Search by Drug Name or NDC
NDC 76329-3316-01 Epinephrine 0.1 mg/mL Details
Epinephrine 0.1 mg/mL
Epinephrine is a INTRACARDIAC; INTRAVENOUS INJECTION in the HUMAN PRESCRIPTION DRUG category. It is labeled and distributed by International Medication Systems, Limited. The primary component is EPINEPHRINE.
MedlinePlus Drug Summary
Epinephrine injection is used along with emergency medical treatment to treat life-threatening allergic reactions caused by insect bites or stings, foods, medications, latex, and other causes. Epinephrine is in a class of medications called alpha- and beta-adrenergic agonists (sympathomimetic agents). It works by relaxing the muscles in the airways and tightening the blood vessels.
Related Packages: 76329-3316-01Last Updated: 12/01/2022
MedLinePlus Full Drug Details: Epinephrine Injection
Product Information
| NDC | 76329-3316 |
|---|---|
| Product ID | 76329-3316_e6e743c3-bb1e-4d6d-853b-b975dc9f1d79 |
| Associated GPIs | 3800003200E520 |
| GCN Sequence Number | 004934 |
| GCN Sequence Number Description | epinephrine SYRINGE 0.1 MG/ML INJECTION |
| HIC3 | J5A |
| HIC3 Description | ADRENERGIC AGENTS,CATECHOLAMINES |
| GCN | 19411 |
| HICL Sequence Number | 002050 |
| HICL Sequence Number Description | EPINEPHRINE |
| Brand/Generic | Generic |
| Proprietary Name | Epinephrine |
| Proprietary Name Suffix | n/a |
| Non-Proprietary Name | Epinephrine |
| Product Type | HUMAN PRESCRIPTION DRUG |
| Dosage Form | INJECTION |
| Route | INTRACARDIAC; INTRAVENOUS |
| Active Ingredient Strength | 0.1 |
| Active Ingredient Units | mg/mL |
| Substance Name | EPINEPHRINE |
| Labeler Name | International Medication Systems, Limited |
| Pharmaceutical Class | Adrenergic alpha-Agonists [MoA], Adrenergic beta-Agonists [MoA], Catecholamine [EPC], Catecholamines [CS], alpha-Adrenergic Agonist [EPC], beta-Adrenergic Agonist [EPC] |
| DEA Schedule | n/a |
| Marketing Category | UNAPPROVED DRUG OTHER |
| Application Number | n/a |
| Listing Certified Through | 2024-12-31 |
Package
Package Images

NDC 76329-3316-01 (76329331601)
| NDC Package Code | 76329-3316-1 |
|---|---|
| Billing NDC | 76329331601 |
| Package | 10 VIAL in 1 PACKAGE (76329-3316-1) / 10 mL in 1 VIAL |
| Marketing Start Date | 1976-12-01 |
| NDC Exclude Flag | N |
| Pricing Information | N/A |
Standard Product Labeling (SPL)/Prescribing Information SPL 353f2d30-4b74-43eb-a2ff-26d593d283f8 Details
SPL UNCLASSIFIED SECTION
DESCRIPTION
Epinephrine is the active principle of the adrenal medulla. Chemically described as (-)-3,4-Dihydroxy-α-[(methyl-amino) methyl] benzyl alcohol, it has the following structural formula:
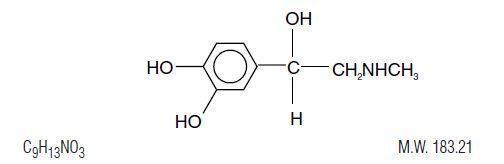
Epinephrine is a sterile aqueous solution of epinephrine prepared with the aid of hydrochloric acid. Each mL of the solution contains 0.1 mg epinephrine and 1 mg sodium bisulfite. Sodium bisulfite is an antioxidant. This solution also contains sodium chloride for adjustment of tonicity, and 10% w/v hydrochloric acid to dissolve epinephrine and to adjust pH to meet USP limits of 2.2 to 5.0. Additionally, the solution also contains sodium citrate and citric acid as buffers for stabilization. The air above the liquid in the individual containers have been displaced by flushing with nitrogen during the filling operation. No antimicrobial preservatives have been added and each vial is intended as a single dose; once the unit is assembled and used, any remaining portion of the solution must be discarded with the entire unit.
CLINICAL PHARMACOLOGY
The actions of epinephrine resemble the effects of stimulation of adrenergic nerves. To a variable degree, it acts on both alpha and beta receptor sites of sympathetic effector cells. Its most prominent actions are on the beta receptors of the heart, vascular and other smooth muscle. When given by rapid I.V. injection it produces a rapid rise in blood pressure, mainly systolic, direct stimulation of cardiac muscle which increases the strength of ventricular contraction, increases the heart rate, and constricts the arterioles in the skin, mucosa and splanchnic areas of circulation.
Epinephrine relaxes the smooth muscle of the bronchi and iris and is a physiologic antagonist of histamine.
The drug also produces an increase in blood sugar and glycogenolysis in the liver.
Blood pressure: When given by slow I.V. injection, epinephrine usually produces a moderate rise in systolic and a fall in diastolic pressure. Although some increase in pulse pressure occurs, there is usually no great elevation in mean blood pressure. Accordingly, the compensatory reflex mechanisms that cause a pronounced increase in blood pressure do not antagonize the direct cardiac actions of epinephrine as much as with catecholamines that have a predominant action on alpha receptors.
Total peripheral resistance: By action of epinephrine on beta receptors of the skeletal muscle vasculature, total peripheral resistance decreases, and blood flow is thereby enhanced. Usually, this vasodilator effect predominates so that the modest rise in systolic pressure which follows slow injection or absorption is the result of direct cardiac stimulation and increase in cardiac output. In some instances, peripheral resistance is not altered or may even rise, owing to a greater ratio of alpha to beta activity in different vascular areas.
Pharmacokinetics: Intravenous injection produces an immediate and intensified response. Following I.V. injection, epinephrine disappears rapidly from the blood stream. Subcutaneously or I.M. administered epinephrine has a rapid onset and short duration of action. Subcutaneous administration during asthmatic attacks may produce bronchodilation within 5 to 10 minutes, and maximal effects may occur within 20 minutes.
The drug becomes fixed in the tissues and is rapidly inactivated chiefly by enzymic transformation to metanephrine or normetanephrine, either of which is subsequently conjugated and excreted in the urine in the form of sulfates and glucuronides. Either sequence results in the formation of 3-methoxy-4-hydroxy-mandelic acid (vanillylmandelic acid, VMA) which is also detectable in the urine.
INDICATIONS AND USAGE
EPINEPHRINE
Epinephrine’s cardiac effects may be of use in the treatment and prophylaxis of cardiac arrest due to various causes in the absence of ventricular fibrillation and attacks of transitory atrioventricular (AV) heart block with syncopal seizures (Stokes-Adams syndrome), but it is not used in cardiac failure or in hemorrhagic, traumatic or in cardiogenic shock. Epinephrine may be used to stimulate the heart in syncope due to complete heart block or carotid sinus hypersensitivity. Epinephrine is also used for resuscitation in cardiac arrest following anesthetic accidents. In cardiopulmonary resuscitation, intracardiac puncture and intramyocardial injection of epinephrine may be effective when external cardiac compression and attempts to restore the circulation by electrical defibillation or use of a pacemaker fail. Epinephrine is seldom used as a vasopressor except in the treatment of anaphylactic shock and under certain conditions in insulin shock.
CONTRAINDICATIONS
Epinephrine should not be used in the presence of cardiac dilatation or coronary insufficiency.
Epinephrine is contraindicated in shock during general anesthesia with halogenated hydrocarbons or cyclopropane, and in individuals with organic brain damage. Epinephrine is also contraindicated with local anesthesia of certain areas; e.g., fingers, toes, because of the danger of vasoconstriction producing sloughing of tissue; also contraindicated in labor because it may delay the second stage.
Epinephrine should not be used in those cases where vasopressor drugs may be contraindicated, e.g., in thyrotoxicosis, diabetes, in obstetrics when maternal blood pressure is in excess of 130/80, and in hypertension and other cardiovascular disorders.
WARNINGS
EPINEPHRINE
Contains sodium bisulfite, a sulfite that may cause allergic-type reactions including anaphylactic symptoms and life-threatening or less severe asthmatic episodes in certain susceptible people. The overall prevalence of sulfite sensitivity in the general population is unknown and probably low. Sulfite sensitivity is seen more frequently in asthmatic rather than in non-asthmatic people.
Use with caution in the following: Elderly; those with cardiovascular disease, hypertension, diabetes, or hyperthyroidism; in psychoneurotic individuals; bronchial asthma and emphysema with degenerative heart disease.
Cardiovascular effects: Inadvertently induced high arterial blood pressure may result in angina pectoris (especially when coronary insufficiency is present), or aortic rupture.
Epinephrine may induce potentially serious cardiac arrhythmias in patients not suffering from heart disease and patients with organic heart disease or who are receiving drugs that sensitize the myocardium. With Epinephrine, a paradoxical but transient lowering of blood pressure, bradycardia and apnea may occur immediately after injection.
Cerebrovascular hemorrhage: Overdosage or inadvertent I.V. injection of epinephrine may cause cerebrovascular hemorrhage resulting from the sharp rise in the blood pressure.
Pulmonary edema: Fatalities may also result from pulmonary edema because of the peripheral constriction and cardiac stimulation produced.
PRECAUTIONS
Although epinephrine can produce ventricular fibrillation, its actions in restoring electrical activity in asystole and in enhancing defibrillation are well documented. However, it should be used with caution in patients with ventricular fibrillation.
In patients with prefibrillatory rhythm, I.V. epinephrine must be used with extreme caution because of its excitatory action on the heart. Since the myocardium is sensitized to the drug by many anesthetic agents, epinephrine may convert asystole to ventricular fibrillation if used in the treatment of anesthetic cardiac accidents.
Hypovolemia: Use is not a substitute for the replacement of blood, plasma, fluids, and electrolytes, which should be restored promptly when loss has occurred.
Drug interactions: Epinephrine must not be administered concomitantly with other sympathomimetic drugs (such as isoproterenol) because of possible additive effects and increased toxicity. Combined effects may induce serious cardiac arrhythmias. They may be administered alternately when the preceding effect of other such drugs has subsided.
Epinephrine is readily destroyed by alkalies and oxidizing agents (e.g., oxygen, chlorine, bromine, iodine, permanganates, chromates, nitrites, and salts of easily reducible metals, especially iron).
The effects of epinephrine may be potentiated by tricyclic antidepressants, certain antihistamines (e.g., diphenhydramine, tripelennamine, chlorpheniramine) and sodium I-thyroxine.
In obstetrics, if vasopressor drugs are used either to correct hypotension or added to the local anesthetic solution, some oxytocic drugs may cause severe persistent hypertension; even rupture of a cerebral blood vessel may occur during the postpartum period.
All vasopressors should be used cautiously in patients taking monoamine oxidase (MAO) inhibitors.
Cyclopropane or halogenated hydrocarbon anesthetics such as halothane which sensitize the myocardium may induce cardiac arrhythmia. (See Contraindications.) When encountered, such arrhythmias may respond to administration of a beta-adrenergic blocking drug.
Diruretic agents may decrease vascular response to pressor drugs such as epinephrine.
Epinephrine may antagonize the neuron blockade produced by guanethidine resulting in decreased antihypertensive effect and requiring increased dosage of the latter.
Use of epinephrine with excessive doses of digitalis, mercurial diuretics or other drugs that sensitize the heart to arrhythmias is not recommended.
Rapidly acting vasodilators such as nitrites or alpha-blocking agents may counteract the marked pressor effects of epinephrine.
Propranolol administered concomitantly with epinephrine may block the beta-adrenergic effects of epinephrine, causing hypertension.
Usage in Pregnancy: Teratogenic Effects - Pregnancy Category C
Epinephrine is teratogenic in small animals when given in doses about 25 times the human dose. There are no adequate and well controlled studies in pregnant women. Use during pregnancy only if the potential benefit justifies the potential risk to the fetus.
ADVERSE REACTIONS
Transient and minor: Anxiety, headache, fear, and palpitations often occur with therapeutic doses, especially in hyperthyroid and hypertensive individuals.
Cardiac arrhythmias and excessive rise in blood pressure may occur with therapeutic doses or inadvertent overdosage.
Local: Repeated local injections can result in necrosis at sites of injection from vascular constriction.
Systemic: Cerebral hemorrhage; hemiplegia; subarachnoid hemorrhage; anginal pain in patients with angina pectoris; anxiety; restlessness; throbbing headache; tremor; weakness; dizziness; pallor; respiratory difficulty; palpitation; apprehensiveness; sweating; nausea; vomiting. “Epinephrine-fastness” can occur with prolonged use.
OVERDOSAGE
Symptoms: Erroneous administration of large doses of epinephrine may lead to precordial distress, vomiting, headache, dyspnea, as well as unusually elevated blood pressure.
Treatment: Most toxic effects can be counteracted by injection of an alpha-adrenergic blocker and a beta-adrenergic blocker. In the event of a sharp rise in blood pressure, rapid acting vasodilators such as the nitrites, or alpha-adrenergic blocking agents can counteract the marked pressor effects. If prolonged hypotension follows, it may be necessary to administer another pressor drug, such as norepinephrine.
If an epinephrine overdose induces pulmonary edema that interferes with respiration, treatment consists of a rapidly acting alpha-adrenergic blocking drug such as phentolamine and/or intermittent positive pressure respiration.
Treatment of cardiac arrhythmias consists of a beta-adrenergic blocking drug such as propranolol.
Epinephrine overdosage can also cause transient bradycardia followed by tachycardia; these may be accompanied by potentially fatal cardiac arrhythmias. Ventricular premature contractions may appear within one minute after injection and may be followed by multifocal ventricular tachycardia (prefibrillation rhythm). Subsidence of the ventricular effects may be followed by atrial tachycardia, and occasionally, by atrioventricular block.
Overdosage sometimes results in extreme pallor and coldness of the skin, metabolic acidosis and kidney failure. Take suitable corrective measures.
DOSAGE AND ADMINISTRATION
EPINEPHRINE
As Cardiac Stimulant:
Intravenous or Intracardiac Injection
Administer by I.V. injection and/or in cardiac arrest, by intracardiac injection into the left ventricular chamber. Intracardiac injection should only be administered by personnel well trained in the technique, if there has not been sufficient time to establish an intravenous route.
Adult Dosage: 1-10 mL (0.1 - 1 mg of epinephrine), repeated every five minutes, if necessary.
Pediatric Dosage: 0.05 - 0.1 mL/ kg of body weight (0.005 - 0.01 mg/ kg) e.g., 2 kg infant would receive 0.1 - 0.2 mL of epinephrine.
NOTE: Do not inject if fibrillation is occurring.
As Vasopressor (Anaphylaxis):
Intravenous Injection
Adult Dosage: 1-2.5 mL (0.1 - 0.25 mg of epinephrine), administered slowly. May be repeated every 5 to 15 minutes as needed.
Pediatric Dosage: 3 mL (0.3 mg of epinephrine), repeated every 15 minutes for 3 or 4 doses, if necessary.
NOTE: Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit.
HOW SUPPLIED
EPINEPHRINE INJECTION, USP, 0.1 mg/ mL
In unit-use packages containing a Luer-Jet™ Luer-Lock Prefilled Syringe
| Stock No. | NDC No. | Size |
|---|---|---|
| 3316 | 76329-3316-1 FOR I.V. USE | 10 mL |
Ten cartons per package.
Syringe Assembly Directions:
USE ASEPTIC TECHNIQUE
Do not assemble until ready to use.

*CAUTION: IMPROPER ENGAGING MAY CAUSE GLASS BREAKAGE AND SUBSEQUENT INJURY.
Protect the solution from light, extreme heat and freezing.
Store at controlled room temperature 15˚ to 30˚C (59˚ to 86˚F).
NOTE: Do not use the injection if its color is pinkish or darker than slightly yellow or if it contains a precipitate.
Rx Only
SPL UNCLASSIFIED SECTION
PRINCIPLE DISPLAY PANEL: Carton
INGREDIENTS AND APPEARANCE
|
EPINEPHRINE
epinephrine injection |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - International Medication Systems, Limited (055750020) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| International Medication Systems, Limited | 055750020 | analysis(76329-3316) , manufacture(76329-3316) , label(76329-3316) | |