials who received either donepezil hydrochloride 5 mg or 10 mg and for which the rate of occurrence was greater for patients treated with donepezil hydrochloride than with placebo. In general, adverse reactions occurred more frequently in female patients and with advancing age.
| Table 3. Adverse Reactions in Pooled Placebo-Controlled Clinical Trials in Mild to Moderate Alzheimer’s Disease
|
||
| Adverse Reaction
| Placebo (n=355)
% | Donepezil Hydrochloride (n=747)
% |
| Percent of Patients with any Adverse Reaction
| 72
| 74
|
| Nausea | 6 | 11 |
| Diarrhea | 5 | 10 |
| Headache | 9 | 10 |
| Insomnia | 6 | 9 |
| Pain, various locations | 8 | 9 |
| Dizziness | 6 | 8 |
| Accident | 6 | 7 |
| Muscle Cramps | 2 | 6 |
| Fatigue | 3 | 5 |
| Vomiting | 3 | 5 |
| Anorexia | 2 | 4 |
| Ecchymosis | 3 | 4 |
| Abnormal Dreams | 0 | 3 |
| Depression | <1 | 3 |
| Weight Loss | 1 | 3 |
| Arthritis | 1 | 2 |
| Frequent Urination | 1 | 2 |
| Somnolence | <1 | 2 |
| Syncope | 1 | 2 |
Severe Alzheimer’s Disease (Donepezil Hydrochloride 5 mg/day and 10 mg/day)
Donepezil hydrochloride has been administered to over 600 patients with severe Alzheimer’s disease during clinical trials of at least 6 months duration, including three double-blind, placebo-controlled trials, two of which had an open label extension.
Adverse Reactions Leading to Discontinuation
The rates of discontinuation from controlled clinical trials of donepezil hydrochloride due to adverse reactions for the donepezil hydrochloride patients were approximately 12% compared to 7% for placebo patients. The most common adverse reactions leading to discontinuation, defined as those occurring in at least 2% of donepezil hydrochloride patients and at twice or more the incidence seen in placebo, were anorexia (2% vs. 1% placebo), nausea (2% vs. <1% placebo), diarrhea (2% vs. 0% placebo) and urinary tract infection (2% vs. 1% placebo).
Most Common Adverse Reactions
The most common adverse reactions, defined as those occurring at a frequency of at least 5% in patients receiving donepezil hydrochloride and at twice or more the placebo rate, are largely predicted by donepezil hydrochloride’s cholinomimetic effects. These include diarrhea, anorexia, vomiting, nausea, and ecchymosis. These adverse reactions were often transient, resolving during continued donepezil hydrochloride treatment without the need for dose modification.
Table 4 lists adverse reactions that occurred in at least 2% of patients in pooled placebo-controlled trials who received donepezil hydrochloride 5 mg or 10 mg and for which the rate of occurrence was greater for patients treated with donepezil hydrochloride than with placebo.
| Table 4. Adverse Reactions in Pooled Controlled Clinical Trials in Severe Alzheimer’s Disease
|
||
| Body System/Adverse Reaction
| Placebo (n=392) % | Donepezil Hydrochloride (n=501) % |
| Percent of Patients with any Adverse Reaction
| 73
| 81
|
| Accident
| 12 | 13 |
| Infection | 9 | 11 |
| Diarrhea | 4 | 10 |
| Anorexia | 4 | 8 |
| Vomiting | 4 | 8 |
| Nausea | 2 | 6 |
| Insomnia | 4 | 5 |
| Ecchymosis | 2 | 5 |
| Headache | 3 | 4 |
| Hypertension | 2 | 3 |
| Pain | 2 | 3 |
| Back Pain | 2 | 3 |
| Eczema | 2 | 3 |
| Hallucinations | 1 | 3 |
| Hostility | 2 | 3 |
| Increase in Creatine Phosphokinase | 1 | 3 |
| Nervousness | 2 | 3 |
| Fever | 1 | 2 |
| Chest Pain | <1 | 2 |
| Confusion | 1 | 2 |
| Dehydration | 1 | 2 |
| Depression | 1 | 2 |
| Dizziness | 1 | 2 |
| Emotional Lability | 1 | 2 |
| Hemorrhage | 1 | 2 |
| Hyperlipemia | <1 | 2 |
| Personality Disorder | 1 | 2 |
| Somnolence | 1 | 2 |
| Syncope | 1 | 2 |
| Urinary Incontinence | 1 | 2 |
Moderate to Severe Alzheimer’s Disease (Donepezil Hydrochloride 23 mg/day)
Donepezil hydrochloride 23 mg/day has been administered to over 1300 individuals globally in clinical trials. Approximately 1050 of these patients have been treated for at least three months and more than 950 patients have been treated for at least six months. The range of patient exposure was from 1 to over 500 days.
Adverse Reactions Leading to Discontinuation
The rate of discontinuation from a controlled clinical trial of donepezil hydrochloride 23 mg/day due to adverse reactions was higher (19%) than for the 10 mg/day treatment group (8%). The most common adverse reactions leading to discontinuation, defined as those occurring in at least 1% of patients and greater than those occurring with 10 mg/day are shown in Table 5.
| Table 5. Most Common Adverse Reactions Leading to Discontinuation in Patients with Moderate to Severe Alzheimer’s Disease
|
||
| Adverse Reaction
| 23 mg/day Donepezil Hydrochloride
(n=963) % | 10 mg/day Donepezil Hydrochloride
(n=471) % |
| Vomiting | 3 | 0 |
| Diarrhea | 2 | 0 |
| Nausea | 2 | 0 |
| Dizziness | 1 | 0 |
The majority of discontinuations due to adverse reactions in the 23 mg group occurred during the first month of treatment.
Most Common Adverse Reactions with Donepezil Hydrochloride 23 mg/day
The most common adverse reactions, defined as those occurring at a frequency of at least 5%, include nausea, diarrhea, vomiting, and anorexia.
Table 6 lists adverse reactions that occurred in at least 2% of patients who received 23 mg/day of donepezil hydrochloride and at a higher frequency than those receiving 10 mg/day of donepezil hydrochloride in a controlled clinical trial that compared the two doses. In this study, there were no important differences in the type of adverse reactions in patients taking donepezil hydrochloride with or without memantine.
| Table 6. Adverse Reactions in a Controlled Clinical Trial in Moderate to Severe Alzheimer’s Disease
|
||
| Adverse Reaction
| 23 mg/day Donepezil Hydrochloride
(n=963) % | 10 mg/day Donepezil Hydrochloride
(n=471) % |
| Percent of Patients with any Adverse Reaction
| 74 | 64 |
| Nausea | 12 | 3 |
| Vomiting | 9 | 3 |
| Diarrhea | 8 | 5 |
| Anorexia | 5 | 2 |
| Dizziness | 5 | 3 |
| Weight Loss | 5 | 3 |
| Headache | 4 | 3 |
| Insomnia | 3 | 2 |
| Urinary Incontinence | 3 | 1 |
| Asthenia | 2 | 1 |
| Contusion | 2 | 0 |
| Fatigue | 2 | 1 |
| Somnolence | 2 | 1 |
6.2. Postmarketing Experience
The following adverse reactions have been identified during post-approval use of donepezil hydrochloride. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Abdominal pain, agitation, aggression, cholecystitis, confusion, convulsions, hallucinations, heart block (all types), hemolytic anemia, hepatitis, hyponatremia, neuroleptic malignant syndrome, pancreatitis, rash, rhabdomyolysis, QTc prolongation, and torsade de pointes.
7. DRUG INTERACTIONS
8. USE IN SPECIFIC POPULATIONS
8.1. Pregnancy
Risk Summary
There are no adequate data on the developmental risks associated with the use of donepezil hydrochloride in pregnant women. In animal studies, developmental toxicity was not observed when donepezil was administered to pregnant rats and rabbits during organogenesis, but administration to rats during the latter part of pregnancy and throughout lactation resulted in increased stillbirths and decreased offspring survival at clinically relevant doses [see Data]. In the U.S. general population, the estimated background risks of major birth defects and miscarriage in clinically recognized pregnancies are 2% to 4% and 15% to 20%, respectively. The background risks of major birth defects and miscarriage for the indicated population are unknown.
Data
Animal Data
Oral administration of donepezil to pregnant rats and rabbits during the period of organogenesis did not produce any teratogenic effects at doses up to 16 mg/kg/day (approximately 6 times the maximum recommended human dose [MRHD] of 23 mg/day on a mg/m2 basis) and 10 mg/kg/day (approximately 7 times the MRHD on a mg/m2 basis), respectively. Oral administration of donepezil (1, 3, 10 mg/kg/day) to rats during late gestation and throughout lactation to weaning produced an increase in stillbirths and reduced offspring survival through postpartum day 4 at the highest dose. The no-effect dose of 3 mg/kg/day is approximately equal to the MRHD on a mg/m2 basis.
8.2 Lactation
Risk Summary
There are no data on the presence of donepezil or its metabolites in human milk, the effects on the breastfed infant, or on milk production.
The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for donepezil and any potential adverse effects on the breastfed infant from donepezil or from the underlying maternal condition.
8.5. Geriatric Use
Alzheimer’s disease is a disorder occurring primarily in individuals over 55 years of age. The mean age of patients enrolled in the clinical studies with donepezil hydrochloride was 73 years; 80% of these patients were between 65 and 84 years old, and 49% of patients were at or above the age of 75. The efficacy and safety data presented in the clinical trials section were obtained from these patients. There were no clinically significant differences in most adverse reactions reported by patient groups ≥ 65 years old and < 65 years old.
8.6. Lower Weight Individuals
In the controlled clinical trial, among patients in the donepezil hydrochloride 23 mg treatment group, those patients weighing <55 kg reported more nausea, vomiting, and decreased weight than patients weighing 55 kg or more. There were more withdrawals due to adverse reactions as well. This finding may be related to higher plasma exposure associated with lower weight.
10. OVERDOSAGE
Because strategies for the management of overdose are continually evolving, it is advisable to contact a Poison Control Center to determine the latest recommendations for the management of an overdose of any drug.
As in any case of overdose, general supportive measures should be utilized. Overdosage with cholinesterase inhibitors can result in cholinergic crisis characterized by severe nausea, vomiting, salivation, sweating, bradycardia, hypotension, respiratory depression, collapse and convulsions. Increasing muscle weakness is a possibility and may result in death if respiratory muscles are involved. Tertiary anticholinergics such as atropine may be used as an antidote for donepezil hydrochloride overdosage. Intravenous atropine sulfate titrated to effect is recommended: an initial dose of 1 to 2 mg IV with subsequent doses based upon clinical response. Atypical responses in blood pressure and heart rate have been reported with other cholinomimetics when co-administered with quaternary anticholinergics such as glycopyrrolate. It is not known whether donepezil hydrochloride and/or its metabolites can be removed by dialysis (hemodialysis, peritoneal dialysis, or hemofiltration).
Dose-related signs of toxicity in animals included reduced spontaneous movement, prone position, staggering gait, lacrimation, clonic convulsions, depressed respiration, salivation, miosis, tremors, fasciculation and lower body surface temperature.
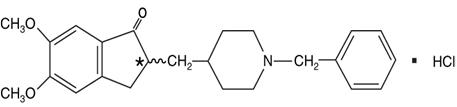
11. DESCRIPTION
Donepezil hydrochloride is a reversible inhibitor of the enzyme acetylcholinesterase, known chemically as (±)-2,3-dihydro-5,6-dimethoxy-2-[[1-(phenylmethyl)-4-piperidinyl]methyl]-1H-inden-1-one hydrochloride. Donepezil hydrochloride is commonly referred to in the pharmacological literature as E2020. It has an empirical formula of C24H29NO3HCl and a molecular weight of 415.96. Donepezil hydrochloride is a white crystalline powder and is freely soluble in chloroform, soluble in water and in glacial acetic acid, slightly soluble in ethanol and in acetonitrile and practically insoluble in ethyl acetate and in n-hexane.
Donepezil hydrochloride tablets, USP are available for oral administration in film-coated tablets containing 5, or 10 mg of donepezil hydrochloride USP .
Inactive ingredients are lactose monohydrate, corn starch, microcrystalline cellulose, hydroxypropyl cellulose, and magnesium stearate. The film coating contains talc, polyethylene glycol, hypromellose and titanium dioxide. Additionally, the 10 mg tablet contains iron oxide yellow as a coloring agent.
12. CLINICAL PHARMACOLOGY
12.1. Mechanism of Action
Current theories on the pathogenesis of the cognitive signs and symptoms of Alzheimer’s disease attribute some of them to a deficiency of cholinergic neurotransmission.
Donepezil hydrochloride is postulated to exert its therapeutic effect by enhancing cholinergic function. This is accomplished by increasing the concentration of acetylcholine through reversible inhibition of its hydrolysis by acetylcholinesterase. There is no evidence that donepezil alters the course of the underlying dementing process.
12.3 Pharmacokinetics
Pharmacokinetics of donepezil are linear over a dose range of 1 to 10 mg given once daily. The rate and extent of absorption of donepezil hydrochloridetablets are not influenced by food.
Based on population pharmacokinetic analysis of plasma donepezil concentrations measured in patients with Alzheimer’s disease, following oral dosing, peak plasma concentration is achieved for donepezil hydrochloride 23 mg tablets in approximately 8 hours, compared with 3 hours for donepezil hydrochloride 10 mg tablets. Peak plasma concentrations were about 2-fold higher for donepezil hydrochloride 23 mg tablets than donepezil hydrochloride 10 mg tablets.
The elimination half life of donepezil is about 70 hours, and the mean apparent plasma clearance (Cl/F) is 0.13 to 0.19 L/hr/kg. Following multiple dose administration, donepezil accumulates in plasma by 4 to 7 fold, and steady state is reached within 15 days. The steady state volume of distribution is 12 to 16 L/kg. Donepezil is approximately 96% bound to human plasma proteins, mainly to albumins (about 75%) and alpha1 - acid glycoprotein (about 21%) over the concentration range of 2 to 1000 ng/mL.
Donepezil is both excreted in the urine intact and extensively metabolized to four major metabolites, two of which are known to be active, and a number of minor metabolites, not all of which have been identified. Donepezil is metabolized by CYP 450 isoenzymes 2D6 and 3A4 and undergoes glucuronidation. Following administration of 14C-labeled donepezil, plasma radioactivity, expressed as a percent of the administered dose, was present primarily as intact donepezil (53%) and as 6-O-desmethyl donepezil (11%), which has been reported to inhibit AChE to the same extent as donepezil in vitro and was found in plasma at concentrations equal to about 20% of donepezil. Approximately 57% and 15% of the total radioactivity was recovered in urine and feces, respectively, over a period of 10 days, while 28% remained unrecovered, with about 17% of the donepezil dose recovered in the urine as unchanged drug. Examination of the effect of CYP2D6 genotype in Alzheimer’s patients showed differences in clearance values among CYP2D6 genotype subgroups. When compared to the extensive metabolizers, poor metabolizers had a 31.5% slower clearance and ultra-rapid metabolizers had a 24% faster clearance.
Hepatic Disease
In a study of 10 patients with stable alcoholic cirrhosis, the clearance of donepezil hydrochloride was decreased by 20% relative to 10 healthy age- and sex-matched subjects.
Renal Disease
In a study of 11 patients with moderate to severe renal impairment (ClC < 18 mL/min/1.73 m2) the clearance of donepezil hydrochloride did not differ from 11 age- and sex-matched healthy subjects.
Age
No formal pharmacokinetic study was conducted to examine age-related differences in the pharmacokinetics of donepezil hydrochloride. Population pharmacokinetic analysis suggested that the clearance of donepezil in patients decreases with increasing age. When compared with 65-year old subjects, 90-year old subjects have a 17% decrease in clearance, while 40-year old subjects have a 33% increase in clearance. The effect of age on donepezil clearance may not be clinically significant.
Gender and Race
No specific pharmacokinetic study was conducted to investigate the effects of gender and race on the disposition of donepezil hydrochloride. However, retrospective pharmacokinetic analysis and population pharmacokinetic analysis of plasma donepezil concentrations measured in patients with Alzheimer’s disease indicates that gender and race (Japanese and Caucasians) did not affect the clearance of donepezil hydrochloride to an important degree.
Body Weight
There was a relationship noted between body weight and clearance. Over the range of body weight from 50 kg to 110 kg, clearance increased from 7.77 L/h to 14.04 L/h, with a value of 10 L/hr for 70 kg individuals.
Drug Interactions
Effect of Donepezil Hydrochloride on the Metabolism of Other Drugs
No in vivo clinical trials have investigated the effect of donepezil hydrochloride on the clearance of drugs metabolized by CYP 3A4 (e.g., cisapride, terfenadine) or by CYP 2D6 (e.g., imipramine). However, in vitro studies show a low rate of binding to these enzymes (mean Ki about 50 to 130 µM), that, given the therapeutic plasma concentrations of donepezil (164 nM), indicates little likelihood of interference. Based on in vitro studies, donepezil shows little or no evidence of direct inhibition of CYP2B6, CYP2C8, and CYP2C19 at clinically relevant concentrations.
Whether donepezil hydrochloride has any potential for enzyme induction is not known. Formal pharmacokinetic studies evaluated the potential of donepezil hydrochloride for interaction with theophylline, cimetidine, warfarin, digoxin, and ketoconazole. No effects of donepezil hydrochloride on the pharmacokinetics of these drugs were observed.
Effect of Other Drugs on the Metabolism of Donepezil Hydrochloride
Ketoconazole and quinidine, strong inhibitors of CYP450 3A and 2D6, respectively, inhibit donepezil metabolism in vitro. Whether there is a clinical effect of quinidine is not known. Population pharmacokinetic analysis showed that in the presence of concomitant CYP2D6 inhibitors donepezil AUC was increased by approximately 17% to 20% in Alzheimer’s disease patients taking donepezil hydrochloride 10 mg and 23 mg. This represented an average effect of weak, moderate, and strong CYP2D6 inhibitors. In a 7-day crossover study in 18 healthy volunteers, ketoconazole (200 mg q.d.) increased mean donepezil (5 mg q.d.) concentrations (AUC0-24 and Cmax) by 36%. The clinical relevance of this increase in concentration is unknown.
Inducers of CYP 3A (e.g., phenytoin, carbamazepine, dexamethasone, rifampin, and phenobarbital) could increase the rate of elimination of donepezil hydrochloride.
Formal pharmacokinetic studies demonstrated that the metabolism of donepezil hydrochloride is not significantly affected by concurrent administration of digoxin or cimetidine.
An in vitro study showed that donepezil was not a substrate of P-glycoprotein.
Drugs Highly Bound to Plasma Proteins
Drug displacement studies have been performed in vitro between this highly bound drug (96%) and other drugs such as furosemide, digoxin, and warfarin. Donepezil hydrochloride at concentrations of 0.3 to 10 micrograms/mL did not affect the binding of furosemide (5 micrograms /mL), digoxin (2 ng/mL), and warfarin (3 micrograms /mL) to human albumin. Similarly, the binding of donepezil hydrochloride to human albumin was not affected by furosemide, digoxin and warfarin.
13. NONCLINICAL TOXICOLOGY
13.1. Carcinogenesis, Mutagenesis, Impairment of Fertility
No evidence of a carcinogenic potential was obtained in an 88-week carcinogenicity study of donepezil conducted in mice at oral doses up to 180 mg/kg/day (approximately 40 times the maximum recommended human dose [MRHD] of 23 mg/day on a mg/m2 basis), or in a 104-week carcinogenicity study in rats at oral doses up to 30 mg/kg/day (approximately 13 times the MRHD on a mg/m2 basis).
Donepezil was negative in a battery of genotoxicity assays (in vitro bacterial reverse mutation, in vitro mouse lymphoma tk, in vitro chromosomal aberration, and in vivo mouse micronucleus).
Donepezil had no effect on fertility in rats at oral doses up to 10 mg/kg/day (approximately 4 times the MRHD on a mg/m2 basis) when administered to males and females prior to and during mating and continuing in females through implantation.
13.2. Animal Toxicology and/or Pharmacology
In an acute dose neurotoxicity study in female rats, oral administration of donepezil and memantine in combination resulted in increased incidence, severity, and distribution of neurodegeneration compared with memantine alone. The no-effect levels of the combination were associated with clinically relevant plasma donepezil and memantine levels.
The relevance of this finding to humans is unknown.
14. CLINICAL STUDIES
14.1. Mild to Moderate Alzheimer's Disease
The effectiveness of donepezil hydrochloride as a treatment for mild to moderate Alzheimer’s disease is demonstrated by the results of two randomized, double-blind, placebo-controlled clinical investigations in patients with Alzheimer’s disease (diagnosed by NINCDS and DSM III-R criteria, Mini-Mental State Examination ≥ 10 and ≤ 26 and Clinical Dementia Rating of 1 or 2). The mean age of patients participating in donepezil hydrochloride trials was 73 years with a range of 50 to 94. Approximately 62% of patients were women and 38% were men. The racial distribution was white 95%, black 3% and other races 2%.
The higher dose of 10 mg did not provide a statistically significantly greater clinical benefit than 5 mg. There is a suggestion, however, based upon order of group mean scores and dose trend analyses of data from these clinical trials, that a daily dose of 10 mg of donepezil hydrochloride might provide additional benefit for some patients. Accordingly, whether or not to employ a dose of 10 mg is a matter of prescriber and patient preference.
Study Outcome Measures
In each study, the effectiveness of treatment with donepezil hydrochloride was evaluated using a dual outcome assessment strategy.
The ability of donepezil hydrochloride to improve cognitive performance was assessed with the cognitive subscale of the Alzheimer’s Disease Assessment Scale (ADAS-cog), a multi-item instrument that has been extensively validated in longitudinal cohorts of Alzheimer’s disease patients. The ADAS-cog examines selected aspects of cognitive performance including elements of memory, orientation, attention, reasoning, language and praxis. The ADAS-cog scoring range is from 0 to 70, with higher scores indicating greater cognitive impairment. Elderly normal adults may score as low as 0 or 1, but it is not unusual for non-demented adults to score slightly higher.
The patients recruited as participants in each study had mean scores on the ADAS-cog of approximately 26 points, with a range from 4 to 61. Experience based on longitudinal studies of ambulatory patients with mild to moderate Alzheimer’s disease suggest that scores on the ADAS-cog increase (worsen) by 6 to 12 points per year. However, smaller changes may be seen in patients with very mild or very advanced disease since the ADAS-cog is not uniformly sensitive to change over the course of the disease. The annualized rate of decline in the placebo patients participating in donepezil hydrochloride trials was approximately 2 to 4 points per year.
The ability of donepezil hydrochloride to produce an overall clinical effect was assessed using a Clinician’s Interview-Based Impression of Change that required the use of caregiver information, the CIBIC-plus. The CIBIC-plus is not a single instrument and is not a standardized instrument like the ADAS-cog. Clinical trials for investigational drugs have used a variety of CIBIC formats, each different in terms of depth and structure.
As such, results from a CIBIC-plus reflect clinical experience from the trial or trials in which it was used and cannot be compared directly with the results of CIBIC-plus evaluations from other clinical trials. The CIBIC-plus used in donepezil hydrochloride trials was a semi-structured instrument that was intended to examine four major areas of patient function: General, Cognitive, Behavioral and Activities of Daily Living. It represents the assessment of a skilled clinician based upon his/her observations at an interview with the patient, in combination with information supplied by a caregiver familiar with the behavior of the patient over the interval rated. The CIBIC-plus is scored as a seven point categorical rating, ranging from a score of 1, indicating “markedly improved,” to a score of 4, indicating “no change” to a score of 7, indicating “markedly worse”. The CIBIC-plus has not been systematically compared directly to assessments not using information from caregivers (CIBIC) or other global methods.
Thirty-Week Study
In a study of 30 weeks duration, 473 patients were randomized to receive single daily doses of placebo, 5 mg/day or 10 mg/day of donepezil hydrochloride. The 30-week study was divided into a 24-week double-blind active treatment phase followed by a 6-week single-blind placebo washout period. The study was designed to compare 5 mg/day or 10 mg/day fixed doses of donepezil hydrochloride to placebo. However, to reduce the likelihood of cholinergic effects, the 10 mg/day treatment was started following an initial 7-day treatment with 5 mg/day doses.
Effects on the ADAS-cog
Figure 1 illustrates the time course for the change from baseline in ADAS-cog scores for all three dose groups over the 30 weeks of the study. After 24 weeks of treatment, the mean differences in the ADAS-cog change scores for donepezil hydrochloride treated patients compared to the patients on placebo were 2.8 and 3.1 points for the 5 mg/day and 10 mg/day treatments, respectively. These differences were statistically significant. While the treatment effect size may appear to be slightly greater for the 10 mg/day treatment, there was no statistically significant difference between the two active treatments.
Following 6 weeks of placebo washout, scores on the ADAS-cog for both the donepezil hydrochloride treatment groups were indistinguishable from those patients who had received only placebo for 30 weeks. This suggests that the beneficial effects of donepezil hydrochloride abate over 6 weeks following discontinuation of treatment and do not represent a change in the underlying disease. There was no evidence of a rebound effect 6 weeks after abrupt discontinuation of therapy.

Figure 2 illustrates the cumulative percentages of patients from each of the three treatment groups who had attained the measure of improvement in ADAS-cog score shown on the X axis. Three change scores, (7-point and 4-point reductions from baseline or no change in score) have been identified for illustrative purposes, and the percent of patients in each group achieving that result is shown in the inset table.
The curves demonstrate that both patients assigned to placebo and donepezil hydrochloride have a wide range of responses, but that the active treatment groups are more likely to show greater improvements. A curve for an effective treatment would be shifted to the left of the curve for placebo, while an ineffective or deleterious treatment would be superimposed upon or shifted to the right of the curve for placebo.

Effects on the CIBIC-plus
Figure 3 is a histogram of the frequency distribution of CIBIC-plus scores attained by patients assigned to each of the three treatment groups who completed 24 weeks of treatment. The mean drug-placebo differences for these groups of patients were 0.35 points and 0.39 points for 5 mg/day and 10 mg/day of donepezil hydrochloride, respectively. These differences were statistically significant. There was no statistically significant difference between the two active treatments.

Fifteen-Week Study
In a study of 15 weeks duration, patients were randomized to receive single daily doses of placebo or either 5 mg/day or 10 mg/day of donepezil hydrochloride for 12 weeks, followed by a 3-week placebo washout period. As in the 30-week study, to avoid acute cholinergic effects, the 10 mg/day treatment followed an initial 7-day treatment with 5 mg/day doses.
Effects on the ADAS-Cog
Figure 4 illustrates the time course of the change from baseline in ADAS-cog scores for all three dose groups over the 15 weeks of the study. After 12 weeks of treatment, the differences in mean ADAS-cog change scores for the donepezil hydrochloride treated patients compared to the patients on placebo were 2.7 and 3 points each, for the 5 and 10 mg/day donepezil hydrochloride treatment groups, respectively. These differences were statistically significant. The effect size for the 10 mg/day group may appear to be slightly larger than that for 5 mg/day. However, the differences between active treatments were not statistically significant.

Following 3 weeks of placebo washout, scores on the ADAS-cog for both the donepezil hydrochloride treatment groups increased, indicating that discontinuation of donepezil hydrochloride resulted in a loss of its treatment effect. The duration of this placebo washout period was not sufficient to characterize the rate of loss of the treatment effect, but, the 30-week study (see above) demonstrated that treatment effects associated with the use of donepezil hydrochloride abate within 6 weeks of treatment discontinuation.
Figure 5 illustrates the cumulative percentages of patients from each of the three treatment groups who attained the measure of improvement in ADAS-cog score shown on the X axis. The same three change scores, (7-point and 4-point reductions from baseline or no change in score) as selected for the 30-week study have been used for this illustration. The percentages of patients achieving those results are shown in the inset table.
As observed in the 30-week study, the curves demonstrate that patients assigned to either placebo or to donepezil hydrochloride have a wide range of responses, but that the donepezil hydrochloride treated patients are more likely to show greater improvements in cognitive performance.

Effects on the CIBIC-plus
Figure 6 is a histogram of the frequency distribution of CIBIC-plus scores attained by patients assigned to each of the three treatment groups who completed 12 weeks of treatment. The differences in mean scores for donepezil hydrochloride treated patients compared to the patients on placebo at Week 12 were 0.36 and 0.38 points for the 5 mg/day and 10 mg/day treatment groups, respectively. These differences were statistically significant.

In both studies, patient age, sex and race were not found to predict the clinical outcome of donepezil hydrochloride treatment.
14.2. Moderate to Severe Alzheimer's Disease
The effectiveness of donepezil hydrochloride in the treatment of patients with moderate to severe Alzheimer’s Disease was established in studies employing doses of 10 mg/day and 23 mg/day. Results of a controlled clinical trial in moderate to severe Alzheimer’s Disease that compared donepezil hydrochloride 23 mg once daily to 10 mg once daily suggest that a 23 mg dose of donepezil hydrochloride provided additional benefit.
Swedish 6 Month Study (10 mg/day)
The effectiveness of donepezil hydrochloride as a treatment for severe Alzheimer’s disease is demonstrated by the results of a randomized, double-blind, placebo-controlled clinical study conducted in Sweden (6 month study) in patients with probable or possible Alzheimer’s disease diagnosed by NINCDS-ADRDA and DSM-IV criteria, MMSE: range of 1 to 10. Two hundred and forty eight (248) patients with severe Alzheimer’s disease were randomized to donepezil hydrochloride or placebo. For patients randomized to donepezil hydrochloride, treatment was initiated at 5 mg once daily for 28 days and then increased to 10 mg once daily. At the end of the 6 month treatment period, 90.5% of the donepezil hydrochloride treated patients were receiving the 10 mg/day dose. The mean age of patients was 84.9 years, with a range of 59 to 99. Approximately 77% of patients were women, and 23% were men. Almost all patients were Caucasian. Probable AD was diagnosed in the majority of the patients (83.6% of donepezil hydrochloride treated patients and 84.2% of placebo treated patients).
Study Outcome Measures
The effectiveness of treatment with donepezil hydrochloride was determined using a dual outcome assessment strategy that evaluated cognitive function using an instrument designed for more impaired patients and overall function through caregiver-rated assessment. This study showed that patients on donepezil hydrochloride experienced significant improvement on both measures compared to placebo.
The ability of donepezil hydrochloride to improve cognitive performance was assessed with the Severe Impairment Battery (SIB). The SIB, a multi-item instrument, has been validated for the evaluation of cognitive function in patients with moderate to severe dementia. The SIB evaluates selective aspects of cognitive performance, including elements of memory, language, orientation, attention, praxis, visuospatial ability, construction, and social interaction. The SIB scoring range is from 0 to 100, with lower scores indicating greater cognitive impairment.
Daily function was assessed using the Modified Alzheimer’s Disease Cooperative Study Activities of Daily Living Inventory for Severe Alzheimer’s Disease (ADCS-ADL-severe). The ADCS-ADL-severe is derived from the Alzheimer’s Disease Cooperative Study Activities of Daily Living Inventory, which is a comprehensive battery of ADL questions used to measure the functional capabilities of patients. Each ADL item is rated from the highest level of independent performance to complete loss. The ADCS-ADL-severe is a subset of 19 items, including ratings of the patient’s ability to eat, dress, bathe, use the telephone, get around (or travel), and perform other activities of daily living; it has been validated for the assessment of patients with moderate to severe dementia. The ADCS-ADL-severe has a scoring range of 0 to 54, with the lower scores indicating greater functional impairment. The investigator performs the inventory by interviewing a caregiver, in this study a nurse staff member, familiar with the functioning of the patient.
Effects on the SIB
Figure 7 shows the time course for the change from baseline in SIB score for the two treatment groups over the 6 months of the study. At 6 months of treatment, the mean difference in the SIB change scores for donepezil hydrochloride treated patients compared to patients on placebo was 5.9 points. Donepezil hydrochloride treatment was statistically significantly superior to placebo.

Figure 8 illustrates the cumulative percentages of patients from each of the two treatment groups who attained the measure of improvement in SIB score shown on the X-axis. While patients assigned both to donepezil hydrochloride and to placebo have a wide range of responses, the curves show that the donepezil hydrochloride group is more likely to show a greater improvement in cognitive performance.

Effects on the ADCS-ADL-severe
Figure 9 illustrates the time course for the change from baseline in ADCS-ADL-severe scores for patients in the two treatment groups over the 6 months of the study. After 6 months of treatment, the mean difference in the ADCS-ADL-severe change scores for donepezil hydrochloride treated patients compared to patients on placebo was 1.8 points. Donepezil hydrochloride treatment was statistically significantly superior to placebo.
Figure 10 shows the cumulative percentages of patients from each treatment group with specified changes from baseline ADCS-ADL-severe scores. While both patients assigned to donepezil hydrochloride and placebo have a wide range of responses, the curves demonstrate that the donepezil hydrochloride group is more likely to show a smaller decline or an improvement.

Japanese 24-Week Study(10 mg/day)
In a study of 24 weeks duration conducted in Japan, 325 patients with severe Alzheimer’s disease were randomized to doses of 5 mg/day or 10 mg/day of donepezil, administered once daily, or placebo. Patients randomized to treatment with donepezil were to achieve their assigned doses by titration, beginning at 3 mg/day, and extending over a maximum of 6 weeks. Two hundred and forty eight (248) patients completed the study, with similar proportions of patients completing the study in each treatment group. The primary efficacy measures for this study were the SIB and CIBIC-plus.
At 24 weeks of treatment, statistically significant treatment differences were observed between the 10 mg/day dose of donepezil and placebo on both the SIB and CIBIC-plus. The 5 mg/day dose of donepezil showed a statistically significant superiority to placebo on the SIB, but not on the CIBIC-plus.
Study of 23 mg/day
The effectiveness of donepezil hydrochloride 23 mg/day as a treatment for moderate to severe Alzheimer’s disease has been demonstrated by the results of a randomized, double-blind, controlled clinical investigation in patients with moderate to severe Alzheimer’s disease. The controlled clinical study was conducted globally in patients with probable Alzheimer’s disease diagnosed by NINCDS-ADRDA and DSM-IV criteria, MMSE: range of 0 to 20. Patients were required to have been on a stable dose of donepezil hydrochloride 10 mg/day for at least 3 months prior to screening. One thousand four hundred and thirty four (1434) patients with moderate to severe Alzheimer’s disease were randomized to 23 mg/day or 10 mg/day. The mean age of patients was 73.8 years, with a range of 47 to 90. Approximately 63% of patients were women, and 37% were men. Approximately 36% of the patients were taking memantine throughout the study.
Study Outcome Measures
The effectiveness of treatment with 23 mg/day was determined using a dual outcome assessment strategy that evaluated cognitive function using an instrument designed for more impaired patients and overall function through caregiver-rated assessment.
The ability of 23 mg/day to improve cognitive performance was assessed with the Severe Impairment Battery (SIB). The SIB, a multi-item instrument, has been validated for the evaluation of cognitive function in patients with moderate to severe dementia. The SIB evaluates selective aspects of cognitive performance, including elements of memory, language, orientation, attention, praxis, visuospatial ability, construction, and social interaction. The SIB scoring range is from 0 to 100, with lower scores indicating greater cognitive impairment.
The ability of 23 mg/day to produce an overall clinical effect was assessed using a Clinician’s Interview-Based Impression of Change that incorporated the use of caregiver information, the CIBIC-plus. The CIBIC-plus used in this trial was a semi-structured instrument that examines four major areas of patient function: General, Cognitive, Behavioral, and Activities of Daily Living. It represents the assessment of a skilled clinician based upon his/her observations at an interview with the patient, in combination with information supplied by a caregiver familiar with the behavior of the patient over the interval rated. The CIBIC-plus is scored as a seven-point categorical rating, ranging from a score of 1, indicating “markedly improved,” to a score of 4, indicating “no change” to a score of 7, indicating “markedly worse.”
Effects on the SIB
Figure 11 shows the time course for the change from baseline in SIB score for the two treatment groups over the 24 weeks of the study. At 24 weeks of treatment, the LS mean difference in the SIB change scores for 23 mg/day-treated patients compared to patients treated with 10 mg was 2.2 units (p = 0.0001). The dose of 23 mg/day was statistically significantly superior to the dose of 10 mg/day.
Figure 11. Time-course of the Change from Baseline in SIB Score for Patients Completing 24 Weeks of Treatment.

Figure 12 illustrates the cumulative percentages of patients from each of the two treatment groups who attained the measure of improvement in SIB score shown on the X-axis. While patients assigned both to 23 mg/day and to 10 mg/day have a wide range of responses, the curves show that the 23 mg-group is more likely to show a greater improvement in cognitive performance. When such curves are shifted to the left, this indicates a greater percentage of patients responding to treatment on the SIB.
Figure 12. Cumulative Percentage of Patients Completing 24 Weeks of Double-blind Treatment with Specified Changes from Baseline SIB Scores.

Effects on the CIBIC-plus
Figure 13 is a histogram of the frequency distribution of CIBIC-plus scores attained by patients at the end of 24 weeks of treatment. The mean difference between the 23 mg/day and 10 mg/day treatment groups was 0.06 units. This difference was not statistically significant.
Figure 13. Frequency Distribution of CIBIC-plus Scores at Week 24.

16. HOW SUPPLIED/STORAGE AND HANDLING
17. PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Patient Information).
Instruct patients and caregivers to take donepezil hydrochloride tablet only once per day, as prescribed.
Instruct patients and caregivers that donepezil hydrochloride tablets can be taken with or without food.
Advise patients and caregivers that donepezil hydrochloride tablets may cause nausea, diarrhea, insomnia, vomiting, muscle cramps, fatigue, and decreased appetite.
Advise patients to notify their healthcare provider if they are pregnant or plan to become pregnant.
Medication Guide available at http://www.alembicusa.com/medicationguide.aspx or call 1-866-210-9797.
Manufactured by:
Alembic Pharmaceuticals Limited
(Formulation Division),
Panelav 389350, Gujarat, India
Manufactured for:
Alembic Pharmaceuticals, Inc.
Bedminster, NJ 07921, USA
Revised: 08/2021
Patient Package Insert
Donepezil Hydrochloride (doe-NEP-e-zil HYE-droe-KLOR-ide)
Tablets, USP
•Tablets: 5 mg and 10 mg
Read this Patient Information that comes with donepezil hydrochloride before you start taking it and each time you get a refill. There may be new information. This leaflet does not take the place of talking with your doctor about Alzheimer’s disease or treatment for it. If you have questions, ask the doctor or pharmacist.
What is donepezil hydrochloride?
Donepezil hydrochloride comes as donepezil hydrochloride film-coated tablets in dosage strengths of 5 mg and 10 mg.
Donepezil hydrochloride is a prescription medicine to treat mild, moderate, and severe Alzheimer’s disease. Donepezil hydrochloride can help with mental function and with doing daily tasks. Donepezil hydrochloride does not work the same in all people. Some people may:
•Seem much better
•Get better in small ways or stay the same
•Get worse over time but slower than expected
•Not change and then get worse as expected
Donepezil hydrochloride does not cure Alzheimer’s disease. All patients with Alzheimer’s disease get worse over time, even if they take donepezil hydrochloride.
Donepezil hydrochloride has not been approved as a treatment for any medical condition in children.
Who should not take donepezil hydrochloride?
Do not take donepezil hydrochloride if you are allergic to any of the ingredients in donepezil hydrochloride or to medicines that contain piperidines. Ask your doctor if you are not sure. See the end of this leaflet for a list of ingredients in donepezil hydrochloride.
What should I tell my doctor before taking donepezil hydrochloride?
Tell the doctor about all of your present or past health problems and conditions. Include:
•Any heart problems including problems with irregular, slow, or fast heartbeats
•Asthma or lung problems
•A seizure
•Stomach ulcers
•Difficulty passing urine
•Liver or kidney problems
•Trouble swallowing tablets
•Present pregnancy or plans to become pregnant. It is not known if donepezil hydrochloride can harm an unborn baby.
•Present breast-feeding. It is not known if donepezil hydrochloride passes into breast milk. Talk to your doctor about the best way to feed your baby if you take donepezil hydrochloride.
Tell the doctor about all the medicines you take, including prescription and non-prescription medicines, vitamins, and herbal products. Donepezil hydrochloride and other medicines may affect each other.
Be particularly sure to tell the doctor if you take aspirin or medicines called nonsteroidal anti-inflammatory drugs (NSAIDs). There are many NSAID medicines, both prescription and non-prescription. Ask the doctor or pharmacist if you are not sure if any of your medicines are NSAIDs. Taking NSAIDs and donepezil hydrochloride together may make you more likely to get stomach ulcers.
Donepezil hydrochloride taken with certain medicines used for anesthesia may cause side effects. Tell the responsible doctor or dentist that you take donepezil hydrochloride before you have:
•surgery
•medical procedures
•dental surgery or procedures.
Know the medicines that you take. Keep a list of all the yours medicines. Show it to your doctor or pharmacist before you start a new medicine.
How should you take donepezil hydrochloride?
•Take donepezil hydrochloride exactly as prescribed by the doctor. Do not stop donepezil hydrochloride or change the dose yourself. Talk with your doctor first.
•Take donepezil hydrochloride one time each day. Donepezil hydrochloride can be taken with or without food.
•If you miss a dose of donepezil hydrochloride, just wait. Take only the next dose at the usual time. Do not take 2 doses at the same time.
•If donepezil hydrochloride is missed for 7 days or more, talk with your doctor before starting again.
•If you take too much donepezil hydrochloride at one time, call your doctor or poison control center, or go to the emergency room right away.
What are the possible side effects of donepezil hydrochloride?
Donepezil hydrochloride may cause the following serious side effects:
•slow heartbeat and fainting. This happens more often in people with heart problems. Call your doctor right away if you feel faint or lightheaded while taking donepezil hydrochloride.
•more stomach acid. This raises the chance of ulcers and bleeding, especially when taking donepezil hydrochloride 23 mg. The risk is higher for peoples who have ulcers, or take aspirin or other NSAIDs.
•worsening of lung problems in people with asthma or other lung disease.
•seizures.
•difficulty passing urine.
Call the doctor right away if you have:
•fainting.
•heartburn or stomach pain that is new or won’t go away.
•nausea or vomiting, blood in the vomit, dark vomit that looks like coffee grounds.
•bowel movements or stools that look like black tar.
•new or worse asthma or breathing problems.
•seizures.
•difficulty passing urine.
The most common side effects of donepezil hydrochloride are:
•nausea
•diarrhea
•not sleeping well
•vomiting
•muscle cramps
•feeling tired
•not wanting to eat
These side effects may get better after you take donepezil hydrochloride for a while. This is not a complete list of side effects with donepezil hydrochloride. For more information, ask your doctor or pharmacist.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
How should donepezil hydrochloride be stored?
Store donepezil hydrochloride at 20° to 25°C (68° to 77°F) [see USP Controlled Room Temperature].
Keep donepezil hydrochloride and all medicines out of the reach of children.
General information about donepezil hydrochloride
Medicines are sometimes prescribed for conditions that are not mentioned in this Patient Information Leaflet. Do not use donepezil hydrochloride for a condition for which it was not prescribed. Do not give donepezil hydrochloride to other people, even if they have the same symptoms or condition. It may harm them.
This leaflet summarizes the most important information about donepezil hydrochloride. If you would like more information, talk with your doctor. You can ask your pharmacist or doctor for information about donepezil hydrochloride that is written for health professionals. For more information call Alembic Pharmaceuticals Limited at 1-866-210-9797.
What are the ingredients in donepezil hydrochloride tablets, USP?
Active ingredient: donepezil hydrochloride USP
Inactive ingredients:
•Donepezil hydrochloride film-coated tablets, USP 5 mg and 10 mg: lactose monohydrate, corn starch, microcrystalline cellulose, hydroxypropyl cellulose, and magnesium stearate. The film coating contains talc, polyethylene glycol, hypromellose and titanium dioxide. Additionally, the 10 mg tablet contains iron oxide yellow as a coloring agent.
Medication Guide available at http://www.alembicusa.com/medicationguide.aspx or call 1-866-210-9797.
Manufactured by:
Alembic Pharmaceuticals Limited
(Formulation Division),
Panelav 389350, Gujarat, India
Manufactured for:
Alembic Pharmaceuticals, Inc.
Bedminster, NJ 07921, USA
Revised: 08/2021
INGREDIENTS AND APPEARANCE
| DONEPEZIL HYDROCHLORIDE
donepezil hydrochloride tablet, film coated |
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
| Labeler - Bryant Ranch Prepack (171714327) |
| Registrant - Bryant Ranch Prepack (171714327) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Bryant Ranch Prepack | 171714327 | REPACK(71335-0876) , RELABEL(71335-0876) | |