Search by Drug Name or NDC
NDC 71713-0202-03 Esomeprazole Magnesium 20 mg/1 Details
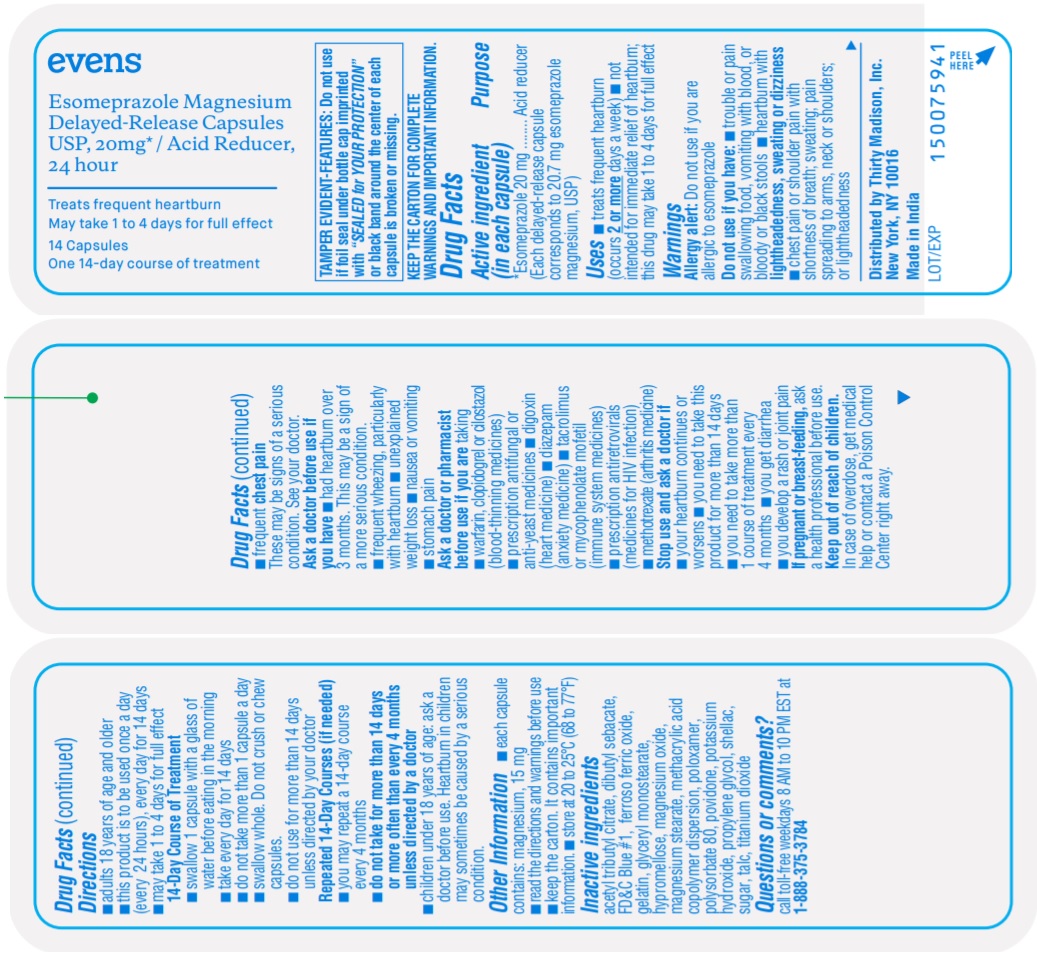
Esomeprazole Magnesium 20 mg/1
Esomeprazole Magnesium is a ORAL CAPSULE, DELAYED RELEASE in the HUMAN OTC DRUG category. It is labeled and distributed by Thirty Madison Inc. The primary component is ESOMEPRAZOLE MAGNESIUM.
MedlinePlus Drug Summary
Prescription esomeprazole is used to treat the symptoms of gastroesophageal reflux disease (GERD), a condition in which backward flow of acid from the stomach causes heartburn and possible injury of the esophagus (the tube between the throat and stomach) in adults and children 1 year of age and older. Prescription esomeprazole is used to treat damage from GERD in adults and children 1 month of age and older. Prescription esomeprazole is used to allow the esophagus to heal and prevent further damage to the esophagus in adults with GERD. Prescription esomeprazole is also used to decrease the chance that people who are taking nonsteroidal anti-inflammatory drugs (NSAIDs) will develop ulcers (sores in the lining of the stomach or intestine) in adults. It is also used with other medications to treat and prevent the return of stomach ulcers caused by a certain type of bacteria (H. pylori) in adults. Prescription esomeprazole is also used to treat conditions in which the stomach produces too much acid such as Zollinger-Ellison syndrome in adults. Nonprescription (over-the-counter) esomeprazole is used to treat frequent heartburn (heartburn that occurs at least 2 or more days a week) in adults. Esomeprazole is in a class of medications called proton pump inhibitors. It works by decreasing the amount of acid made in the stomach.
Related Packages: 71713-0202-03Last Updated: 12/01/2022
MedLinePlus Full Drug Details: Esomeprazole
Product Information
| NDC | 71713-0202 |
|---|---|
| Product ID | 71713-202_277a1c13-e5dc-71a8-5d5c-4b853d8870d0 |
| Associated GPIs | |
| GCN Sequence Number | n/a |
| GCN Sequence Number Description | n/a |
| HIC3 | n/a |
| HIC3 Description | n/a |
| GCN | n/a |
| HICL Sequence Number | n/a |
| HICL Sequence Number Description | n/a |
| Brand/Generic | n/a |
| Proprietary Name | Esomeprazole Magnesium |
| Proprietary Name Suffix | n/a |
| Non-Proprietary Name | Esomeprazole Magnesium |
| Product Type | HUMAN OTC DRUG |
| Dosage Form | CAPSULE, DELAYED RELEASE |
| Route | ORAL |
| Active Ingredient Strength | 20 |
| Active Ingredient Units | mg/1 |
| Substance Name | ESOMEPRAZOLE MAGNESIUM |
| Labeler Name | Thirty Madison Inc |
| Pharmaceutical Class | Cytochrome P450 2C19 Inhibitors [MoA], Proton Pump Inhibitor [EPC], Proton Pump Inhibitors [MoA] |
| DEA Schedule | n/a |
| Marketing Category | ANDA |
| Application Number | ANDA207673 |
| Listing Certified Through | 2024-12-31 |
Package
Package Images

NDC 71713-0202-03 (71713020203)
| NDC Package Code | 71713-202-03 |
|---|---|
| Billing NDC | 71713020203 |
| Package | 3 BOTTLE in 1 CARTON (71713-202-03) / 14 CAPSULE, DELAYED RELEASE in 1 BOTTLE |
| Marketing Start Date | 2019-05-31 |
| NDC Exclude Flag | N |
| Pricing Information | N/A |
Standard Product Labeling (SPL)/Prescribing Information SPL ecf8010c-3ed7-838b-d500-1a0e1dce3e94 Details
Active ingredient(s) in each capsule
Uses
Warnings
Allergy alert: Do not use if you are allergic to esomeprazole
Do not use if you have:
- trouble or pain swallowing food, vomiting with blood, or bloody or black stools
- heartburn with lightheadedness, sweating or dizziness
- chest pain or shoulder pain with shortness of breath; sweating; pain spreading to arms, neck or shoulders; or lightheadness
- frequent chest pain.
These may be signs of a serious condition. See your doctor.
Ask a doctor before use if you have
- had heartburn over 3 months. This may be a sign of a more serious condition.
- frequent wheezing, particularly with heartburn
- unexplained weight loss
- nausea or vomiting
- stomach pain
Ask a doctor or pharmacist before use if you are taking
- warfarin, clopidogrel or cilostazol (blood-thinning medicines)
- prescription ant fungal or anti-yeast medicines
- digoxin (heart medicine)
- diazepam (anxiety medicine)
- tacrolimus or mycophenolate mofetil (immune system medicine)
- prescription antiretrovirals (medicines for HIV infection)
- methotrexate (arthritis medicine)
Directions
- adults 18 years of age and older
- this product is to be used once a day (every 24 hours), every day for 14 days
- may take 1 to 4 days for full effect
14-Day Course of Treatment
- swallow 1 capsule with a glass of water before eating in the morning
- take every day for 14 days
- do not take more than 1 capsule a day
- swallow whole. Do not crush or chew capsules.
- do not use for more than 14 days unless directed by your doctor
Repeated 14-Day Courses (if needed)
- you may repeat a 14-day course every 4 months
- do not take for more than 14 days or more often than every 4 months unless directed by a doctor
- children under 18 years of age: ask a doctor before use. Heartburn in children may sometimes be caused by a serious condition.
Other information
Inactive ingredients
acetyl tributyl citrate, dibutyl sebacate, FD&C Blue #1, ferroso ferric oxide, gelatin, glyceryl monostearate, hypromellose, magnesium oxide, magnesium stearate, methacrylic acid copolymer dispersion, poloxamer, polysorbate 80, povidone, potassium hydroxide, propylene glycol, shellac, sugar, talc, titanium dioxide
SPL UNCLASSIFIED SECTION
Tips for Managing Heartburn
- Avoid foods or drinks that are more likely to cause heartburn, such as rich, spicy, fatty and fried foods, chocolate, caffeine, alcohol and even some acidic fruits and vegetables.
- Eat slowly and do not eat big meals.
- Do not eat late at night or just before bedtime.
- Do not lie flat or bend over soon after eating.
- Raise the head of your bed.
- Wear loose-fitting clothing around your stomach.
- If you are overweight, lose weight.
- If you smoke, quit smoking.
INGREDIENTS AND APPEARANCE
| ESOMEPRAZOLE MAGNESIUM
esomeprazole magnesium capsule, delayed release |
||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||
| Labeler - Thirty Madison Inc (080774087) |