Search by Drug Name or NDC
NDC 71610-0511-30 Trazodone Hydrochloride 100 mg/1 Details
Trazodone Hydrochloride 100 mg/1
Trazodone Hydrochloride is a ORAL TABLET in the HUMAN PRESCRIPTION DRUG category. It is labeled and distributed by Aphena Pharma Solutions - Tennessee, LLC. The primary component is TRAZODONE HYDROCHLORIDE.
MedlinePlus Drug Summary
Trazodone is used to treat depression. Trazodone is in a class of medications called serotonin modulators. It works by increasing the amount of serotonin, a natural substance in the brain that helps maintain mental balance.
Related Packages: 71610-0511-30Last Updated: 12/01/2022
MedLinePlus Full Drug Details: Trazodone
Product Information
| NDC | 71610-0511 |
|---|---|
| Product ID | 71610-511_e02c5c89-bea0-4bca-b774-2c9faaec5e73 |
| Associated GPIs | 58120080100310 |
| GCN Sequence Number | 046242 |
| GCN Sequence Number Description | trazodone HCl TABLET 100 MG ORAL |
| HIC3 | H7E |
| HIC3 Description | SEROTONIN-2 ANTAGONIST/REUPTAKE INHIBITORS (SARIS) |
| GCN | 16392 |
| HICL Sequence Number | 001652 |
| HICL Sequence Number Description | TRAZODONE HCL |
| Brand/Generic | Generic |
| Proprietary Name | Trazodone Hydrochloride |
| Proprietary Name Suffix | n/a |
| Non-Proprietary Name | Trazodone Hydrochloride |
| Product Type | HUMAN PRESCRIPTION DRUG |
| Dosage Form | TABLET |
| Route | ORAL |
| Active Ingredient Strength | 100 |
| Active Ingredient Units | mg/1 |
| Substance Name | TRAZODONE HYDROCHLORIDE |
| Labeler Name | Aphena Pharma Solutions - Tennessee, LLC |
| Pharmaceutical Class | Serotonin Reuptake Inhibitor [EPC] |
| DEA Schedule | n/a |
| Marketing Category | ANDA |
| Application Number | ANDA205253 |
| Listing Certified Through | 2024-12-31 |
Package
Package Images

NDC 71610-0511-30 (71610051130)
| NDC Package Code | 71610-511-30 |
|---|---|
| Billing NDC | 71610051130 |
| Package | 30 TABLET in 1 BOTTLE (71610-511-30) |
| Marketing Start Date | 2020-12-29 |
| NDC Exclude Flag | N |
| Pricing Information | N/A |
Standard Product Labeling (SPL)/Prescribing Information SPL b716575a-5b96-49c5-bcb2-56b05fbb7920 Details
HIGHLIGHTS OF PRESCRIBING INFORMATION
TRAZODONE HYDROCHLORIDE tablets, for oral use
Initial U.S. Approval: 1981
WARNING: SUICIDAL THOUGHTS and BEHAVIORS
See full prescribing information for complete boxed warning.
INDICATIONS AND USAGE
Trazodone is a selective serotonin reuptake inhibitor indicated for the treatment of major depressive disorder (MDD) (1).
DOSAGE AND ADMINISTRATION
- Starting dose: 150 mg in divided doses daily. May be increased by 50 mg per day every three to four days. Maximum dose: 400 mg per day in divided doses (2).
- Trazodone hydrochloride tablets should be taken shortly after a meal or light snack (2).
- Tablets should be swallowed whole or broken in half along the score line, and should not be chewed or crushed (2).
- When discontinued, gradual dose reduction is recommended (2).
DOSAGE FORMS AND STRENGTHS
- Scored tablets of 50mg, 100mg, 150 mg and 300 mg (3).
CONTRAINDICATIONS
WARNINGS AND PRECAUTIONS
- Serotonin Syndrome: Increased risk when co-administered with other serotonergic agents (e.g., SSRI, SNRI, triptans), but also when taken alone. If it occurs, discontinue trazodone and initiate supportive treatment (5.2).
- Cardiac Arrhythmias: Increases the QT interval. Avoid use with drugs that also increase the QT interval and in patients with risk factors for prolonged QT interval (5.3)
- Orthostatic Hypotension and Syncope: Warn patients of risk and symptoms of hypotension (5.4).
- Increased Risk of Bleeding: Concomitant use of aspirin, nonsteroidal anti-inflammatory drugs (NSAIDs), other antiplatelet drugs, warfarin, and other anticoagulants may increase this risk (5.5).
- Priapism: Cases of painful and prolonged penile erections and priapism have been reported. Immediate medical attention should be sought if signs and symptoms of prolonged penile erections or priapism are observed (5.6).
- Activation of Mania or Hypomania: Screen for bipolar disorder and monitor for mania or hypomania (5.7).
- Potential for Cognitive and Motor Impairment: Has potential to impair judgment, thinking, and motor skills. Advise patients to use caution when operating machinery (5.9).
- Angle-Closure Glaucoma: Avoid use of antidepressants, including trazodone, in patients with untreated anatomically narrow angles. (5.10).
ADVERSE REACTIONS
Most common adverse reactions (incidence ≥ 5% and twice that of placebo) are: edema, blurred vision, syncope, drowsiness, fatigue, diarrhea, nasal congestion, weight loss (6).
To report SUSPECTED ADVERSE REACTIONS, contact Zydus Pharmaceuticals (USA) Inc. at 1-877-993-8779 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS
- CNS Depressants: Trazodone may enhance effects of alcohol, barbiturates, or other CNS depressants (7).
- CYP3A4 Inhibitors: Consider trazodone dose reduction based on tolerability (2.5, 7).
- CYP3A4 Inducers: Increase in trazodone dosage may be necessary (2.5, 7).
- Digoxin or Phenytoin: Monitor for increased digoxin or phenytoin serum levels (7).
- Warfarin: Monitor for increased or decreased prothrombin time (7).
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 12/2020
Table Of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
WARNING: SUICIDAL THOUGHTS and BEHAVIORS
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Dose Selection
2.2 Improtant Administration Instructions
2.3 Screen for Bipolar Disorder Prior to Starting Trazodone
2.4 Switchning to or from Monoamine Oxidase Inhibitor Antidepressant
2.5 Dosage Recommendations for Concomitant Use with Strong CYP3A4 Inhibitors or Inducers
2.6 Discontinuation of Treatment with Trazodone
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Suicidal Thoughts and Behaviors in Pediatric and Young Adult Patients
5.2 Serotonin Syndrome
5.3 Cardiac Arrhythmias
5.4 Orthostatic Hypotension and Syncope
5.5 Increased Risk of Bleeding
5.6 Priapism
5.7 Activation of Mania or Hypomania
5.8 Discontinuation Syndrome
5.9 Potential for Cognitive and Motor Impairment
5.10 Angle-Closure Glaucoma
5.11 Hyponatremia
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Postmarketing Experience
7 DRUG INTERACTIONS
7.1 Drugs Having Clinically Important Interactions with Trazodone
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Renal Impairment
8.7 Hepatic Impairment
9 DRUG ABUSE AND DEPENDENCE
9.1 Controlled Substance
9.2 Abuse
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- *
- Sections or subsections omitted from the full prescribing information are not listed.
WARNING
Antidepressants increased the risk of suicidal thoughts and behaviors in pediatric and young adult patients in short-term studies. Closely monitor all antidepressant-treated patients for clinical worsening, and for emergence of suicidal thoughts and behaviors [see Warnings and Precautions (5.1)]. Trazodone is not approved for use in pediatric patients [see Use in Specific Populations (8.4)].
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Dose Selection
An initial dose of 150 mg/day in divided doses is suggested. The dosage should be initiated at a low-dose and increased gradually, noting the clinical response and any evidence of intolerance. Occurrence of drowsiness may require the administration of a major portion of the daily dose at bedtime or a reduction of dosage.
The dose may be increased by 50 mg/day every 3 to 4 days. The maximum dose for outpatients usually should not exceed 400 mg/day in divided doses. Inpatients (i.e., more severely depressed patients) may be given up to but not in excess of 600 mg/day in divided doses.
Once an adequate response has been achieved, dosage may be gradually reduced, with subsequent adjustment depending on therapeutic response.
2.2 Improtant Administration Instructions
Trazodone hydrochloride tablets can be swallowed whole or administered as a half tablet by breaking the tablet along the score line.
Trazodone hydrochloride tablets should be taken shortly after a meal or light snack.
2.3 Screen for Bipolar Disorder Prior to Starting Trazodone
Prior to initiating treatment with trazodone hydrochloride tablets or another antidepressant, screen patients for a personal or family history of bipolar disorder, mania, or hypomania [see Warnings and Precautions (5.7)].
2.4 Switchning to or from Monoamine Oxidase Inhibitor Antidepressant
At least 14 days must elapse between discontinuation of a monoamine oxidase inhibitor (MAOI) antidepressant and initiation of trazodone hydrochloride tablets. In addition, at least 14 days must elapse after stopping trazodone hydrochloride tablets before starting an MAOI antidepressant [see Contraindications (4), Warnings and Precautions (5.2)].
2.5 Dosage Recommendations for Concomitant Use with Strong CYP3A4 Inhibitors or Inducers
Coadministration with Strong CYP3A4 Inhibitors
Consider reducing trazodone dose based on tolerability when trazodone is coadministered with a strong CYP3A4 inhibitor [see Drug Interactions (7.1)].
Coadministration with Strong CYP3A4 Inducers
Consider increasing trazodone dose based on therapeutic response when trazodone is coadministered with a strong CYP3A4 inducer [see Drug Interactions (7.1)].
2.6 Discontinuation of Treatment with Trazodone
Adverse reactions may occur upon discontinuation of trazodone [See Warnings and Precautions (5.8)]. Gradually reduce the dosage rather than stopping trazodone abruptly whenever possible.
3 DOSAGE FORMS AND STRENGTHS
Trazodone hydrochloride tablets, USP are available in the following strengths:
Trazodone hydrochloride tablets USP, 50 mg are white to off-white, round-shape, biconvex beveled tablets, bisect on one side and plain on other side. The bisected side of tablet is debossed with '8' on upper side of bisect and '05' on lower side of bisect.
Trazodone hydrochloride tablets USP, 100 mg are white to off-white, round-shape, biconvex beveled tablets, bisect on one side and plain on other side. The bisected side of tablet is debossed with '8' on upper side of bisect and '06' on lower side of bisect.
Trazodone hydrochloride tablets USP, 150 mg are white to off-white, oval-shape, flat faced beveled tablets having one full bisect and two trisect notches on one side and two trisects on other side. The full bisected side of tablet is debossed with '8' on one side of bisect and '07' on other bisect segments.
Trazodone hydrochloride tablets USP, 300 mg are white to off-white, oval-shape, flat faced beveled tablets having one full bisect and two trisect notches on one side and two trisects on other side. The full bisected side of tablet is debossed with '8' on one side of bisect and '08' on other bisect segment.
5 WARNINGS AND PRECAUTIONS
5.1 Suicidal Thoughts and Behaviors in Pediatric and Young Adult Patients
In pooled analyses of placebo-controlled trials of antidepressant drugs (SSRIs and other antidepressant classes) that included approximately 77,000 adult patients and over 4,400 pediatric patients, the incidence of suicidal thoughts and behaviors in pediatric and young adult patients was greater in antidepressant-treated patients than in placebo-treated patients. The drug-placebo differences in the number of cases of suicidal thoughts and behaviors per 1,000 patients treated are provided in Table 1.
No suicides occurred in any of the pediatric studies. There were suicides in the adult studies, but the number was not sufficient to reach any conclusion about antidepressant drug effect on suicide.
| Age Range
(years) | Drug-Placebo Difference in Number of Patients of
Suicidal Thoughts or Behaviors per 1,000 Patients Treated |
| Increases Compared to Placebo
|
|
| < 18 | 14 additional patients |
| 18 to 24 | 5 additional patients |
| Decreases Compared to Placebo
|
|
| 25 to 64 | 1 fewer patient |
| ≥ 65 | 6 fewer patients |
It is unknown whether the risk of suicidal thoughts and behaviors in pediatric and young adult patients extends to longer-term use, i.e., beyond four months. However, there is substantial evidence from placebo-controlled maintenance trials in adults with MDD that antidepressants delay the recurrence of depression.
Monitor all antidepressant-treated patients for clinical worsening and emergence of suicidal thoughts and behaviors, especially during the initial few months of drug therapy and at times of dosage changes. Counsel family members or caregivers of patients to monitor for changes in behavior and to alert the healthcare provider. Consider changing the therapeutic regimen, including possibly discontinuing trazodone, in patients whose depression is persistently worse, or who are experiencing emergent suicidal thoughts or behaviors.
5.2 Serotonin Syndrome
Serotonin-norepinephrine reuptake inhibitors (SNRIs) and SSRIs, including trazodone, can precipitate serotonin syndrome, a potentially life-threatening condition. The risk is increased with concomitant use of other serotonergic drugs (including triptans, tricyclic antidepressants, fentanyl, lithium, tramadol, tryptophan, buspirone, and St. John's Wort) and with drugs that impair metabolism of serotonin, i.e., MAOIs [see Contraindications (4), Drug Interactions (7.1)]. Serotonin syndrome can also occur when these drugs are used alone.
Serotonin syndrome signs and symptoms may include mental status changes (e.g., agitation, hallucinations, delirium, and coma), autonomic instability (e.g., tachycardia, labile blood pressure, dizziness, diaphoresis, flushing, hyperthermia), neuromuscular symptoms (e.g., tremor, rigidity, myoclonus, hyperreflexia, incoordination), seizures, and gastrointestinal symptoms (e.g., nausea, vomiting, diarrhea).
The concomitant use of trazodone with MAOIs is contraindicated. In addition, do not initiate trazodone in a patient being treated with MAOIs such as linezolid or intravenous methylene blue. No reports involved the administration of methylene blue by other routes (such as oral tablets or local tissue injection). If it is necessary to initiate treatment with an MAOI such as linezolid or intravenous methylene blue in a patient taking trazodone, discontinue trazodone before initiating treatment with the MAOI [see Contraindications (4), Drug Interactions (7.1)].
Monitor all patients taking trazodone for the emergence of serotonin syndrome. Discontinue treatment with trazodone and any concomitant serotonergic agents immediately if the above symptoms occur, and initiate supportive symptomatic treatment. If concomitant use of trazodone with other serotonergic drugs is clinically warranted, inform patients of the increased risk for serotonin syndrome and monitor for symptoms.
5.3 Cardiac Arrhythmias
Clinical studies indicate that trazodone hydrochloride may be arrhythmogenic in patients with preexisting cardiac disease. Arrhythmias identified include isolated PVCs, ventricular couplets, tachycardia with syncope, and torsade de pointes. Postmarketing events, including torsade de pointes have been reported at doses of 100 mg or less with the immediate-release form of trazodone. Trazodone should also be avoided in patients with a history of cardiac arrhythmias, as well as other circumstances that may increase the risk of the occurrence of torsade de pointes and/or sudden death, including symptomatic bradycardia, hypokalemia or hypomagnesemia, and the presence of congenital prolongation of the QT interval. Trazodone is not recommended for use during the initial recovery phase of myocardial infarction. Caution should be used when administering trazodone to patients with cardiac disease and such patients should be closely monitored, since antidepressant drugs (including trazodone) may cause cardiac arrhythmias [see Adverse Reactions (6.2)].
Trazodone prolongs the QT/QTc interval. The use of trazodone should be avoided in patients with known QT prolongation or in combination with other drugs that are inhibitors of CYP3A4 (e.g., itraconazole, clarithromycin, voriconazole), or known to prolong QT interval including Class 1A antiarrhythmics (e.g., quinidine, procainamide) or Class 3 antiarrhythmics (e.g., amiodarone, sotalol), certain antipsychotic medications (e.g., ziprasidone, chlorpromazine, thioridazine), and certain antibiotics (e.g., gatifloxacin). Concomitant administration of drugs may increase the risk of cardiac arrhythmia [see Drug Interactions (7.1)].
5.4 Orthostatic Hypotension and Syncope
Hypotension, including orthostatic hypotension and syncope has been reported in patients receiving trazodone hydrochloride. Concomitant use with an antihypertensive may require a reduction in the dose of the antihypertensive drug.
5.5 Increased Risk of Bleeding
Drugs that interfere with serotonin reuptake inhibition, including trazodone, increase the risk of bleeding events. Concomitant use of aspirin, nonsteroidal anti-inflammatory drugs (NSAIDS), other antiplatelet drugs, warfarin, and other anticoagulants may add to this risk. Case reports and epidemiological studies (case-control and cohort design) have demonstrated an association between use of drugs that interfere with serotonin reuptake and the occurrence of gastrointestinal bleeding. Bleeding events related to drugs that interfere with serotonin reuptake have ranged from ecchymosis, hematoma, epistaxis, and petechiae to life-threatening hemorrhages.
Inform patients about the risk of bleeding associated with the concomitant use of trazodone and antiplatelet agents or anticoagulants. For patients taking warfarin, carefully monitor coagulation indices when initiating, titrating, or discontinuing trazodone.
5.6 Priapism
Cases of priapism (painful erections greater than 6 hours in duration) have been reported in men receiving trazodone. Priapism, if not treated promptly, can result in irreversible damage to the erectile tissue. Men who have an erection lasting greater than 4 hours, whether painful or not, should immediately discontinue the drug and seek emergency medical attention [see Adverse Reactions (6.2), Overdosage (10)].
Trazodone should be used with caution in men who have conditions that might predispose them to priapism (e.g., sickle cell anemia, multiple myeloma, or leukemia), or in men with anatomical deformation of the penis (e.g., angulation, cavernosal fibrosis, or Peyronie's disease).
5.7 Activation of Mania or Hypomania
In patients with bipolar disorder, treating a depressive episode with trazodone or another antidepressant may precipitate a mixed/manic episode. Activation of mania/hypomania has been reported in a small proportion of patients with major affective disorder who were treated with antidepressants. Prior to initiating treatment with trazodone, screen patients for any personal or family history of bipolar disorder, mania, or hypomania [see Dosage and Administration (2.3)].
5.8 Discontinuation Syndrome
Adverse reactions after discontinuation of serotonergic antidepressants, particularly after abrupt discontinuation, include: nausea, sweating, dysphoric mood, irritability, agitation, dizziness, sensory disturbances (e.g., paresthesia, such as electric shock sensations), tremor, anxiety, confusion, headache, lethargy, emotional lability, insomnia, hypomania, tinnitus, and seizures. A gradual reduction in dosage rather than abrupt cessation is recommended whenever possible [See Dosage and Administration (2.6)].
5.9 Potential for Cognitive and Motor Impairment
Trazodone may cause somnolence or sedation and may impair the mental and/or physical ability required for the performance of potentially hazardous tasks. Patients should be cautioned about operating hazardous machinery, including automobiles, until they are reasonably certain that the drug treatment does not affect them adversely.
5.10 Angle-Closure Glaucoma
The pupillary dilation that occurs following use of many antidepressant drugs including trazodone may trigger an angle closure attack in a patient with anatomically narrow angles who does not have a patent iridectomy. Avoid use of antidepressants, including trazodone, in patients with untreated anatomically narrow angles.
5.11 Hyponatremia
Hyponatremia may occur as a result of treatment with SNRIs and SSRIs, including trazodone. Cases with serum sodium lower than 110 mmol/L have been reported. Signs and symptoms of hyponatremia include headache, difficulty concentrating, memory impairment, confusion, weakness, and unsteadiness, which can lead to falls. Signs and symptoms associated with more severe and/or acute cases have included hallucination, syncope, seizure, coma, respiratory arrest, and death. In many cases, this hyponatremia appears to be the result of the syndrome of inappropriate antidiuretic hormone secretion (SIADH).
In patients with symptomatic hyponatremia, discontinue trazodone and institute appropriate medical intervention. Elderly patients, patients taking diuretics, and those who are volume-depleted may be at greater risk of developing hyponatremia with SSRIs and SNRIs [see Use in Specific Populations (8.5)].
6 ADVERSE REACTIONS
The following serious adverse reactions are described elsewhere in the labeling:
- Suicidal Thoughts and Behavior in Children, Adolescents and Young Adults [see Boxed Warning and Warnings and Precautions (5.1)]
- Serotonin Syndrome [see Warnings and Precautions (5.2)]
- Cardiac Arrythmias (see Warnings and Precautions (5.3)]
- Orthostatic Hypotension and Syncope [see Warnings and Precautions (5.4)]
- Increased Risk of Bleeding [see Warnings and Precautions (5.5)]
- Priapism [see Warnings and Precautions (5.6)]
- Activation of Mania or Hypomania [see Warnings and Precautions (5.7)]
- Discontinuation Syndrome [see Warnings and Precautions (5.8)]
- Potential for Cognitive and Motor Impairment [see Warnings and Precautions (5.9)]
- Angle-Closure Glaucoma [see Warnings and Precautions (5.10)]
- Hyponatremia [see Warnings and Precautions (5.11)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
|
| Inpatients
| Outpatients
|
||
| Trazodone
| Placebo
| Trazodone
| Placebo
|
|
|
| N=142
| N=95
| N=157
| N=158
|
| Allergic
| ||||
| Skin Condition/Edema | 3% | 1% | 7% | 1% |
| Autonomic
| ||||
| Blurred Vision | 6% | 4% | 15% | 4% |
| Constipation | 7% | 4% | 8% | 6% |
| Dry Mouth | 15% | 8% | 34% | 20% |
| Cardiovascular
| ||||
| Hypertension | 20% | 1% | 1% | *
|
| Hypotension | 7% | 1% | 4% | 0 |
| Syncope | 3% | 2% | 5% | 1% |
| CNS
| ||||
| Confusion | 5% | 0 | 6% | 8% |
| Decreased Concentration | 3% | 2% | 1% | 0 |
| Disorientation | 2% | 0 | *
| 0 |
| Dizziness/Light-Headedness | 20% | 5% | 28% | 15% |
| Drowsiness | 24% | 6% | 41% | 20% |
| Fatigue | 11% | 4% | 6% | 3% |
| Headache | 10% | 5% | 20% | 16% |
| Nervousness | 15% | 11% | 6% | 8% |
| Gastrointestinal
| ||||
| Abdominal/Gastric Disorder | 4% | 4% | 6% | 4% |
| Diarrhea | 0 | 1% | 5% | 1% |
| Nausea/Vomiting | 10% | 1% | 13% | 10% |
| Musculoskeletal
| ||||
| Aches/Pains | 6% | 3% | 5% | 3% |
| Neurological
| ||||
| Incoordination | 5% | 0 | 2% | * |
| Tremors | 3% | 1% | 5% | 4% |
| Other
| ||||
| Eyes Red/Tired/Itching | 3% | 0 | 0 | 0 |
| Head Full-Heavy | 3% | 0 | 0 | 0 |
| Malaise | 3% | 0 | 0 | 0 |
| Nasal/Sinus Congestion | 3% | 0 | 6% | 3% |
| Weight Gain | 1% | 0 | 5% | 2% |
| Weight Loss | *
| 3% | 6% | 3% |
Other adverse reactions occurring at an incidence of <2% with the use of trazodone hydrochloride in the controlled clinical studies: akathisia, allergic reaction, anemia, chest pain, delayed urine flow, early menses, flatulence, hallucinations/delusions, hematuria, hypersalivation, hypomania, impaired memory, impaired speech, impotence, increased appetite, increased libido, increased urinary frequency, missed periods, muscle twitches, numbness, paresthesia, retrograde ejaculation, shortness of breath, and tachycardia/palpitations. Occasional sinus bradycardia has occurred in long-term studies.
6.2 Postmarketing Experience
The following adverse reactions have been identified during post-approval use of trazodone. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to estimate their frequency or establish a causal relationship to drug exposure:
Blood and lymphatic system disorders: hemolytic anemia, leukocytosis
Cardiac disorders: cardiospasm, congestive heart failure, conduction block, orthostatic hypotension and syncope, palpitations, bradycardia, atrial fibrillation, myocardial infarction, cardiac arrest, arrhythmia, ventricular ectopic activity, including ventricular tachycardia and QT prolongation. Prolonged QT interval, torsade de pointes, and ventricular tachycardia have been reported at doses of 100 mg per day or less [see Warnings and Precautions (5.3)].
Endocrine disorders: inappropriate ADH syndrome
Eye disorders: diplopia
Gastrointestinal disorders: increased salivation, nausea/vomiting
General disorders and administration site conditions: chills, edema, unexplained death, weakness
Hepatobiliary disorders: cholestasis, jaundice, hyperbilirubinemia, liver enzyme alterations
Investigations: increased amylase
Metabolism and nutrition disorders: methemoglobinemia
Nervous system disorders: aphasia, ataxia, cerebrovascular accident, extrapyramidal symptoms, grand mal seizures, paresthesia, tardive dyskinesia, vertigo
Psychiatric disorders: abnormal dreams, agitation, anxiety, hallucinations, insomnia, paranoid reaction, psychosis, stupor
Renal and urinary disorders: urinary incontinence, urinary retention
Reproductive system and breast disorders: breast enlargement or engorgement, clitorism, lactation, priapism [see Warnings and Precautions (5.6)]
Respiratory, thoracic and mediastinal disorders: apnea
Skin and subcutaneous tissue disorders: alopecia, hirsutism, leukonychia, pruritus, psoriasis, rash, urticaria
Vascular disorders: vasodilation
7 DRUG INTERACTIONS
MAOIs should not be used within 14 days of trazodone [see Warnings and Precautions (5.8)].
Central Nervous System (CNS) Depressants
Trazodone may enhance the response to alcohol, barbiturates, and other CNS depressants.
Cytochrome P450 3A4 Inhibitors
In vitro drug metabolism studies suggest that there is a potential for drug interactions when trazodone is given with cytochrome P450 3A4 (CYP3A4) inhibitors. The effect of short-term administration of ritonavir (200 mg twice daily, 4 doses) on the pharmacokinetics of a single dose of trazodone (50 mg) has been studied in 10 healthy subjects. The Cmax of trazodone increased by 34%, the AUC increased 2.4 fold, the half-life increased by 2.2 fold, and the clearance decreased by 52%. Adverse effects including nausea, hypotension, and syncope were observed when ritonavir and trazodone were coadministered. It is likely that ketoconazole, indinavir, and other CYP3A4 inhibitors such as itraconazole may lead to substantial increases in trazodone plasma concentrations with the potential for adverse effects. If trazodone is used with a potent CYP3A4 inhibitor, the risk of cardiac arrhythmia may be increased [see Warnings and Precautions (5.4)] and a lower dose of trazodone should be considered.
Cytochrome P450 Inducers (e.g., Carbamazepine)
Carbamazepine induces CYP3A4. Following coadministration of carbamazepine 400 mg per day with trazodone 100 mg to 300 mg daily, carbamazepine reduced plasma concentrations of trazodone and m-chlorophenlypiperazine (an active metabolite) by 76% and 60% respectively, compared to pre-carbamazepine values. Patients should be closely monitored to see if there is a need for an increased dose of trazodone when taking both drugs.
Digoxin and Phenytoin
Increased serum digoxin or phenytoin levels have been reported in patients receiving trazodone concurrently with either of these drugs. Monitor serum levels and adjust dosages as needed.
Serotonergic Drugs
Based on the mechanism of action of trazodone and the potential for serotonin syndrome, caution is advised when trazodone is coadministered with other drugs that may affect the neurotransmitter systems [see Warnings and Precautions (5.2)].
NSAIDs, Aspirin, or Other Drugs Affecting Coagulation or Bleeding
Due to a possible association between serotonin modulating drugs and gastrointestinal bleeding, patients should be monitored for and cautioned about the potential risk of bleeding associated with the concomitant use of trazodone and NSAIDs, aspirin, or other drugs that affect coagulation or bleeding [see Warnings and Precautions (5.7)].
Warfarin
There have been reports of altered (either increased or decreased) prothrombin times in taking both warfarin and trazodone.
7.1 Drugs Having Clinically Important Interactions with Trazodone
| Monoamine Oxidase Inhibitors (MAOIs)
|
|
| Clinical Impact: | The concomitant use of MAOIs and serotonergic drugs including trazodone increases the risk of serotonin syndrome. |
| Intervention: | Trazodone is contraindicated in patients taking MAOIs, including MAOIs such as linezolid or intravenous methylene blue [see Contraindications (4), Dosage and Administration (2.3, 2.4), and Warnings and Precautions (5.2)].
|
| Examples: | isocarboxazid, moclobemide, phenelzine, selegiline, tranylcypromine |
| Other Serotonergic Drugs
|
|
| Clinical Impact: | The concomitant use of serotonergic drugs including trazodone and other serotonergic drugs increases the risk of serotonin syndrome. |
| Intervention: | Monitor patients for signs and symptoms of serotonin syndrome, particularly during trazodone initiation. If serotonin syndrome occurs, consider discontinuation of trazodone and/or concomitant serotonergic drugs [see Warnings and Precautions (5.2)].
|
| Examples: | triptans, antidepressants (tricyclic and serotonin uptake inhibitors), fentanyl, lithium, tramadol, tryptophan, buspirone, and St. John's Wort |
| Antiplatelet Agents and Anticoagulants
|
|
| Clinical Impact: | Serotonin release by platelets plays an important role in hemostasis. The concurrent use of an antiplatelet agent or anticoagulant with trazodone may potentiate the risk of bleeding. |
| Intervention: | Inform patients of the increased risk of bleeding with the concomitant use of trazodone and antiplatelet agents and anticoagulants. For patients taking warfarin, carefully monitor the international normalized ratio (INR) when initiating or discontinuing trazodone [see Warnings and Precautions (5.5)].
|
| Examples: | warfarin, rivaroxaban, dabigatran, clopidogrel |
| Strong CYP3A4 Inhibitors
|
|
| Clinical Impact: | The concomitant use of trazodone and strong CYP3A4 inhibitors increased the exposure of trazodone compared to the use of trazodone alone.
|
| Intervention: | If trazodone is used with a potent CYP3A4 inhibitor, the risk of adverse reactions, including cardiac arrhythmias, may be increased and a lower dose of trazodone should be considered [see Dosage and Administration (2.5), Warnings and Precautions (5.3)].
|
| Examples: | itraconazole, ketoconazole, clarithromycin, indinavir |
| Strong CYP3A4 Inducers
|
|
| Clinical Impact: | The concomitant use of trazodone and strong CYP3A4 inducers decreased the exposure of trazodone compared to the use of trazodone alone.
|
| Intervention: | Patients should be closely monitored to see if there is a need for an increased dose of trazodone when taking CYP3A4 inducers [see Dosage and Administration (2.5)].
|
| Examples: | rifampin, carbamazepine, phenytoin, St. John's wort |
| Digoxin and Phenytoin
|
|
| Clinical Impact: | Digoxin and phenytoin are narrow therapeutic index drugs. Concomitant use of trazodone can increase digoxin or phenytoin concentrations. |
| Intervention: | Measure serum digoxin or phenytoin concentrations before initiating concomitant use of trazodone. Continue monitoring and reduce digoxin or phenytoin dose as necessary. |
| Examples: | digoxin, phenytoin |
| Central Nervous System (CNS) Depressants
|
|
| Clinical Impact: | Trazodone may enhance the response CNS depressants. |
| Intervention: | Patients should be counseled that trazodone may enhance the response to alcohol, barbiturates, and other CNS depressants. |
| Examples: | alcohol, barbiturates |
| QT Interval Prolongation
|
|
| Clinical Impact: | Concomitant use of drugs that prolong the QT interval may add to the QT effects of trazodone and increase the risk of cardiac arrhythmia. |
| Intervention: | Avoid the use of trazodone in combination with other drugs known to prolong QTc [see Warnings and Precautions (5.3)] . |
| Examples: | Class 1A antiarrhythmics: quinidine, procainamide, disopyramide; Class 3 antiarrhythmics: amiodarone, sotalol; Antipsychotics: ziprasidone, chlorpromazine, thioridazine; Antibiotics: gatifloxacin |
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
There is a pregnancy exposure registry that monitors pregnancy outcomes in women exposed to antidepressants during pregnancy. Healthcare providers are encouraged to register patients by calling the National Pregnancy Registry for Antidepressants at 1-844-405-6185 or visiting online at https://womensmentalhealth.org/clinical-and-research-programs/ pregnancyregistry/ antidepressants/
Risk Summary
Published prospective cohort studies, case series, and case reports over several decades with trazodone hydrochloride tablets use in pregnant women have not identified any drug-associated risks of major birth defects, miscarriage, or adverse maternal or fetal outcomes (see Data). Trazodone hydrochloride has been shown to cause increased fetal resorption and other adverse effects on the fetus in the rat when given at dose levels approximately 7.3 times to 11 times the maximum recommended human dose (MRHD) of 400 mg/day in adults on a mg/m2 basis. There was also an increase in congenital anomalies in the rabbit at approximately 7.3 times to 22 times the MRHD on a mg/m2 basis (see Data).
The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively.
Clinical Considerations
Disease-associated maternal and/or embryofetal risk
A prospective, longitudinal study followed 201 pregnant women with a history of major depressive disorder who were euthymic and taking antidepressants at the beginning of pregnancy. The women who discontinued antidepressants during pregnancy were more likely to experience a relapse of major depression that women who continued antidepressants. Consider the risk of untreated depression when discontinuing or changing treatment with antidepressant medication during pregnancy and postpartum.
Data
Human Data
While available studies cannot definitively establish the absence of risk, published data from prospective cohort studies, case series, and case reports over several decades have not identified an association with trazodone use during pregnancy and major birth defects, miscarriage, or other adverse maternal or fetal outcomes. All available studies have methodological limitations, including small sample size and inconsistent comparator groups.
Animal Data
No teratogenic effects were observed when trazodone was given to pregnant rats and rabbits during the period of organogenesis at oral doses up to 450 mg/kg/day. This dose is 11 times and 22 times, in rats and rabbits, respectively, the maximum recommended human dose (MRHD) of 400 mg/day in adults on a mg/m2 basis. Increased fetal resorption and other adverse effects on the fetus in rats at 7.3 times to 11 times the MRHD and increase in congenital anomalies in rabbits at 7.3 times to 22 times the MRHD on a mg/m2 basis were observed. No further details on these studies are available.
8.2 Lactation
Data from published literature report the transfer of trazodone into human milk. There are no data on the effect of trazodone on milk production. Limited data from postmarketing reports have not identified and association of adverse effects on the breastfed child. The developmental and health benefits of breastfeeding should be considered along with the mother's clinical need for trazodone hydrochloride tablets and any potential adverse effects on the breastfed child from trazodone hydrochloride tablets or from the underlying maternal condition.
8.4 Pediatric Use
Safety and effectiveness in the pediatric population have not been established. Antidepressants increased the risk of suicidal thoughts and behaviors in pediatric patients [see Boxed Warning, Warnings and Precautions (5.1)].
8.5 Geriatric Use
Reported clinical literature and experience with trazodone has not identified differences in responses between elderly and younger patients. However, as experience in the elderly with trazodone hydrochloride is limited, it should be used with caution in geriatric patients.
Serotonergic antidepressants have been associated with cases of clinically significant hyponatremia in elderly patients who may be at greater risk for this adverse reaction [see Warnings and Precautions (5.11)].
10 OVERDOSAGE
Death from overdose has occurred in patients ingesting trazodone and other CNS depressant drugs concurrently (alcohol; alcohol and chloral hydrate and diazepam; amobarbital; chlordiazepoxide; or meprobamate).
The most severe reactions reported to have occurred with overdose of trazodone alone have been priapism, respiratory arrest, seizures, and ECG changes, including QT prolongation. The reactions reported most frequently have been drowsiness and vomiting. Overdosage may cause an increase in incidence or severity of any of the reported adverse reactions.
There is no specific antidote for trazodone hydrochloride overdose. In managing overdosage, consider the possibility of multiple drug involvement. For current information on the management of poisoning or overdose, contact a poison control center (1-800-222-1222 or www.poison.org).
11 DESCRIPTION
Trazodone hydrochloride is a selective serotonin reuptake inhibitor and 5HT2 receptor antagonist. Trazodone hydrochloride, USP is a triazolopyridine derivative designated as 2-[3-[4-(3-chlorophenyl)-1-piperazinyl]propyl]- 1,2,4-triazolo[4, 3-a]pyridin-3(2H)-one hydrochloride. It is a white to off-white, crystalline powder which is sparingly soluble in chloroform and in water. The structural formula is represented as follows:
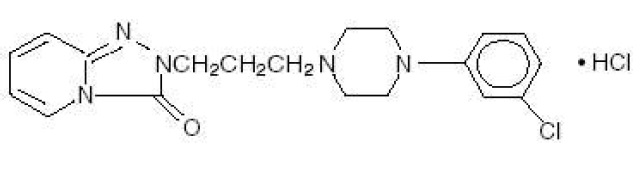
Molecular Formula: C19H22ClN5O•HCl
Molecular Weight: 408.33
Each trazodone hydrochloride tablet, USP for oral administration contains 50 mg, 100 mg, 150 mg or 300 mg of trazodone hydrochloride, USP. In addition, each tablet contains the following inactive ingredients: colloidal silicon dioxide, magnesium stearate, microcrystalline cellulose, pregelatinized starch, sodium lauryl sulfate, and sodium starch glycolate.
The USP Dissolution Test is pending.
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
The mechanism of trazodone's antidepressant action is not fully understood, but is thought to be related to its enhancement of serotonergic activity in the CNS. Trazodone is both a selective serotonin reuptake inhibitor (SSRI) and a 5HT2 receptor antagonist and the net result of this action on serotonergic transmission and its role in trazodone's antidepressant effect is unknown.
12.2 Pharmacodynamics
Preclinical studies have shown that trazodone selectively inhibits neuronal reuptake of serotonin (Ki = 367 nM) and acts as an antagonist at 5-HT-2A (Ki = 35.6 nM) serotonin receptors. Trazodone is also an antagonist at several other monoaminergic receptors including 5-HT2B (Ki = 78.4 nM), 5-HT2C (Ki = 224 nM), α1A (Ki = 153 nM), α2C (Ki = 155 nM) receptors and it is a partial agonist at 5HT1A (Ki = 118 nM) receptor.
Trazodone antagonizes alpha 1-adrenergic receptors, a property which may be associated with postural hypotension.
12.3 Pharmacokinetics
In humans, trazodone hydrochloride is absorbed after oral administration without selective localization in any tissue. When trazodone hydrochloride is taken shortly after ingestion of food, there may be an increase in the amount of drug absorbed, a decrease in maximum concentration and a lengthening in the time to maximum concentration. Peak plasma levels occur approximately one hour after dosing when trazodone hydrochloride is taken on an empty stomach or 2 hours after dosing when taken with food.
Metabolism
In vitro studies in human liver microsomes show that trazodone is metabolized, via oxidative cleavage, to an active metabolite, m-chlorophenylpiperazine (mCPP) by CYP3A4. Other metabolic pathways that may be involved in the metabolism of trazodone have not been well characterized. Trazodone is extensively metabolized; less than 1% of an oral dose is excreted unchanged in the urine.
Elimination
In some patients trazodone may accumulate in the plasma.
Protein Binding
Trazodone is 89 to 95% protein bound in vitro at concentrations attained with therapeutic doses in humans.
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
No drug- or dose-related occurrence of carcinogenesis was evident in rats receiving trazodone in daily oral doses up to 7.3 times the maximum recommended human dose (MRHD) of 400 mg/day in adults on a mg/m2basis.
Mutagenesis
No genotoxicity studies were conducted with trazodone.
Impairment of Fertility
Trazodone has no effect on fertility in rats at doses up to 7.3 times the MRHD in adults on a mg/m2 basis.
14 CLINICAL STUDIES
16 HOW SUPPLIED/STORAGE AND HANDLING
Trazodone Hydrochloride Tablets USP, 50 mg are white to off-white, round-shape, biconvex beveled tablets, bisect on one side and plain on other side. The bisected side of tablet is debossed with '8' on upper side of bisect and '05' on lower side of bisect and are supplied as follows:
NDC 68382-805-06 in bottle of 30 tablets
NDC 68382-805-16 in bottle of 90 tablets
NDC 68382-805-01 in bottle of 100 tablets
NDC 68382-805-05 in bottle of 500 tablets
NDC 68382-805-10 in bottle of 1,000 tablets
NDC 68382-805-77 in unit-dose blister carton of 100 (10 x 10) unit-dose tablets
Trazodone Hydrochloride Tablets USP, 100 mg are white to off-white, round-shape, biconvex beveled tablets, bisect on one side and plain on other side. The bisected side of tablet is debossed with '8' on upper side of bisect and '06' on lower side of bisect and are supplied as follows:
NDC 68382-806-06 in bottle of 30 tablets
NDC 68382-806-16 in bottle of 90 tablets
NDC 68382-806-01 in bottle of 100 tablets
NDC 68382-806-05 in bottle of 500 tablets
NDC 68382-806-10 in bottle of 1,000 tablets
NDC 68382-806-77 in unit-dose blister carton of 100 (10 x 10) unit-dose tablets
Trazodone Hydrochloride Tablets USP, 150 mg are white to off-white, oval-shape, flat faced beveled tablets having one full bisect and two trisect notches on one side and two trisects on other side. The full bisected side of tablet is debossed with '8' on one side of bisect and '07' on other bisect segments and are supplied as follows:
NDC 68382-807-06 in bottle of 30 tablets
NDC 68382-807-16 in bottle of 90 tablets
NDC 68382-807-01 in bottle of 100 tablets
NDC 68382-807-05 in bottle of 500 tablets
NDC 68382-807-10 in bottle of 1,000 tablets
NDC 68382-807-77 in unit-dose blister carton of 100 (10 x 10) unit-dose tablets
Directions for using the correct score when breaking the tablet, please refer to the
following:
-For 50 mg, break the score on either the left or right side of the tablet (one-third of a
tablet).

-For 75 mg, break the score down the middle of the tablet (one-half of a tablet).

-For 100 mg, break the score on either the left or right side of the tablet (two-thirds of a
tablet).

-For 150 mg, use the entire tablet.

Trazodone Hydrochloride Tablets USP, 300 mg are white to off-white, oval-shape, flat
faced beveled tablets having one full bisect and two trisect notches on one side and two
trisects on other side. The full bisected side of tablet is debossed with '8' on one side of
bisect and '08' on other bisect segment and are supplied as follows:
NDC 68382-808-06 in bottle of 30 tablets
NDC 68382-808-16 in bottle of 90 tablets
NDC 68382-808-01 in bottle of 100 tablets
NDC 68382-808-05 in bottle of 500 tablets
NDC 68382-808-10 in bottle of 1,000 tablets
NDC 68382-808-77 in unit-dose blister carton of 100 (10 x 10) unit-dose tablets
Directions for using the correct score when breaking the tablet, please refer to the
following:
-For 100 mg, break the score on either the left or right side of the tablet (one-third of a
tablet).

-For 150 mg, break the score down the middle of the tablet (one-half of a tablet).

-For 200 mg, break the score on either the left or right side of the tablet (two-thirds of a
tablet).

-For 300 mg, use the entire tablet.

Store at 20°C to 25°C (68°F to 77°F) [See USP Controlled Room Temperature].
Dispense with a child-resistant closure in a tight, light-resistant container.
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Medication Guide).
Suicidal Thoughts and Behaviors
Advise patients and caregivers to look for the emergence of suicidality, especially early during treatment and when the dosage is adjusted up or down and instruct them to report such symptoms to the healthcare provider [see Box Warning and Warnings and Precautions (5.1)].
Dosage and Administration
Advise patients that trazodone hydrochloride tablets should be taken shortly after a meal or light snack. Advise patients regarding the importance of following dosage titration instructions [see Dosage and Administration (2)].
Serotonin Syndrome
Caution patients about the risk of serotonin syndrome, particularly with the concomitant use of trazodone hydrochloride tablets with other serotonergic drugs including triptans, tricyclic antidepressants, fentanyl, lithium, tramadol, tryptophan, buspirone, St. John's Wort, and with drugs that impair metabolism of serotonin (in particular, MAOIs, both those intended to treat psychiatric disorders and also others, such as linezolid). Patients should contact their health care provider or report to the emergency room if they experience signs or symptoms of serotonin syndrome [see Warnings and Precautions (5.2) and Drug Interactions (7)].
Activation of Mania/Hypomania
Advise patients and their caregivers to observe for signs of activation of mania/hypomania and instruct them to report such symptoms to the healthcare provider [see Warnings and Precautions (5.7)].
Increased Risk of Bleeding
Inform patients about the concomitant use of trazodone hydrochloride tablets with aspirin, NSAIDs, other antiplatelet drugs, warfarin, or other anticoagulants because the combined use of drugs that interfere with serotonin reuptake and these medications has been associated with an increased risk of bleeding. Advise them to inform their health care providers if they are taking or planning to take any prescription or over-the-counter medications that increase the risk of bleeding [see Warnings and Precautions (5.5)].
Discontinuation Syndrome
Advise patients not to abruptly discontinue trazodone hydrochloride tablets and to discuss any tapering regimen with their healthcare provider. Adverse reactions can occur when trazodone hydrochloride tablets are discontinued [see Warnings and Precautions (5.8)].
Concomitant Medications
Advise patients to inform their health care providers if they are taking, or plan to take any prescription or over-the-counter medications since there is a potential for interactions [see Drug Interactions (7.1)].
Pregnancy
Advise patients to notify their healthcare provider if they become pregnant or intend to become pregnant during therapy with trazodone hydrochloride tablets. Advise patients that there is a pregnancy exposure registry that monitors pregnancy outcomes in women exposed to trazodone hydrochloride tablets during pregnancy [see Use in Special Populations (8.1)].
Medication Guide available at www.zydususa.com/medguides or call 1-877-993-8779.
SPL UNCLASSIFIED SECTION
SPL UNCLASSIFIED SECTION
| MEDICATION GUIDE
Trazodone Hydrochloride (traz' oh done hye'' droe klor' ide) Tablets USP, for oral use |
| What is the most important information I should know about trazodone hydrochloride tablets?
Antidepressant medicines, depression or other serious mental illnesses, and suicidal thoughts or actions: Talk to your healthcare provider about:
2. Depression and other serious mental illnesses are the most important causes of suicidal thoughts and actions . Some people may have a higher risk of having suicidal thoughts or actions . These include people who have or have a family history of bipolar illness (also called manic- depressive illness) or suicidal thoughts or actions. 3. How can I watch for and try to prevent suicidal thoughts and actions? Pay close attention to any changes, especially sudden changes in mood, behaviors, thoughts, or feelings. This is very important when an antidepressant medicine is started or when the dose is changed. Call your healthcare provider right away to report new or sudden changes in mood, behavior, thoughts or feelings. Keep all follow-up visits with your healthcare provider as scheduled. Call your healthcare provider between visits as needed, especially if you are worried about symptoms. Call a healthcare provider right away if you have any of the following symptoms, especially if they are new, worse, or worry you: Thoughts about suicide or dying Attempts to commit suicide New or worse depression New or worse anxiety Feeling very agitated or restless Panic attacks Trouble sleeping (insomnia) New or worse irritability Acting aggressive, being angry or violent Acting on dangerous impulses An extreme increase in activity and talking (mania) Other unusual changes in behavior or mood |
| What else do I need to know about antidepressant medicines?
Never stop an antidepressant medicine without first talking to a healthcare provider . Stopping an antidepressant medicine suddenly can cause other symptoms. Antidepressants are medicines used to treat depression and other illnesses. It is important to discuss all the risks of treating depression and also the risks of not treating it. You should discuss all treatment choices with your healthcare provider, not just the use of antidepressants. Antidepressant medicines have other side effects . Talk to your healthcare provider about the side effects of your medicines. Antidepressant medicines can interact with other medicines. Know all of the medicines that you take. Keep a list of all medicines to show your healthcare provider. Do not start new medicines without first checking with your healthcare provider. It is not known if trazodone hydrochloride tablets are safe and effective in children. |
| What are trazodone hydrochloride tablets?
Trazodone hydrochloride tablets are prescription medicine used in adults to treat major depressive disorder (MDD). Trazodone hydrochloride tablets belong to a class of medicines known as SSRIs (or selective serotonin reuptake inhibitors). |
Do not take trazodone hydrochloride tablets:
|
| Before you take trazodone hydrochloride tablets tell your healthcare provider about all of your medical conditons, including if you:
have heart problems, including QT prolongation or a family history of it have ever had a heart attack have bipolar disorder have liver or kidney problems have other serious medical conditions are pregnant or plan to become pregnant. It is not known if trazodone hydrochloride tablets will harm your unborn baby. Talk to your healthcare provider about the risk to your unborn baby if you take trazodone hydrochloride tablets. ○ If you become pregnant during treatment with trazodone hydrochloride tablets, talk to your healthcare provider about registering with the National Pregnancy Registry for Antidepressants. You can register by calling 1-844-405-6185. are breastfeeding or plan to breastfeed. Trazodone hydrochloride passes into your breast milk. Talk to your healthcare provider about the best way to feed your baby if you take trazodone hydrochloride tablets. have taken a Monoamine Oxidase Inhibitor (MAOI) or if you have stopped taking an MAOI in the last 2 weeks. Tell your healthcare provider about all the medicines you take , including prescription and over-the-counter medicines, vitamins, and herbal supplements. Using trazodone hydrochloride tablets with certain other medicines can affect each other causing serious side effects. Especially tell your healthcare provider if you take: triptans used to treat migraine headache medicines used to treat mood, anxiety, psychotic or thought disorders, including tricyclics, lithium, SSRIs, SNRIs, buspirone, or antipsychotics tramadol over-the-counter supplements such as tryptophan or St. John's Wort nonsteroidal anti-inflammatory drugs (NSAIDS) aspirin warfarin (Coumadin, Jantoven) phenytoin (Mesantoin) diuretics Know the medicines you take. Keep a list of them and show it to your healthcare provider and pharmacist when you get a new medicine. |
| How should I take trazodone hydrochloride tablets?
Take trazodone hydrochloride tablets exactly as your healthcare provider tells you. Trazodone hydrochloride tablets should be taken shortly after a meal or light snack. If you feel drowsy after taking trazodone hydrochloride tablets, talk to your healthcare provider. Your healthcare provider may change your dose or the time of day you take your trazodone hydrochloride tablets. Do not stop taking trazodone hydrochloride tablets without talking to your healthcare provider. Trazodone hydrochloride tablets should be swallowed whole or broken in half along the score line. Do not chew or crush trazodone hydrochloride tablets. Tell your healthcare provider if you cannot swallow trazodone either whole or as a half tablet.
|
| What should I avoid while taking trazodone hydrochloride tablets?
Do not drive, operate heavy machinery, or do other dangerous activities until you know how trazodone hydrochloride tablets affect you. Trazodone hydrochloride tablets can slow your thinking and motor skills. Do not drink alcohol or take other medicines that make you sleepy or dizzy while taking trazodone hydrochloride tablets until you talk with your healthcare provider. Trazodone hydrochloride tablets may make your sleepiness or dizziness worse if you take it with alcohol or other medicines that cause sleepiness or dizziness. |
| What are the possible side effects of trazodone hydrochloride tablets?
Trazodone hydrochloride tablets can cause serious side effects or death, including:
○ changes in vision ○ swelling or redness in or around the eye Only some people are at risk for these problems. You may want to undergo an eye examination to see if you are at risk and receive preventative treatment if you are.
The most common side effects of trazodone hydrochloride tablets include:
|
| How should I store trazodone hydrochloride tablets?
Store trazodone hydrochloride tablets between 68°F to 77°F (20°C to 25°C). Keep in tight container Keep out of the light Safely throw away medicine that is out of date or no longer needed. Keep trazodone hydrochloride tablets and all medicines out of the reach of children. |
| General information about the safe and effective use of trazodone hydrochloride tablets.
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use trazodone hydrochloride tablets for a condition for which it was not prescribed. Do not give trazodone hydrochloride tablets to other people, even if they have the same symptoms that you have. It may harm them. You can ask your pharmacist or healthcare provider for information about trazodone hydrochloride tablets that is written for health professionals . Please address medical inquiries to [email protected] or Tel.: 1-877-993-8779. |
| What are the ingredients in trazodone hydrochloride tablets?
Active ingredient: trazodone hydrochloride, USP Inactive ingredients: colloidal silicon dioxide, magnesium stearate, microcrystalline cellulose, pregelatinized starch, sodium lauryl sulfate, and sodium starch glycolate. This Medication Guide has been approved by the U.S. Food and Drug Administration. Medication Guide available at www.zydususa.com/medguides or call 1-877-993-8779. |
| Manufactured by:
Cadila Healthcare Limited Ahmedabad, India. Distributed by: Zydus Pharmaceuticals (USA) Inc. Pennington, NJ 08534 |
| Rev.: 09/20 |
| This Medication Guide has been approved by the U.S. Food and Drug Administration. |
Repackaging Information
Please reference the How Supplied section listed above for a description of individual tablets. This drug product has been received by Aphena Pharma - TN in a manufacturer or distributor packaged configuration and repackaged in full compliance with all applicable cGMP regulations. The package configurations available from Aphena are listed below:
| Count | 100 mg |
| 30 | 71610-511-30 |
| 90 | 71610-511-60 |
| 180 | 71610-511-80 |
Store between 20°-25°C (68°-77°F). See USP Controlled Room Temperature. Dispense in a tight light-resistant container as defined by USP. Keep this and all drugs out of the reach of children.
Repackaged by:

Cookeville, TN 38506
20210127JH
INGREDIENTS AND APPEARANCE
| TRAZODONE HYDROCHLORIDE
trazodone hydrochloride tablet |
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
| Labeler - Aphena Pharma Solutions - Tennessee, LLC (128385585) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Aphena Pharma Solutions - Tennessee, LLC | 128385585 | REPACK(71610-511) | |