Search by Drug Name or NDC
NDC 00037-6822-10 PROCTOFOAM 100; 100 mg/10g; mg/10g Details
PROCTOFOAM 100; 100 mg/10g; mg/10g
PROCTOFOAM is a TOPICAL AEROSOL, FOAM in the HUMAN PRESCRIPTION DRUG category. It is labeled and distributed by Meda Pharmaceuticals Inc.. The primary component is HYDROCORTISONE ACETATE; PRAMOXINE HYDROCHLORIDE.
MedlinePlus Drug Summary
Rectal hydrocortisone is used along with other medications to treat proctitis (swelling in the rectum) and ulcerative colitis (a condition which causes swelling and sores in the lining of the large intestine and rectum). It is also used to relieve itching and swelling from hemorrhoids and other rectal problems. Hydrocortisone is in a class of medications called corticosteroids. It works by activating natural substances in the skin to reduce swelling, redness, and itching.
Related Packages: 00037-6822-10Last Updated: 11/30/2022
MedLinePlus Full Drug Details: Hydrocortisone Rectal
Pramoxine is used to temporarily relieve pain and itching from insect bites; poison ivy, poison oak, or poison sumac; minor cuts, scrapes, or burns; minor skin irritation or rashes; or dry, itchy skin. Pramoxine also may be used to treat soreness, burning, itching, and pain from hemorrhoids (''piles'') and other minor rectal irritations or itching. Pramoxine is in a class of medications called topical anesthetics. It works by stopping nerves from sending pain signals.
Related Packages: 00037-6822-10Last Updated: 11/30/2022
MedLinePlus Full Drug Details: Pramoxine
Product Information
| NDC | 00037-6822 |
|---|---|
| Product ID | 0037-6822_ed540cbc-cfaa-4eee-ba7a-0f324c779df4 |
| Associated GPIs | 89991002313910 |
| GCN Sequence Number | 006847 |
| GCN Sequence Number Description | hydrocortisone/pramoxine FOAM 1 %-1 % RECTAL |
| HIC3 | Q3I |
| HIC3 Description | HEMORRHOID PREP,ANTI-INFLAM STEROID-LOCAL ANESTHET |
| GCN | 82430 |
| HICL Sequence Number | 015163 |
| HICL Sequence Number Description | HYDROCORTISONE ACETATE/PRAMOXINE HCL |
| Brand/Generic | Brand |
| Proprietary Name | PROCTOFOAM |
| Proprietary Name Suffix | HC |
| Non-Proprietary Name | pramoxine hydrochloride hydrocortisone acetate |
| Product Type | HUMAN PRESCRIPTION DRUG |
| Dosage Form | AEROSOL, FOAM |
| Route | TOPICAL |
| Active Ingredient Strength | 100; 100 |
| Active Ingredient Units | mg/10g; mg/10g |
| Substance Name | HYDROCORTISONE ACETATE; PRAMOXINE HYDROCHLORIDE |
| Labeler Name | Meda Pharmaceuticals Inc. |
| Pharmaceutical Class | Corticosteroid Hormone Receptor Agonists [MoA], Corticosteroid [EPC] |
| DEA Schedule | n/a |
| Marketing Category | ANDA |
| Application Number | ANDA086195 |
| Listing Certified Through | 2024-12-31 |
Package
Package Images

NDC 00037-6822-10 (00037682210)
| NDC Package Code | 0037-6822-10 |
|---|---|
| Billing NDC | 00037682210 |
| Package | 1 CANISTER in 1 CARTON (0037-6822-10) / 10 g in 1 CANISTER |
| Marketing Start Date | 2014-08-15 |
| NDC Exclude Flag | N |
| Pricing Information | |
| Price Per Unit | 17.5901 |
| Pricing Unit | GM |
| Effective Date | 2024-01-30 |
| NDC Description | PROCTOFOAM-HC 1%-1% FOAM |
| Pharmacy Type Indicator | C/I |
| OTC | N |
| Explanation Code | 2, 5, 6 |
| Classification for Rate Setting | B |
| As of Date | 2024-02-21 |
Standard Product Labeling (SPL)/Prescribing Information SPL 3831e931-ec0a-11e3-ac10-0800200c9a66 Details
DESCRIPTION
Proctofoam® HC (hydrocortisone acetate 1% and pramoxine hydrochloride 1%) is a topical aerosol foam for anal use containing hydrocortisone acetate 1% and pramoxine hydrochloride 1% in a hydrophilic base containing cetyl alcohol, emulsifying wax, methylparaben, poly-oxyethylene-10-stearyl ether, propylene glycol, propylparaben, purified water, trolamine and inert propellants: isobutane and propane.
Proctofoam® HC contains a synthetic corticosteroid used as an anti-inflammatory/antipruritic agent and a local anesthetic.
Hydrocortisone acetate
Molecular weight: 404.50. Solubility of hydrocortisone acetate in water: 1 mg/100 mL.
Chemical name: pregn-4-ene-3,20-dione, 21-(acetyloxy)-11,17-dihydroxy-,(11β)-.
Pramoxine hydrochloride
Molecular weight: 329.86. Pramoxine hydrochloride is freely soluble in water. Chemical name: morpholine, 4-[3-(4-butoxyphenoxy) propyl]-, hydrochloride.
CLINICAL PHARMACOLOGY
Topical corticosteroids share anti-inflammatory, antipruritic and vasoconstrictive actions.
The mechanism of anti-inflammatory activity of the topical corticosteroids is unclear. Various laboratory methods, including vasoconstrictor assays, are used to compare and predict potencies and/or clinical efficacies of the topical corticosteroids. There is some evidence to suggest that a recognizable correlation exists between vasoconstrictor potency and therapeutic efficacy in man.
Pramoxine hydrochloride is a surface or local anesthetic which is not chemically related to the "caine" types of local anesthetics. Its unique chemical structure is likely to minimize the danger of cross-sensitivity reactions in patients allergic to other local anesthetics.
Pharmacokinetics
The extent of percutaneous absorption of topical corticosteroids is determined by many factors including the vehicle, the integrity of the epidermal barrier, and the use of occlusive dressings.
Topical corticosteroids can be absorbed through normal intact skin. Inflammation and/or other disease processes in the skin increase the percutaneous absorption of topical corticosteroids. Occlusive dressings substantially increase the percutaneous absorption of topical corticosteroids. Thus, occlusive dressings may be a valuable therapeutic adjunct for treatment of resistant dermatoses. (See DOSAGE AND ADMINISTRATION.)
Once absorbed through the skin, topical corticosteroids are handled through pharmacokinetic pathways similar to systemically administered corticosteroids. Corticosteroids are bound to plasma proteins in varying degrees. Corticosteroids are metabolized primarily in the liver and are then excreted by the kidneys. Some of the topical corticosteroids and their metabolites are also excreted into the bile.
INDICATIONS AND USAGE
CONTRAINDICATIONS
WARNINGS
Do not insert any part of the aerosol container directly into the anus. Avoid contact with the eyes. Contents of the container are under pressure. Do not burn or puncture the aerosol container. Do not store at temperatures above 120°F (49°C). If there is no evidence of clinical improvement within two or three weeks after starting Proctofoam® HC therapy, or if the patient's condition worsens, discontinue the drug. Keep this and all medicines out of the reach of children.
PRECAUTIONS
General
Systemic absorption of topical corticosteroids has produced reversible hypothalamic-pituitary-adrenal (HPA) axis suppression, manifestations of Cushing's syndrome, hyperglycemia, and glucosuria in some patients.
Conditions which augment systemic absorption include the application of the more potent steroids, use over large surface areas, prolonged use, and the addition of occlusive dressings. Therefore, patients receiving a large dose of a potent topical steroid applied to a large surface area or under an occlusive dressing should be evaluated periodically for evidence of HPA axis suppression by using the urinary free cortisol and ACTH stimulation tests. If HPA axis suppression is noted, an attempt should be made to withdraw the drug, to reduce the frequency of application or to substitute a less potent steroid.
Recovery of HPA axis function is generally prompt and complete upon discontinuation of the drug. Infrequently, signs and symptoms of steroid withdrawal may occur, requiring supplemental systemic corticosteroids. Pediatric patients may absorb proportionally larger amounts of topical corticosteroids and thus be more susceptible to systemic toxicity. (see PRECAUTIONS: Pediatric Use.)
If irritation develops, topical corticosteroids should be discontinued and appropriate therapy instituted.
In the presence of dermatological infections, the use of an appropriate antifungal or antibacterial agent should be instituted. If a favorable response does not occur promptly, the corticosteroid should be discontinued until the infection has been adequately controlled.
Information for the Patient
Patients using topical corticosteroids should receive the following information and instructions:
- 1.
- This medication is to be used as directed by the physician. It is for anal or perianal use only. Avoid contact with eyes.
- 2.
- Be advised not to use this medication for any disorder other than for which it has been prescribed.
- 3.
- Report any signs of adverse reactions.
Laboratory Tests
The following tests may be helpful in evaluating the HPA axis suppression:
Urinary free cortisol test
ACTH stimulation test
Carcinogenesis, Mutagenesis, Impairment of Fertility
Long-term animal studies have not been performed to evaluate the carcinogenic potential or the effect on fertility of topical corticosteroids.
Studies to determine mutagenicity with prednisolone and hydrocortisone have revealed negative results.
Pregnancy
Teratogenic Effects
Corticosteroids are generally teratogenic in laboratory animals when administered systemically at relatively low dosage levels. The more potent corticosteroids have been shown to be teratogenic after dermal application in laboratory animals. There are no adequate, well-controlled studies of teratogenic effects from topically applied corticosteroids in pregnant women. Therefore, topical corticosteroids should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus. Drugs of this class should not be used extensively on pregnant patients, in large amounts, or for prolonged periods of time.
Nursing Mothers
It is not known whether topical administration of corticosteroids could result in sufficient systemic absorption to produce detectable quantities in breast milk. Systemically administered corticosteroids are secreted into breast milk in quantities not likely to have a deleterious effect on the infant. Nevertheless, caution should be exercised when topical corticosteroids are administered to a nursing woman.
Pediatric Use
Pediatric patients may demonstrate greater susceptibility to topical corticosteroid-induced HPA axis suppression and Cushing's syndrome than mature patients because of a larger skin surface area to body weight ratio.
Hypothalamic-pituitary-adrenal (HPA) axis suppression, Cushing's syndrome, and intracranial hypertension have been reported in pediatric patients receiving topical corticosteroids. Manifestations of adrenal suppression in pediatric patients include linear growth retardation, delayed weight gain, low plasma cortisol levels, and absence of response to ACTH stimulation. Manifestations of intracranial hypertension include bulging fontanelles, headaches, and bilateral papilledema.
Administration of topical corticosteroids to pediatric patients should be limited to the least amount compatible with an effective therapeutic regimen. Chronic corticosteroid therapy may interfere with the growth and development of pediatric patients.
Geriatric Use
Reported clinical experience has not identified differences in responses between the elderly and younger patients. In general, dose selection for an elderly patient should be cautious using the least amount compatible with an effective therapeutic regimen and reflecting the greater frequency of decreased hepatic, renal or cardiac function, and of concomitant disease or other drug therapy.
ADVERSE REACTIONS
The following local adverse reactions are reported infrequently with topical corticosteroids, but may occur more frequently with the use of occlusive dressings. These reactions are listed in approximate decreasing order of occurrence: burning, itching, irritation, dryness, folliculitis, hypertrichosis, acneiform eruptions, hypopigmentation, perioral dermatitis, allergic contact dermatitis, maceration of the skin, secondary infection, skin atrophy, striae and miliaria.
To report SUSPECTED ADVERSE REACTIONS, contact Meda Pharmaceuticals Inc. at 1-866-210-5948 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
OVERDOSAGE
Topically applied corticosteroids can be absorbed in sufficient amounts to produce systemic effects. (See PRECAUTIONS.)
DOSAGE AND ADMINISTRATION
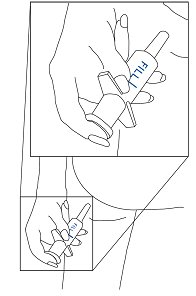
Apply to affected area 3 to 4 times daily. Use the applicator supplied for anal administration. For perianal use, transfer a small quantity to a tissue and rub in gently.
Directions for Use.
- 1.
- Place cap on top of container. Shake foam container vigorously for 5-10 seconds before each use. Do not remove container cap during use of the product.
- 2.
- Hold container upright on a level surface and gently place the tip of the applicator onto the nose of the container cap. CONTAINER MUST BE HELD UPRIGHT TO OBTAIN PROPER FLOW OF MEDICATION.
- 3.
- Pull plunger past the fill line on the applicator barrel.
- 4.
- Hold the container and applicator at eye level. Place the index and middle fingers on the container cap flanges and the thumb beneath the container. Support the applicator with your other hand. Prime the container by pressing down firmly on flanges and then release. With initial priming, a burst of air may come out of the container. It usually requires 1-2 pumps for foam to appear.
- 5.
- To fill applicator barrel, press down firmly on cap flanges, hold for 1-2 seconds, and release. Wait 5-10 seconds to allow foam to expand in applicator barrel. Repeat until foam reaches fill line. It usually requires 3-4 pumps for foam to reach fill line. Remove applicator from container cap. Note: If foam goes beyond fill line, it will continue to expand and flow backwards resulting in foam build-up under cap.
- 6.
- Hold applicator firmly by barrel, making sure thumb and middle finger are positioned securely underneath and resting against barrel wings. Place index finger over the plunger. Gently insert tip into anus. Once in place, push plunger to expel foam, then withdraw applicator. CAUTION: Do not insert any part of the aerosol container directly into the anus. Apply to anus only with enclosed applicator. Do not insert any part of the applicator past the anus into rectum.
- 7.
- After each use, applicator parts should be pulled apart for thorough cleaning with warm water. Since some foam will appear under the cap, the cap and underlying tip should be pulled apart and rinsed to help prevent build-up of foam and possible blockage.
HOW SUPPLIED
Proctofoam® HC is supplied in an aerosol container with a special anal applicator. When used correctly, the aerosol container will deliver a minimum of 14 applications.
NDC 0037-6822-10 10 g
Store upright at controlled room temperature 20°-25°C (68°-77°F). DO NOT REFRIGERATE.
Distributed by:
Meda Pharmaceuticals Inc.
Somerset, New Jersey 08873-4120
(c)2020 Mylan Specialty L.P.
Proctofoam is a registered trademark of Alaven Pharmaceutical LLC, a Mylan company.
IN-682210-03
141135-0420
Distributed by:
Meda Pharmaceuticals Inc.
Somerset, New Jersey 08873-4120
Proctofoam is a registered trademark of Alaven Pharmaceutical LLC, a Mylan company.
To report SUSPECTED ADVERSE REACTIONS, contact Meda Pharmaceuticals Inc. at 1-866-210-5948 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
IS-682210-03
141134-0420
PRINCIPAL DISPLAY PANEL - 10 g
NDC 0037-6822-10
STORE UPRIGHT
proctofoam® HC
(hydrocortisone acetate 1% and
pramoxine hydrochloride 1%)
topical aerosol
Rx Only
10 g net wt
Description
proctofoam® HC (hydrocortisone acetate 1% and
pramoxine hydrochloride 1%) is a topical aerosol foam for
anal use containing hydrocortisone acetate 1% and
pramoxine hydrochloride 1% in a hydrophilic base
containing cetyl alcohol, emulsifying wax, methylparaben
polyoxyethylene-10-stearyl ether, propylene glycol
propylparaben, purified water, trolamine, and inert
propellants: isobutane and propane.
Dosage: Apply to affected areas 3 to 4 times daily. Use
the applicator supplied for anal administration. For
perianal use, transfer a small quantity to a tissue and rub
gently. See patient instructions (inside) for full
directions for use. Please see package insert for
complete prescribing information.
Caution: Do not insert any part of the aerosol container
directly into the anus. Apply to anus only with the
enclosed applicator. Do not insert any part of applicator
past the anus into rectum.
WARNINGS: Contents of the container are under
pressure. Do not burn or puncture the aerosol container.
Do not store at temperatures above 120°F (49°C).
KEEP OUT OF REACH OF CHILDREN.
Store upright at controlled room temperature
20° - 25°C (68° - 77°F).
DO NOT REFRIGERATE.
117713-0622 UC-682210-05
Distributed by:
Meda Pharmaceuticals Inc
Somerset, New Jersey
08873-4120
©2022 Viatris Inc.
PROCTOFOAM is a registered
trademark of
Alaven Pharmaceutical LLC,
a Viatris company.
INGREDIENTS AND APPEARANCE
| PROCTOFOAM
HC
pramoxine hydrochloride hydrocortisone acetate aerosol, foam |
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
| Labeler - Meda Pharmaceuticals Inc. (051229602) |