Search by Drug Name or NDC
NDC 71335-1382-02 LOW DOSE ASPIRIN 81 mg/1 Details
LOW DOSE ASPIRIN 81 mg/1
LOW DOSE ASPIRIN is a ORAL TABLET, COATED in the HUMAN OTC DRUG category. It is labeled and distributed by Bryant Ranch Prepack. The primary component is ASPIRIN.
MedlinePlus Drug Summary
Prescription aspirin is used to relieve the symptoms of rheumatoid arthritis (arthritis caused by swelling of the lining of the joints), osteoarthritis (arthritis caused by breakdown of the lining of the joints), systemic lupus erythematosus (condition in which the immune system attacks the joints and organs and causes pain and swelling) and certain other rheumatologic conditions (conditions in which the immune system attacks parts of the body). Nonprescription aspirin is used to reduce fever and to relieve mild to moderate pain from headaches, menstrual periods, arthritis, toothaches, and muscle aches. Nonprescription aspirin is also used to prevent heart attacks in people who have had a heart attack in the past or who have angina (chest pain that occurs when the heart does not get enough oxygen). Nonprescription aspirin is also used to reduce the risk of death in people who are experiencing or who have recently experienced a heart attack. Nonprescription aspirin is also used to prevent ischemic strokes (strokes that occur when a blood clot blocks the flow of blood to the brain) or mini-strokes (strokes that occur when the flow of blood to the brain is blocked for a short time) in people who have had this type of stroke or mini-stroke in the past. Aspirin will not prevent hemorrhagic strokes (strokes caused by bleeding in the brain). Aspirin is in a group of medications called salicylates. It works by stopping the production of certain natural substances that cause fever, pain, swelling, and blood clots. Aspirin is also available in combination with other medications such as antacids, pain relievers, and cough and cold medications. This monograph only includes information about the use of aspirin alone. If you are taking a combination product, read the information on the package or prescription label or ask your doctor or pharmacist for more information.
Related Packages: 71335-1382-02Last Updated: 12/01/2022
MedLinePlus Full Drug Details: Aspirin
Product Information
| NDC | 71335-1382 |
|---|---|
| Product ID | 71335-1382_d67fd0a4-2590-4cf5-a3e8-dec5f8c3fa7b |
| Associated GPIs | 64100010000601 |
| GCN Sequence Number | 016995 |
| GCN Sequence Number Description | aspirin TABLET DR 81 MG ORAL |
| HIC3 | M9P |
| HIC3 Description | PLATELET AGGREGATION INHIBITORS |
| GCN | 00161 |
| HICL Sequence Number | 001820 |
| HICL Sequence Number Description | ASPIRIN |
| Brand/Generic | Generic |
| Proprietary Name | LOW DOSE ASPIRIN |
| Proprietary Name Suffix | n/a |
| Non-Proprietary Name | ASPIRIN |
| Product Type | HUMAN OTC DRUG |
| Dosage Form | TABLET, COATED |
| Route | ORAL |
| Active Ingredient Strength | 81 |
| Active Ingredient Units | mg/1 |
| Substance Name | ASPIRIN |
| Labeler Name | Bryant Ranch Prepack |
| Pharmaceutical Class | Anti-Inflammatory Agents, Non-Steroidal [CS], Cyclooxygenase Inhibitors [MoA], Decreased Platelet Aggregation [PE], Decreased Prostaglandin Production [PE], Nonsteroidal Anti-inflammatory Drug [EPC], Platelet Aggregation Inhibitor [EPC] |
| DEA Schedule | n/a |
| Marketing Category | OTC MONOGRAPH NOT FINAL |
| Application Number | part343 |
| Listing Certified Through | 2023-12-31 |
Package
Package Images
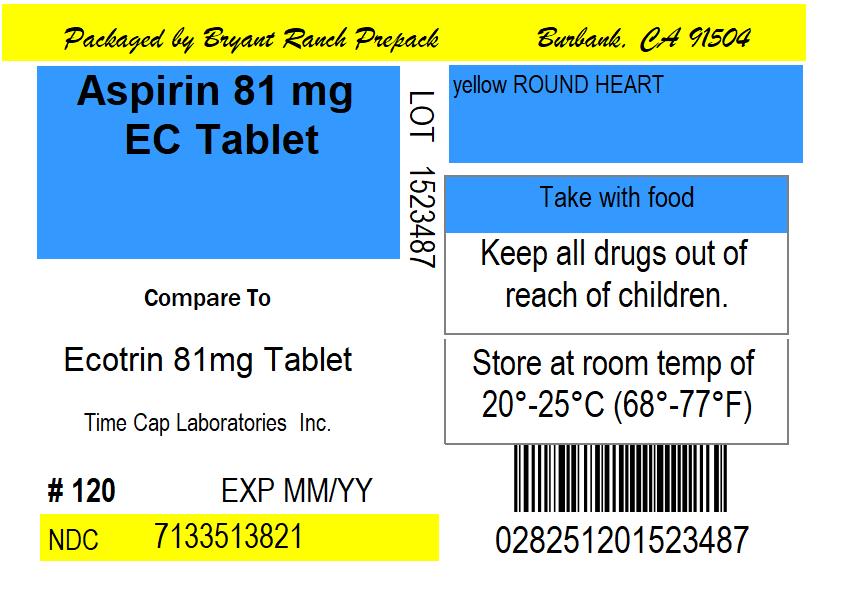
NDC 71335-1382-02 (71335138202)
| NDC Package Code | 71335-1382-2 |
|---|---|
| Billing NDC | 71335138202 |
| Package | 30 TABLET, COATED in 1 BOTTLE (71335-1382-2) |
| Marketing Start Date | 2020-03-12 |
| NDC Exclude Flag | N |
| Pricing Information | N/A |
Standard Product Labeling (SPL)/Prescribing Information SPL a272bf44-54df-448a-8bff-e31f9488b416 Details
SPL UNCLASSIFIED SECTION
SPL UNCLASSIFIED SECTION
SPL UNCLASSIFIED SECTION
Warnings
Reye’s syndrome: Children and teenagers who have or are recovering from chicken pox or flu-like symptoms should not use this product. When using this product, if changes in behavior with nausea and vomiting occur, consult a doctor because these symptoms could be an early sign of Reye's syndrome, a rare but serious illness.
Allergy alert: Aspirin may cause a severe allergic reaction, which may include:
- hives
- facial swelling
- shock
- asthma (wheezing)
Stomach bleeding warning: This product contains an NSAID, which may cause severe stomach bleeding. The chance is higher if you:
- are age 60 or older
- have had stomach ulcers or bleeding problems
- take a blood thinning (anticoagulant) or steroid drug
- take other drugs containing prescription or nonprescription NSAIDs (aspirin, ibuprofen, naproxen, or others)
- have 3 or more alcoholic drinks every day while using this product
- take more or for a longer time than directed
SPL UNCLASSIFIED SECTION
SPL UNCLASSIFIED SECTION
SPL UNCLASSIFIED SECTION
SPL UNCLASSIFIED SECTION
Stop use and ask a doctor if
- an allergic reaction occurs. Seek medical help right away.
- you experience any of the following signs of stomach bleeding:
feel faint
have bloody or black stools
vomit blood
have stomach pain that does not get better
- pain gets worse or lasts more than 10 days
- fever gets worse or lasts more than 3 days
- redness or swelling is present
- new symptoms occur
- ringing in the ears or a loss of hearing occurs
SPL UNCLASSIFIED SECTION
SPL UNCLASSIFIED SECTION
SPL UNCLASSIFIED SECTION
OTHER INFORMATION
SPL UNCLASSIFIED SECTION
Inactive ingredients anhydrous lactose, carnauba wax, colloidal silicon dioxide, croscarmellose sodium, D&C yellow #10 aluminum lake, iron oxide ochre, methacrylic acid and ethyl acrylate copolymer, microcrystalline cellulose, polysorbate 80, simethicone, sodium hydroxide, sodium lauryl sulfate, starch, talc, titanium dioxide, triethyl citrate
HOW SUPPLIED
NDC: 71335-1382-8: 15 Tablets in a BOTTLE
NDC: 71335-1382-1: 120 Tablets in a BOTTLE
NDC: 71335-1382-2: 30 Tablets in a BOTTLE
NDC: 71335-1382-3: 100 Tablets in a BOTTLE
NDC: 71335-1382-4: 20 Tablets in a BOTTLE
NDC: 71335-1382-5: 90 Tablets in a BOTTLE
NDC: 71335-1382-6: 60 Tablets in a BOTTLE
NDC: 71335-1382-7: 36 Tablets in a BOTTLE
NDC: 71335-1382-9: 10 Tablets in a BOTTLE
INGREDIENTS AND APPEARANCE
| LOW DOSE ASPIRIN
aspirin tablet, coated |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Labeler - Bryant Ranch Prepack (171714327) |
| Registrant - Bryant Ranch Prepack (171714327) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Bryant Ranch Prepack | 171714327 | REPACK(71335-1382) , RELABEL(71335-1382) | |