Search by Drug Name or NDC
NDC 00093-7386-05 Venlafaxine Hydrochloride 150 mg/1 Details
Venlafaxine Hydrochloride 150 mg/1
Venlafaxine Hydrochloride is a ORAL CAPSULE, EXTENDED RELEASE in the HUMAN PRESCRIPTION DRUG category. It is labeled and distributed by Teva Pharmaceuticals USA, Inc.. The primary component is VENLAFAXINE HYDROCHLORIDE.
MedlinePlus Drug Summary
Venlafaxine is used to treat depression. Venlafaxine extended-release (long-acting) capsules are also used to treat generalized anxiety disorder (GAD; excessive worrying that is difficult to control), social anxiety disorder (extreme fear of interacting with others or performing in front of others that interferes with normal life), and panic disorder (sudden, unexpected attacks of extreme fear and worry about these attacks). Venlafaxine is in a class of medications called selective serotonin and norepinephrine reuptake inhibitors (SNRIs). It works by increasing the amounts of serotonin and norepinephrine, natural substances in the brain that help maintain mental balance.
Related Packages: 00093-7386-05Last Updated: 11/30/2022
MedLinePlus Full Drug Details: Venlafaxine
Product Information
| NDC | 00093-7386 |
|---|---|
| Product ID | 0093-7386_79d62d73-0002-4826-8ef3-d9872d14aa4c |
| Associated GPIs | 58180090107050 |
| GCN Sequence Number | 046405 |
| GCN Sequence Number Description | venlafaxine HCl CAP ER 24H 150 MG ORAL |
| HIC3 | H7C |
| HIC3 Description | SEROTONIN-NOREPINEPHRINE REUPTAKE-INHIB (SNRIS) |
| GCN | 16818 |
| HICL Sequence Number | 008847 |
| HICL Sequence Number Description | VENLAFAXINE HCL |
| Brand/Generic | Generic |
| Proprietary Name | Venlafaxine Hydrochloride |
| Proprietary Name Suffix | n/a |
| Non-Proprietary Name | Venlafaxine Hydrochloride |
| Product Type | HUMAN PRESCRIPTION DRUG |
| Dosage Form | CAPSULE, EXTENDED RELEASE |
| Route | ORAL |
| Active Ingredient Strength | 150 |
| Active Ingredient Units | mg/1 |
| Substance Name | VENLAFAXINE HYDROCHLORIDE |
| Labeler Name | Teva Pharmaceuticals USA, Inc. |
| Pharmaceutical Class | Norepinephrine Uptake Inhibitors [MoA], Serotonin Uptake Inhibitors [MoA], Serotonin and Norepinephrine Reuptake Inhibitor [EPC] |
| DEA Schedule | n/a |
| Marketing Category | ANDA |
| Application Number | ANDA076565 |
| Listing Certified Through | 2024-12-31 |
Package
Package Images



NDC 00093-7386-05 (00093738605)
| NDC Package Code | 0093-7386-05 |
|---|---|
| Billing NDC | 00093738605 |
| Package | 500 CAPSULE, EXTENDED RELEASE in 1 BOTTLE (0093-7386-05) |
| Marketing Start Date | 2012-03-01 |
| NDC Exclude Flag | N |
| Pricing Information | |
| Price Per Unit | 0.13853 |
| Pricing Unit | EA |
| Effective Date | 2024-02-21 |
| NDC Description | VENLAFAXINE HCL ER 150 MG CAP |
| Pharmacy Type Indicator | C/I |
| OTC | N |
| Explanation Code | 1 |
| Classification for Rate Setting | G |
| As of Date | 2024-02-21 |
Standard Product Labeling (SPL)/Prescribing Information SPL 9a30e1b5-272b-4109-ad6c-a3c9a895822f Details
HIGHLIGHTS OF PRESCRIBING INFORMATION
VENLAFAXINE HYDROCHLORIDE extended-release capsules, for oral use
Initial U.S. Approval: 1997
WARNING: SUICIDAL THOUGHTS AND BEHAVIORS
See full prescribing information for complete boxed warning.
• Increased risk of suicidal thoughts and behavior in pediatric patients and young adults taking antidepressants. Closely monitor all antidepressant-treated patients for clinical worsening and emergence of suicidal thoughts and behaviors (5.1).
• Venlafaxine hydrochloride extended-release capsules are not approved for use in pediatric patients (8.4).
RECENT MAJOR CHANGES
INDICATIONS AND USAGE
DOSAGE AND ADMINISTRATION
| Indication | Starting Dose | Target Dose | Maximum Dose |
| MDD (2.2) | 37.5 to 75 mg/day | 75 mg/day | 225 mg/day |
| GAD (2.3) | 37.5 to 75 mg/day | 75 mg/day | 225 mg/day |
| SAD (2.4) | 75 mg/day | 75 mg/day | 75 mg/day |
| PD (2.5) | 37.5 mg/day | 75 mg/day | 225 mg/day |
- Take once daily with food. Capsules should be taken whole; do not divide, crush, chew, or dissolve (2.1).
- When discontinuing treatment, reduce the dose gradually (2.10, 5.7).
- Renal impairment: reduce the total daily dose by 25% to 50% in patients with renal impairment. Reduce the total daily dose by 50% or more in patients undergoing dialysis or with severe renal impairment (2.9).
- Hepatic impairment: reduce the daily dose by 50% in patients with mild to moderate hepatic impairment. In patients with severe hepatic impairment or hepatic cirrhosis, it may be necessary to reduce the dose by more than 50% (2.8).
DOSAGE FORMS AND STRENGTHS
- Extended-release capsules: 37.5 mg, 75 mg, and 150 mg (3).
CONTRAINDICATIONS
WARNINGS AND PRECAUTIONS
- Serotonin Syndrome: Increased risk when co-administered with other serotonergic agents (e.g., SSRIs, SNRIs, triptans), but also when taken alone. If it occurs, discontinue venlafaxine hydrochloride extended-release capsules and initiate supportive treatment (4, 5.2, 7.1).
- Elevated Blood Pressure: Control hypertension before initiating treatment. Monitor blood pressure regularly during treatment (5.3).
- Increased Risk of Bleeding: Concomitant use of aspirin, NSAIDs, other antiplatelet drugs, warfarin, and other anticoagulants may increase risk (5.4).
- Angle-Closure Glaucoma: Angle-closure glaucoma has occurred in patients with untreated anatomically narrow angles, treated with antidepressants (5.5).
- Activation of Mania or Hypomania: Screen patients for bipolar disorder (5.6).
- Discontinuation Syndrome: Taper dose and monitor for discontinuation symptoms (5.7).
- Seizures: Can occur. Use cautiously in patients with seizure disorder (5.8).
- Hyponatremia: Can occur in association with SIADH (5.9).
- Interstitial Lung Disease and Eosinophilic Pneumonia: Can occur (5.12).
- Sexual Dysfunction: venlafaxine hydrochloride extended-release capsules may cause symptoms of sexual dysfunction (5.13).
ADVERSE REACTIONS
Most common adverse reactions (incidence ≥ 5% and at least twice the rate of placebo): nausea, somnolence, dry mouth, sweating, abnormal ejaculation, anorexia, constipation, impotence (men), and libido decreased (6.1).
To report SUSPECTED ADVERSE REACTIONS, contact Teva at 1-888-838-2872 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
USE IN SPECIFIC POPULATIONS
Pregnancy: Third trimester use may increase risk for symptoms of poor neonatal adaptation (respiratory distress, temperature instability, feeding difficulty, hypotonia, tremor, irritability) in the neonate (8.1).
See 17 for PATIENT COUNSELING INFORMATION and Medication Guide.
Revised: 9/2022
Table Of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
WARNING: SUICIDAL THOUGHTS AND BEHAVIORS
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 General Administration Information
2.2 Major Depressive Disorder
2.3 Generalized Anxiety Disorder
2.4 Social Anxiety Disorder (Social Phobia)
2.5 Panic Disorder
2.6 Screen for Bipolar Disorder Prior to Starting Venlafaxine Hydrochloride Extended-Release Capsules
2.7 Switching Patients from Venlafaxine Tablets
2.8 Dosage Recommendations for Patients with Hepatic Impairment
2.9 Dosage Recommendations for Patients with Renal Impairment
2.10 Discontinuing Treatment with Venlafaxine Hydrochloride Extended-Release Capsules
2.11 Switching Patients to or from a Monoamine Oxidase Inhibitor (MAOI) Antidepressant
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Suicidal Thoughts and Behaviors in Adolescents and Young Adults
5.2 Serotonin Syndrome
5.3 Elevated Blood Pressure
5.4 Increased Risk of Bleeding
5.5 Angle-Closure Glaucoma
5.6 Activation of Mania or Hypomania
5.7 Discontinuation Syndrome
5.8 Seizures
5.9 Hyponatremia
5.10 Weight and Height Changes in Pediatric Patients
5.11 Appetite Changes in Pediatric Patients
5.12 Interstitial Lung Disease and Eosinophilic Pneumonia
5.13 Sexual Dysfunction
6 ADVERSE REACTIONS
6.1 Clinical Studies Experience
6.2 Postmarketing Experience
7 DRUG INTERACTIONS
7.1 Drugs Having Clinically Important Interactions with Venlafaxine Hydrochloride Extended-Release Capsules
7.2 Other Drug Interactions with Venlafaxine Hydrochloride Extended-Release Capsules
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Hepatic Impairment
8.7 Renal Impairment
9 DRUG ABUSE AND DEPENDENCE
9.1 Controlled Substance
9.2 Abuse
9.3 Dependence
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
14.1 Major Depressive Disorder
14.2 Generalized Anxiety Disorder
14.3 Social Anxiety Disorder (also known as Social Phobia)
14.4 Panic Disorder
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- *
- Sections or subsections omitted from the full prescribing information are not listed.
WARNING
Antidepressants increased the risk of suicidal thoughts and behavior in pediatric and young adult patients in short-term studies. Closely monitor all antidepressant-treated patients for clinical worsening, and emergence of suicidal thoughts and behaviors [see Warnings and Precautions (5.1].
Venlafaxine hydrochloride extended-release capsules are not approved for use in pediatric patients [see Use in Specific Populations (8.4)].
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 General Administration Information
Administer venlafaxine hydrochloride extended-release capsules as a single dose with food, either in the morning or in the evening at approximately the same time each day [see Clinical Pharmacology (12.3)]. Swallow capsules whole with fluid. Do not divide, crush, chew, or place in water.
The capsule may also be administered by carefully opening the capsule and sprinkling the entire contents on a spoonful of applesauce. This drug/food mixture should be swallowed immediately without chewing and followed with a glass of water to ensure complete swallowing of the pellets (spheroids).
2.2 Major Depressive Disorder
For most patients, the recommended starting dose for venlafaxine hydrochloride extended-release capsules is 75 mg per day, administered in a single dose. For some patients, it may be desirable to start at 37.5 mg per day for 4 to 7 days to allow new patients to adjust to the medication before increasing to 75 mg per day. Patients not responding to the initial 75 mg per day dose may benefit from dose increases to a maximum of 225 mg per day. Dose increases should be in increments of up to 75 mg per day, as needed, and should be made at intervals of not less than 4 days. In the clinical studies establishing efficacy, upward titration was permitted at intervals of 2 weeks or more.
2.3 Generalized Anxiety Disorder
For most patients, the recommended starting dose for venlafaxine hydrochloride extended-release capsules is 75 mg per day, administered in a single dose. For some patients, it may be desirable to start at 37.5 mg per day for 4 to 7 days to allow new patients to adjust to the medication before increasing to 75 mg per day. Patients not responding to the initial 75 mg per day dose may benefit from dose increases to a maximum of 225 mg per day. Dose increases should be in increments of up to 75 mg per day, as needed, and should be made at intervals of not less than 4 days.
2.4 Social Anxiety Disorder (Social Phobia)
The recommended dose is 75 mg per day, administered in a single dose. There was no evidence that higher doses confer any additional benefit.
2.5 Panic Disorder
The recommended starting dose is 37.5 mg per day of venlafaxine hydrochloride extended-release capsules for 7 days. Patients not responding to 75 mg per day may benefit from dose increases to a maximum of approximately 225 mg per day. Dose increases should be in increments of up to 75 mg per day, as needed, and should be made at intervals of not less than 7 days.
2.6 Screen for Bipolar Disorder Prior to Starting Venlafaxine Hydrochloride Extended-Release Capsules
Prior to initiating treatment with venlafaxine hydrochloride extended-release capsules, screen patients for a personal or family history of bipolar disorder, mania, or hypomania [see Warnings and Precautions (5.6)].
2.7 Switching Patients from Venlafaxine Tablets
Patients with depression who are currently being treated with venlafaxine tablets (immediate release) may be switched to venlafaxine hydrochloride extended-release capsules at the nearest equivalent dose (mg per day), e.g., 37.5 mg venlafaxine twice a day to 75 mg venlafaxine hydrochloride extended-release capsules once daily. However, individual dosage adjustments may be necessary.
2.8 Dosage Recommendations for Patients with Hepatic Impairment
Reduce the venlafaxine hydrochloride extended-release capsules total daily dose by 50% in patients with mild (Child-Pugh Class A) to moderate (Child-Pugh Class B) hepatic impairment. Reduce the total daily dose by 50% or more in patients with severe hepatic impairment (Child-Pugh Class C) or hepatic cirrhosis [see Use in Specific Populations (8.6)].
2.9 Dosage Recommendations for Patients with Renal Impairment
Reduce the venlafaxine hydrochloride extended-release capsules total daily dose by 25% to 50% in patients with mild (CLcr 60 to 89 mL/min) or moderate (CLcr 30 to 59 mL/min) renal impairment. Reduce the total daily dose by 50% or more in patients undergoing hemodialysis or with severe renal impairment (CLcr < 30 mL/min). Because there was much individual variability in clearance between patients with renal impairment, individualization of dosage is recommended in some patients [see Use in Specific Populations (8.7)].
2.10 Discontinuing Treatment with Venlafaxine Hydrochloride Extended-Release Capsules
A gradual reduction in the dose, rather than abrupt cessation, is recommended when discontinuing therapy with venlafaxine hydrochloride extended-release capsules. In clinical studies with venlafaxine hydrochloride extended-release capsules, tapering was achieved by reducing the daily dose by 75 mg at one-week intervals. Individualization of tapering may be necessary. In some patients, discontinuation may need to occur over a period of several months [see Warnings and Precautions (5.7)].
2.11 Switching Patients to or from a Monoamine Oxidase Inhibitor (MAOI) Antidepressant
At least 14 days must elapse between discontinuation of an MAOI antidepressant and initiation of venlafaxine hydrochloride extended-release capsules. In addition, at least 7 days must elapse after stopping venlafaxine hydrochloride extended-release capsules before starting an MAOI antidepressant [see Contraindications (4), Warnings and Precautions (5.2), and Drug Interactions (7.1)].
3 DOSAGE FORMS AND STRENGTHS
Venlafaxine hydrochloride extended-release capsules, USP are available in the following strengths:
- 37.5 mg extended-release capsules - light-gray opaque cap and buff opaque body with “93” and “7384” on both body and cap.
- 75 mg extended-release capsules - buff opaque cap and buff opaque body with “93” and “7385” on both body and cap.
- 150 mg extended-release capsules - light-orange opaque cap and light-orange opaque body with “93” and “7386” on both body and cap.
4 CONTRAINDICATIONS
Venlafaxine hydrochloride extended-release capsules are contraindicated in patients:
- with known hypersensitivity to venlafaxine hydrochloride, desvenlafaxine succinate or to any excipients in the formulation [see Adverse Reactions (6.2)].
- taking, or within 14 days of stopping, MAOIs (including the MAOIs linezolid and intravenous methylene blue) because of the risk of serotonin syndrome [see Dosage and Administration (2.11), Warnings and Precautions (5.2), and Drug Interactions (7.1)].
5 WARNINGS AND PRECAUTIONS
5.1 Suicidal Thoughts and Behaviors in Adolescents and Young Adults
In pooled analyses of placebo-controlled trials of antidepressant drugs (SSRIs and other antidepressant classes) that included approximately 77,000 adult patients and 4,500 pediatric patients, the incidence of suicidal thoughts and behaviors in antidepressant-treated patients age 24 years and younger was greater than in placebo-treated patients. There was considerable variation in risk of suicidal thoughts and behaviors among drugs, but there was an increased risk identified in young patients for most drugs studied. There were differences in absolute risk of suicidal thoughts and behaviors across the different indications, with the highest incidence in patients with MDD. The drug-placebo differences in the number of cases of suicidal thoughts and behaviors per 1,000 patients treated are provided in Table 1.
|
Age Range |
Drug-Placebo Difference in Number of Patients of Suicidal Thoughts and Behaviors per 1,000 Patients Treated |
|
Increases Compared to Placebo |
|
|
< 18 years old |
14 additional patients |
|
18 to 24 years old |
5 additional patients |
|
Decreases Compared to Placebo |
|
|
25 to 64 years old |
1 fewer patient |
|
≥ 65 years old |
6 fewer patients |
|
*Venlafaxine hydrochloride extended-release capsules are not approved in pediatric patients. |
|
It is unknown whether the risk of suicidal thoughts and behaviors in children, adolescents, and young adults extends to longer-term use, i.e., beyond four months. However, there is substantial evidence from placebo-controlled maintenance trials in adults with MDD that antidepressants delay the recurrence of depression and that depression itself is a risk factor for suicidal thoughts and behaviors.
Monitor all antidepressant-treated patients for any indication for clinical worsening and emergence of suicidal thoughts and behaviors, especially during the initial few months of drug therapy, and at times of dosage changes. Counsel family members or caregivers of patients to monitor for changes in behavior and to alert the healthcare provider. Consider changing the therapeutic regimen, including possibly discontinuing venlafaxine hydrochloride extended-release capsules, in patients whose depression is persistently worse, or who are experiencing emergent suicidal thoughts or behaviors.
5.2 Serotonin Syndrome
Serotonin-norepinephrine reuptake inhibitors (SNRIs), including venlafaxine hydrochloride extended-release capsules can precipitate serotonin syndrome, a potentially life-threatening condition. The risk is increased with concomitant use of other serotonergic drugs (including triptans, tricyclic antidepressants, fentanyl, lithium, tramadol, tryptophan, buspirone, amphetamines, and St. John’s Wort) and with drugs that impair metabolism of serotonin, i.e., MAOIs [see Contraindications (4), Drug Interactions (7.1)]. Serotonin syndrome can also occur when these drugs are used alone.
Serotonin syndrome signs and symptoms may include mental status changes (e.g., agitation, hallucinations, delirium, coma), autonomic instability (e.g., tachycardia, labile blood pressure, dizziness, diaphoresis, flushing, hyperthermia), neuromuscular symptoms (e.g., tremor, rigidity, myoclonus, hyperreflexia, incoordination), seizures, and gastrointestinal symptoms (e.g., nausea, vomiting, diarrhea).
The concomitant use of venlafaxine hydrochloride extended-release capsules with MAOIs is contraindicated. In addition, do not initiate venlafaxine hydrochloride extended-release capsules in a patient being treated with MAOIs such as linezolid or intravenous methylene blue. No reports involved the administration of methylene blue by other routes (such as oral tablets or local tissue injection). If it is necessary to initiate treatment with an MAOI such as linezolid or intravenous methylene blue in a patient taking venlafaxine hydrochloride extended-release capsules, discontinue venlafaxine hydrochloride extended-release capsules before initiating treatment with the MAOI [see Contraindications (4), Drug Interactions (7.1)].
Monitor all patients taking venlafaxine hydrochloride extended-release capsules for the emergence of serotonin syndrome. Discontinue treatment with venlafaxine hydrochloride extended-release capsules and any concomitant serotonergic agents immediately if the above symptoms occur, and initiate supportive symptomatic treatment. If concomitant use of venlafaxine hydrochloride extended-release capsules with other serotonergic drugs is clinically warranted, inform patients of the increased risk for serotonin syndrome and monitor for symptoms.
5.3 Elevated Blood Pressure
In controlled trials, there were dose-related increases in systolic and diastolic blood pressure, as well as cases of sustained hypertension [see Adverse Reactions (6.1)].
Monitor blood pressure before initiating treatment with venlafaxine hydrochloride extended-release capsules and regularly during treatment. Control pre-existing hypertension before initiating treatment with venlafaxine hydrochloride extended-release capsules. Use caution in treating patients with pre-existing hypertension or cardiovascular or cerebrovascular conditions that might be compromised by increases in blood pressure. Sustained blood pressure elevation can lead to adverse outcomes. Cases of elevated blood pressure requiring immediate treatment have been reported with venlafaxine hydrochloride extended-release capsules. Consider dose reduction or discontinuation of treatment for patients who experience a sustained increase in blood pressure.
Across all clinical studies with venlafaxine tablets, 1.4% of patients in the venlafaxine hydrochloride extended-release capsule treated groups experienced a ≥ 15 mm Hg increase in supine diastolic blood pressure (SDBP)
≥ 105 mm Hg, compared to 0.9% of patients in the placebo groups. Similarly, 1% of patients in the venlafaxine hydrochloride extended-release capsule treated groups experienced a ≥ 20 mm Hg increase in supine systolic blood pressure (SSBP) with blood pressure ≥ 180 mm Hg, compared to 0.3% of patients in the placebo groups [see Adverse Reactions (6.1)]. Treatment with venlafaxine hydrochloride extended-release capsules was associated with sustained hypertension (defined as SDBP ≥ 90 mm Hg and ≥ 10 mm Hg above baseline for three consecutive on-therapy visits [see Adverse Reactions (6.1)]. An insufficient number of patients received mean doses of venlafaxine hydrochloride extended-release capsules over 300 mg per day in clinical studies to fully evaluate the incidence of sustained increases in blood pressure at these higher doses.
5.4 Increased Risk of Bleeding
Drugs that interfere with serotonin reuptake inhibition, including venlafaxine hydrochloride extended-release capsules, may increase the risk of bleeding events, ranging from ecchymoses, hematomas, epistaxis, petechiae, and gastrointestinal hemorrhage to life-threatening hemorrhage. Concomitant use of aspirin, Nonsteroidal Anti-Inflammatory Drugs (NSAIDs), warfarin, and other anti-coagulants or other drugs known to affect platelet function may add to this risk. Case reports and epidemiological studies (case-control and cohort design) have demonstrated an association between use of drugs that interfere with serotonin reuptake and the occurrence of gastrointestinal bleeding.
Inform patients about the risk of bleeding associated with the concomitant use of venlafaxine hydrochloride extended-release capsules nonsteroidal anti-inflammatory drugs (NSAIDs), aspirin, or other drugs that affect coagulation. For patients taking warfarin, carefully monitor coagulation indices when initiating, titrating, or discontinuing venlafaxine hydrochloride extended-release capsules.
5.5 Angle-Closure Glaucoma
The pupillary dilation that occurs following use of many antidepressant drugs including venlafaxine hydrochloride extended-release capsules may trigger an angle closure attack in a patient with anatomically narrow angles who does not have a patent iridectomy. Avoid use of antidepressants, including venlafaxine hydrochloride extended-release capsules, in patients with untreated anatomically narrow angles.
5.6 Activation of Mania or Hypomania
In patients with bipolar disorder, treating a depressive episode with venlafaxine hydrochloride extended-release capsules or another antidepressant may precipitate a mixed/manic episode. Mania or hypomania was reported in venlafaxine hydrochloride extended-release capsule treated patients in the premarketing studies in MDD, SAD, and PD (see Table 2). Prior to initiating treatment with venlafaxine hydrochloride extended-release capsules, screen for any personal or family history of bipolar disorder, mania, or hypomania.
|
Indication |
Venlafaxine Hydrochloride Extended-Release Capsules |
Placebo |
|
MDD |
0.3 |
0.0 |
|
GAD |
0.0 |
0.2 |
|
SAD |
0.2 |
0.0 |
|
PD |
0.1 |
0.0 |
5.7 Discontinuation Syndrome
Discontinuation symptoms have been systematically evaluated in patients taking venlafaxine, including prospective analyses of clinical studies in GAD and retrospective surveys of studies in MDD and SAD. Abrupt discontinuation or dose reduction of venlafaxine at various doses has been found to be associated with the appearance of new symptoms, the frequency of which increased with increased dose level and with longer duration of treatment. Reported symptoms include agitation, anorexia, anxiety, confusion, impaired coordination and balance, diarrhea, dizziness, dry mouth, dysphoric mood, fasciculation, fatigue, flu-like symptoms, headaches, hypomania, insomnia, nausea, nervousness, nightmares, sensory disturbances (including shock-like electrical sensations), somnolence, sweating, tremor, vertigo, and vomiting.
There have been postmarketing reports of serious discontinuation symptoms which can be protracted and severe. Completed suicide, suicidal thoughts, aggression and violent behavior have been observed in patients during reduction in venlafaxine hydrochloride extended-release capsules dosage, including during discontinuation. Other postmarketing reports describe visual changes (such as blurred vision or trouble focusing) and increased blood pressure after stopping or reducing the dose of venlafaxine hydrochloride extended-release capsules.
During marketing of venlafaxine hydrochloride extended-release capsules, other SNRIs, and SSRIs, there have been reports of adverse events occurring upon discontinuation of these drugs, particularly when abrupt, including the following: irritability, lethargy, emotional lability, tinnitus, and seizures.
Patients should be monitored for these symptoms when discontinuing treatment with venlafaxine hydrochloride extended-release capsules. A gradual reduction in the dose, rather than abrupt cessation, is recommended. If intolerable symptoms occur following a decrease in the dose or upon discontinuation of treatment, then resuming the previously prescribed dose may be considered. Subsequently, the healthcare provider may continue decreasing the dose, but at a more gradual rate. In some patients, discontinuation may need to occur over a period of several months [see Dosage and Administration (2.10)].
5.8 Seizures
Cases of seizure have been reported with venlafaxine therapy. Venlafaxine hydrochloride extended-release capsules has not been systematically evaluated in patients with seizure disorder. Venlafaxine hydrochloride extended-release capsules should be prescribed with caution in patients with a seizure disorder.
5.9 Hyponatremia
Hyponatremia can occur as a result of treatment with SNRIs, including venlafaxine hydrochloride extended-release capsules. In many cases, the hyponatremia appears to be the result of the Syndrome of Inappropriate Antidiuretic Hormone (SIADH) secretion. Cases with serum sodium lower than 110 mmol/L have been reported. Elderly patients may be at greater risk of developing hyponatremia with SNRIs. Also, patients taking diuretics, or those who are otherwise volume-depleted, may be at greater risk [see Use in Specific Populations (8.5) and Clinical Pharmacology (12.3)]. Consider discontinuation of venlafaxine hydrochloride extended-release capsules in patients with symptomatic hyponatremia, and institute appropriate medical intervention.
Signs and symptoms of hyponatremia include headache, difficulty concentrating, memory impairment, confusion, weakness, and unsteadiness, which may lead to falls. Signs and symptoms associated with more severe and/or acute cases have included hallucination, syncope, seizure, coma, respiratory arrest, and death.
5.10 Weight and Height Changes in Pediatric Patients
Weight Changes
The average change in body weight and incidence of weight loss (percentage of patients who lost 3.5% or more) in the placebo-controlled pediatric studies in MDD, GAD, and SAD are shown in Tables 3 and 4.
|
Indication (Duration) |
Venlafaxine Hydrochloride |
Placebo |
|
MDD and GAD (4 pooled studies, 8 weeks) |
-0.45 (n = 333) |
+0.77 (n = 333) |
|
SAD (16 weeks) |
-0.75 (n = 137) |
+0.76 (n = 148) |
|
a Venlafaxine hydrochloride extended-release capsules are not approved for use in pediatric patients. |
||
|
Indication (Duration) |
Venlafaxine Hydrochloride |
Placebo |
|
MDD and GAD (4 pooled studies, 8 weeks) |
18b (n = 333) |
3.6 (n = 333) |
|
SAD (16 weeks) |
47b (n = 137) |
14 (n = 148) |
|
a Venlafaxine hydrochloride extended-release capsules are not approved for use in pediatric patients. b p < 0.001 versus placebo |
||
Weight loss was not limited to patients with anorexia [see Warnings and Precautions (5.11)].
The risks associated with longer term venlafaxine hydrochloride extended-release capsule use were assessed in an open-label MDD study of children and adolescents who received venlafaxine hydrochloride extended-release capsules for up to six months. The children and adolescents in the study had increases in weight that were less than expected, based on data from age- and sex-matched peers. The difference between observed weight gain and expected weight gain was larger for children (< 12 years old) than for adolescents (≥ 12 years old).
Venlafaxine hydrochloride extended-release capsules are not approved for use in pediatric patients [Use in Specific Populations (8.4)].
Height Changes
Table 5 shows the average height increase in pediatric patients in the short-term, placebo-controlled MDD, GAD, and SAD studies. The differences in height increases in GAD and MDD studies were most notable in patients younger than 12 years old.
|
Indication (Duration) |
Venlafaxine Hydrochloride |
Placebo |
|
MDD (8 weeks) |
0.8 (n = 146) |
0.7 (n = 147) |
|
GAD (8 weeks) |
0.3b (n = 122) |
1.0 (n = 132) |
|
SAD (16 weeks) |
1.0 (n = 109) |
1.0 (n = 112) |
|
a Venlafaxine hydrochloride extended-release capsules are not approved for use in pediatric patients. b p = 0.041 |
||
In the six-month, open-label MDD study, children and adolescents had height increases that were less than expected, based on data from age- and sex-matched peers. The difference between observed and expected growth rates was larger for children (< 12 years old) than for adolescents (≥ 12 years old) [see Use in Specific Populations (8.4)].
5.11 Appetite Changes in Pediatric Patients
Decreased appetite (reported as anorexia) was more commonly observed in venlafaxine hydrochloride extended-release capsule treated patients versus placebo-treated patients in the premarketing evaluation of venlafaxine hydrochloride extended-release capsules for MDD, GAD, and SAD (see Table 6).
Venlafaxine hydrochloride extended-release capsules are not approved for use in pediatric patients [see Use in Specific Populations (8.4)].
|
Indication (Duration) |
Venlafaxine Hydrochloride Extended-Release Capsules Incidence |
Discontinuation |
Placebo Incidence |
Discontinuation |
|
MDD and GAD (pooled, 8 weeks) |
10 |
0.0 |
3 |
- |
|
SAD (16 weeks) |
22 |
0.7 |
3 |
0.0 |
|
a The discontinuation rates for weight loss were 0.7% for patients receiving either venlafaxine hydrochloride extended-release capsules or placebo. b Venlafaxine hydrochloride extended-release capsules are not approved for use in pediatric patients. |
||||
5.12 Interstitial Lung Disease and Eosinophilic Pneumonia
Interstitial lung disease and eosinophilic pneumonia associated with venlafaxine therapy have been rarely reported. The possibility of these events should be considered in venlafaxine hydrochloride extended-release capsules-treated patients who present with progressive dyspnea, cough or chest discomfort. Such patients should undergo a prompt medical evaluation, and discontinuation of venlafaxine hydrochloride extended-release capsules should be considered.
5.13 Sexual Dysfunction
Use of SNRIs, including venlafaxine hydrochloride extended-release capsules, may cause symptoms of sexual dysfunction [see Adverse Reactions (6.1)]. In male patients, SNRI use may result in ejaculatory delay or failure, decreased libido, and erectile dysfunction. In female patients, SNRI use may result in decreased libido and delayed or absent orgasm.
It is important for prescribers to inquire about sexual function prior to initiation of venlafaxine hydrochloride extended-release capsules and to inquire specifically about changes in sexual function during treatment, because sexual function may not be spontaneously reported. When evaluating changes in sexual function, obtaining a detailed history (including timing of symptom onset) is important because sexual symptoms may have other causes, including the underlying psychiatric disorder. Discuss potential management strategies to support patients in making informed decisions about treatment.
6 ADVERSE REACTIONS
The following adverse reactions are discussed in more detail in other sections of the labeling:
- Hypersensitivity [see Contraindications (4)]
- Suicidal Thoughts and Behaviors in Adolescents and Young Adults [see Warnings and Precautions (5.1)]
- Serotonin Syndrome [see Warnings and Precautions (5.2)]
- Elevated Blood Pressure [see Warnings and Precautions (5.3)]
- Increased Risk of Bleeding [see Warnings and Precautions (5.4)]
- Angle Closure Glaucoma [see Warnings and Precautions (5.5)]
- Activation of Mania/Hypomania [see Warnings and Precautions (5.6)]
- Discontinuation Syndrome [see Warnings and Precautions (5.7)]
- Seizure [see Warnings and Precautions (5.8)]
- Hyponatremia [see Warnings and Precautions (5.9)]
- Weight and Height changes in Pediatric Patients [see Warnings and Precautions (5.10)]
- Appetite Changes in Pediatric Patients [see Warnings and Precautions (5.11)]
- Interstitial Lung Disease and Eosinophilic Pneumonia [see Warnings and Precautions (5.12)]
- Sexual Dysfunction [see Warnings and Precautions (5.13)]
6.1 Clinical Studies Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical studies of another drug and may not reflect the rates observed in clinical practice.
Most Common Adverse Reactions
The most commonly observed adverse reactions in the clinical study database in venlafaxine hydrochloride extended-release capsule treated patients in MDD, GAD, SAD, and PD (incidence ≥ 5% and at least twice the rate of placebo) were: nausea (30.0%), somnolence (15.3%), dry mouth (14.8%), sweating (11.4%), abnormal ejaculation (9.9%), anorexia (9.8%), constipation (9.3%), impotence (5.3%), and decreased libido (5.1%).
Adverse Reactions Reported as Reasons for Discontinuation of Treatment
Combined across short-term, placebo-controlled premarketing studies for all indications, 12% of the 3,558 patients who received venlafaxine hydrochloride extended-release capsules (37.5 mg to 225 mg) discontinued treatment due to an adverse experience, compared with 4% of the 2,197 placebo-treated patients in those studies.
The most common adverse reactions leading to discontinuation in ≥ 1% of the venlafaxine hydrochloride extended-release capsule treated patients in the short-term studies (up to 12 weeks) across indications are shown in Table 7.
|
Body System Adverse Reaction |
Venlafaxine Hydrochloride Extended-Release Capsules n = 3,558 |
Placebo n = 2,197 |
|
Body as a whole | ||
|
Asthenia |
1.7 |
0.5 |
|
Headache |
1.5 |
0.8 |
|
Digestive system | ||
|
Nausea |
4.3 |
0.4 |
|
Nervous system | ||
|
Dizziness |
2.2 |
0.8 |
|
Insomnia |
2.1 |
0.6 |
|
Somnolence |
1.7 |
0.3 |
|
Skin and appendages |
1.5 |
0.6 |
|
Sweating |
1.0 |
0.2 |
Common Adverse Reactions in Placebo-controlled Studies
The number of patients receiving multiple doses of venlafaxine hydrochloride extended-release capsules during the premarketing assessment for each approved indication is shown in Table 8. The conditions and duration of exposure to venlafaxine in all development programs varied greatly, and included (in overlapping categories) open and double-blind studies, uncontrolled and controlled studies, inpatient (venlafaxine tablets only) and outpatient studies, fixed-dose, and titration studies.
|
Indication |
Venlafaxine Hydrochloride Extended-Release Capsules |
|
MDD |
705a |
|
GAD |
1,381 |
|
SAD |
819 |
|
PD |
1,314 |
|
a In addition, in the premarketing assessment of venlafaxine tablets, multiple doses were administered to 2,897 patients in studies for MDD. |
|
The incidences of common adverse reactions (those that occurred in ≥ 2% of venlafaxine hydrochloride extended-release capsule treated patients [357 MDD patients, 1,381 GAD patients, 819 SAD patients, and 1,001 PD patients] and more frequently than placebo) in venlafaxine hydrochloride extended-release capsule treated patients in short-term, placebo-controlled, fixed- and flexible-dose clinical studies (doses 37.5 mg to 225 mg per day) are shown in Table 9.
The adverse reaction profile did not differ substantially between the different patient populations.
|
Body System |
Venlafaxine Hydrochloride |
Placebo |
|
Adverse Reaction |
n = 3,558 |
n = 2,197 |
|
Body as a whole | ||
|
Asthenia |
12.6 |
7.8 |
|
Cardiovascular system | ||
|
Hypertension |
3.4 |
2.6 |
|
Palpitation |
2.2 |
2.0 |
|
Vasodilatation |
3.7 |
1.9 |
|
Digestive system | ||
|
Anorexia |
9.8 |
2.6 |
|
Constipation |
9.3 |
3.4 |
|
Diarrhea |
7.7 |
7.2 |
|
Dry mouth |
14.8 |
5.3 |
|
Nausea |
30.0 |
11.8 |
|
Vomiting |
4.3 |
2.7 |
|
Nervous system | ||
|
Abnormal dreams |
2.9 |
1.4 |
|
Dizziness |
15.8 |
9.5 |
|
Insomnia |
17.8 |
9.5 |
|
Libido decreased |
5.1 |
1.6 |
|
Nervousness |
7.1 |
5.0 |
|
Paresthesia |
2.4 |
1.4 |
|
Somnolence |
15.3 |
7.5 |
|
Tremor |
4.7 |
1.6 |
|
Respiratory system | ||
|
Yawn |
3.7 |
0.2 |
|
Skin and appendages | ||
|
Sweating (including night sweats) |
11.4 |
2.9 |
|
Special senses | ||
|
Abnormal vision |
4.2 |
1.6 |
|
Urogenital system | ||
|
Abnormal ejaculation/orgasm (men)a |
9.9 |
0.5 |
|
Anorgasmia (men)a |
3.6 |
0.1 |
|
Anorgasmia (women)b |
2.0 |
0.2 |
|
Impotence (men)a |
5.3 |
1.0 |
|
a Percentages based on the number of men (venlafaxine hydrochloride extended-release capsules, n = 1,440; placebo, n = 923) b Percentages based on the number of women (venlafaxine hydrochloride extended-release capsules, n = 2,118; placebo, n = 1,274) |
||
Other Adverse Reactions Observed in Clinical Studies
Body as a whole – Photosensitivity reaction, chills
Cardiovascular system – Postural hypotension, syncope, hypotension, tachycardia
Digestive system – Gastrointestinal hemorrhage [see Warnings and Precautions (5.4)], bruxism
Hemic/Lymphatic system – Ecchymosis [see Warnings and Precautions (5.4)]
Metabolic/Nutritional – Hypercholesterolemia, weight gain [see Warnings and Precautions (5.10)], weight loss [see Warnings and Precautions (5.10)]
Nervous system – Seizures [see Warnings and Precautions (5.8)], manic reaction [see Warnings and Precautions (5.6)], agitation, confusion, akathisia, hallucinations, hypertonia, myoclonus, depersonalization, apathy
Skin and appendages – Urticaria, pruritus, rash, alopecia
Special senses – Mydriasis, abnormality of accommodation, tinnitus, taste perversion
Urogenital system – Urinary retention, urination impaired, urinary incontinence, urinary frequency increased, menstrual disorders associated with increased bleeding or increased irregular bleeding (e.g., menorrhagia, metrorrhagia)
Vital Sign Changes
In placebo-controlled premarketing studies, there were increases in mean blood pressure (see Table 10). Across most indications, a dose-related increase in mean supine systolic and diastolic blood pressure was evident in patients treated with venlafaxine hydrochloride extended-release capsules. Across all clinical studies in MDD, GAD, SAD and PD, 1.4% of patients in the venlafaxine hydrochloride extended-release capsules groups experienced an increase in SDBP of ≥ 15 mm Hg along with a blood pressure ≥ 105 mm Hg, compared to 0.9% of patients in the placebo groups. Similarly, 1% of patients in the venlafaxine hydrochloride extended-release capsules groups experienced an increase in SSBP of ≥ 20 mm Hg with a blood pressure ≥ 180 mm Hg, compared to 0.3% of patients in the placebo groups.
|
Indication (Duration) |
Venlafaxine Hydrochloride Extended-release Capsules |
Placebo |
||||
|
≤ 75 mg per day |
> 75 mg per day |
|||||
|
SSBP |
SDBP |
SSBP |
SDBP |
SSBP |
SDBP |
|
|
MDD | ||||||
|
(8 to 12 weeks) |
-0.28 |
0.37 |
2.93 |
3.56 |
-1.08 |
-0.10 |
|
GAD | ||||||
|
(8 weeks) |
-0.28 |
0.02 |
2.40 |
1.68 |
-1.26 |
-0.92 |
|
(6 months) |
1.27 |
-0.69 |
2.06 |
1.28 |
-1.29 |
-0.74 |
|
SAD | ||||||
|
(12 weeks) |
-0.29 |
-1.26 |
1.18 |
1.34 |
-1.96 |
-1.22 |
|
(6 months) |
-0.98 |
-0.49 |
2.51 |
1.96 |
-1.84 |
-0.65 |
|
PD | ||||||
|
(10 to 12 weeks) |
-1.15 |
0.97 |
-0.36 |
0.16 |
-1.29 |
-0.99 |
Venlafaxine hydrochloride extended-release capsule treatment was associated with sustained hypertension (defined as Supine Diastolic Blood Pressure [SDBP] ≥ 90 mm Hg and ≥ 10 mm Hg above baseline for three consecutive on-therapy visits (see Table 11). An insufficient number of patients received mean doses of venlafaxine hydrochloride extended-release capsules over 300 mg per day in clinical studies to fully evaluate the incidence of sustained increases in blood pressure at these higher doses.
|
Indication |
Dose Range (mg per day) |
Incidence (%) |
|
MDD |
75 to 375a |
19/705 (3) |
|
GAD |
37.5 to 225 |
5/1011 (0.5) |
|
SAD |
75 to 225 |
5/771 (0.6) |
|
PD |
75 to 225 |
9/973 (0.9) |
|
a Maximum recommended dosage for venlafaxine hydrochloride extended-release capsules is 225 mg once daily. |
||
Venlafaxine hydrochloride extended-release capsules were associated with mean increases in pulse rate compared with placebo in premarketing placebo-controlled studies (see Table 12) [see Warnings and Precautions (5.3, 5.4)].
|
Indication (Duration) |
Venlafaxine Hydrochloride |
Placebo |
|
MDD (12 weeks) |
2 |
1 |
|
GAD (8 weeks) |
2 |
< 1 |
|
SAD (12 weeks) |
3 |
1 |
|
PD (12 weeks) |
1 |
< 1 |
Laboratory Changes
Serum Cholesterol
Venlafaxine hydrochloride extended-release capsules were associated with mean final increases in serum cholesterol concentrations compared with mean final decreases for placebo in premarketing MDD, GAD, SAD and PD clinical studies (Table 13).
|
Indication (Duration) |
Venlafaxine Hydrochloride |
Placebo |
|
MDD (12 weeks) |
+1.5 |
-7.4 |
|
GAD (8 weeks) (6 months) |
+1.0 +2.3 |
-4.9 -7.7 |
|
SAD (12 weeks) (6 months) |
+7.9 +5.6 |
-2.9 -4.2 |
|
PD (12 weeks) |
5.8 |
-3.7 |
Venlafaxine hydrochloride extended-release capsule treatment for up to 12 weeks in premarketing placebo-controlled trials for major depressive disorder was associated with a mean final on-therapy increase in serum cholesterol concentration of approximately 1.5 mg/dL compared with a mean final decrease of 7.4 mg/dL for placebo. Venlafaxine hydrochloride extended-release capsule treatment for up to 8 weeks and up to 6 months in premarketing placebo-controlled GAD trials was associated with mean final on-therapy increases in serum cholesterol concentration of approximately 1.0 mg/dL and 2.3 mg/dL, respectively while placebo subjects experienced mean final decreases of 4.9 mg/dL and 7.7 mg/dL, respectively. Venlafaxine hydrochloride extended-release capsule treatment for up to 12 weeks and up to 6 months in premarketing placebo-controlled Social Anxiety Disorder trials was associated with mean final on-therapy increases in serum cholesterol concentration of approximately 7.9 mg/dL and 5.6 mg/dL, respectively, compared with mean final decreases of 2.9 mg/dL and 4.2 mg/dL, respectively, for placebo. Venlafaxine hydrochloride extended-release capsule treatment for up to 12 weeks in premarketing placebo-controlled panic disorder trials was associated with mean final on-therapy increases in serum cholesterol concentration of approximately 5.8 mg/dL compared with a mean final decrease of 3.7 mg/dL for placebo.
Patients treated with venlafaxine tablets (immediate release) for at least 3 months in placebo-controlled 12-month extension trials had a mean final on-therapy increase in total cholesterol of 9.1 mg/dL compared with a decrease of 7.1 mg/dL among placebo-treated patients. This increase was duration dependent over the study period and tended to be greater with higher doses. Clinically relevant increases in serum cholesterol, defined as 1) a final on-therapy increase in serum cholesterol ≥ 50 mg/dL from baseline and to a value ≥ 261 mg/dL, or 2) an average on-therapy increase in serum cholesterol ≥ 50 mg/dL from baseline and to a value ≥ 261 mg/dL, were recorded in 5.3% of venlafaxine-treated patients and 0.0% of placebo-treated patients.
Serum Triglycerides
Venlafaxine hydrochloride extended-release capsules were associated with mean final on-therapy increases in fasting serum triglycerides compared with placebo in premarketing clinical studies of SAD and PD up to 12 weeks (pooled data) and 6 months duration (Table 14).
|
Indication (Duration) |
Venlafaxine Hydrochloride Extended-Release Capsules |
Placebo |
|
SAD (12 weeks) |
8.2 |
0.4 |
|
SAD (6 months) |
11.8 |
1.8 |
|
PD (12 weeks) |
5.9 |
0.9 |
|
PD (6 months) |
9.3 |
0.3 |
6.2 Postmarketing Experience
The following adverse reactions have been identified during post-approval use of venlafaxine hydrochloride extended-release capsules. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Body as a whole – Anaphylaxis, angioedema
Cardiovascular system – QT prolongation, ventricular fibrillation, ventricular tachycardia (including torsade de pointes), takotsubo cardiomyopathy
Digestive system – Pancreatitis
Hemic/Lymphatic system – Mucous membrane bleeding [see Warnings and Precautions (5.4)], blood dyscrasias (including agranulocytosis, aplastic anemia, neutropenia and pancytopenia), prolonged bleeding time, thrombocytopenia
Metabolic/Nutritional – Hyponatremia [see Warnings and Precautions (5.9)], Syndrome of Inappropriate Antidiuretic Hormone (SIADH) secretion [see Warnings and Precautions (5.9)], abnormal liver function tests, hepatitis, prolactin increased
Musculoskeletal – Rhabdomyolysis
Nervous system – Neuroleptic Malignant Syndrome (NMS) [see Warnings and Precautions (5.2)], serotonergic syndrome [see Warnings and Precautions (5.2)], delirium, extrapyramidal reactions (including dystonia and dyskinesia), impaired coordination and balance, tardive dyskinesia
Respiratory system – Dyspnea, interstitial lung disease, pulmonary eosinophilia [see Warnings and Precautions (5.12)]
Skin and appendages – Stevens-Johnson syndrome, toxic epidermal necrolysis, erythema multiforme
Special senses – Angle closure glaucoma [see Warnings and Precautions (5.5)]
7 DRUG INTERACTIONS
7.1 Drugs Having Clinically Important Interactions with Venlafaxine Hydrochloride Extended-Release Capsules
|
Monoamine Oxidase Inhibitors (MAOI) |
|
|
Clinical Impact |
The concomitant use of SNRIs, including venlafaxine hydrochloride extended-release capsules, with MAOIs increases the risk of serotonin syndrome. |
|
Intervention |
Concomitant use of venlafaxine hydrochloride extended-release capsules are contraindicated in patients taking MAOIs, including MAOIs such as linezolid or intravenous methylene blue [see Dosage and Administration (2.11), Contraindications (4) and Warnings and Precautions (5.2)] |
|
Other Serotonergic Drugs |
|
|
Clinical Impact |
Concomitant use of venlafaxine hydrochloride extended-release capsules with other serotonergic drugs increases the risk of serotonin syndrome. |
|
Intervention |
Monitor for symptoms of serotonin syndrome when venlafaxine hydrochloride extended-release capsules are used concomitantly with other drugs that may affect the serotonergic neurotransmitter systems. If serotonin syndrome occurs, consider discontinuation of venlafaxine hydrochloride extended-release capsules and/or concomitant serotonergic drugs [see Dosage and Administration (2.11) and Warnings and Precautions (5.2)]. |
|
Drugs that Interfere with Hemostasis |
|
|
Clinical Impact |
Concomitant use of venlafaxine hydrochloride extended-release capsules with an antiplatelet or anticoagulant drug may potentiate the risk of bleeding. This may be due to the effect of venlafaxine hydrochloride extended-release capsules on the release of serotonin by platelets. |
|
Intervention |
Closely monitor for bleeding for patients receiving an antiplatelet or anticoagulant drug when venlafaxine hydrochloride extended-release capsules are initiated or discontinued [see Warnings and Precautions (5.4)]. |
|
Effect of CYP3A Inhibitors |
|
|
Clinical Impact |
Concomitant use of a CYP3A inhibitor increases the Cmax and AUC of venlafaxine and O-desmethylvenlafaxine (ODV) [see Clinical Pharmacology (12.3)], which may increase the risk of toxicity of venlafaxine hydrochloride extended-release capsules |
|
Intervention |
Consider reducing the dose of venlafaxine hydrochloride extended-release capsules. |
|
CYP2D6 Substrates |
|
|
Clinical Impact |
Concomitant use of venlafaxine hydrochloride extended-release capsules increases Cmax and AUC of a CYP2D6 substrate, which may increase the risk of toxicity of the CYP2D6 substrate [see Clinical Pharmacology (12.3)]. |
|
Intervention |
Consider reduction in dose of concomitant CYP2D6 substrates. |
7.2 Other Drug Interactions with Venlafaxine Hydrochloride Extended-Release Capsules
Central Nervous System (CNS)-Active Drugs
The risk of using venlafaxine concomitantly with other CNS-active drugs (including alcohol) has not been systematically evaluated. Consequently, caution is advised when venlafaxine hydrochloride extended-release capsules are taken concomitantly in combination with other CNS-active drugs.
Weight Loss Agents
Concomitant use of venlafaxine hydrochloride extended-release capsules and weight loss agents is not recommended. The safety and efficacy of venlafaxine therapy in combination with weight loss agents, including phentermine, have not been established. Venlafaxine hydrochloride extended-release capsules are not indicated for weight loss alone or in combination with other products.
Laboratory Test Interference
False-positive urine immunoassay screening tests for phencyclidine (PCP) and amphetamine have been reported in patients taking venlafaxine due to lack of specificity of the screening tests. False positive test results may be expected for several days following discontinuation of venlafaxine therapy. Confirmatory tests, such as gas chromatography/mass spectrometry, will distinguish venlafaxine from PCP and amphetamine.
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Pregnancy Exposure Registry
There is a pregnancy exposure registry that monitors pregnancy outcomes in women exposed to antidepressants, including venlafaxine hydrochloride extended-release capsules, during pregnancy. Healthcare providers are encouraged to register patients by calling the National Pregnancy Registry for Antidepressants at 1-844-405-6185 or visiting online at https://womensmentalhealth.org/clinical-and-research-programs/pregnancyregistry/antidepressants/.
Risk Summary
Available data from published epidemiologic studies on venlafaxine use in pregnant women have not identified a drug-associated risk of major birth defects, miscarriage or adverse fetal outcomes (see Data). Available data from observational studies with venlafaxine have identified a potential increased risk for preeclampsia when used during mid to late pregnancy; exposure to SNRIs near delivery may increase the risk for postpartum hemorrhage (see Clinical Considerations). There are risks associated with untreated depression in pregnancy and poor neonatal adaptation in newborns with exposure to SNRIs, including venlafaxine hydrochloride extended-release capsules, during pregnancy (see Clinical Considerations).
In animal studies, there was no evidence of malformations or fetotoxicity following administration of venlafaxine during organogenesis at doses up to 2.5 times (rat) or 4 times (rabbit) the maximum recommended human daily dose on a mg/m2 basis. Postnatal mortality and decreased pup weights were observed following venlafaxine administration to pregnant rats during gestation and lactation at 2.5 times (mg/m2) the maximum human daily dose.
The estimated background risk of major birth defects and miscarriage for the indicated populations is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively.
Clinical Considerations
Disease-Associated Maternal and/or Embryo/Fetal Risk
Women who discontinue antidepressants during pregnancy are more likely to experience a relapse of major depression than women who continue antidepressants. This finding is from a prospective, longitudinal study that followed 201 pregnant women with a history of major depression who were euthymic and taking antidepressants at the beginning of pregnancy. Consider the risk of untreated depression when discontinuing or changing treatment with antidepressant medication during pregnancy and postpartum.
Maternal Adverse Reactions
Exposure to venlafaxine in mid to late pregnancy may increase the risk for preeclampsia, and exposure to SNRIs near delivery may increase the risk for postpartum hemorrhage.
Fetal/Neonatal Adverse Reactions
Neonates exposed to SNRIs late in the third trimester have developed complications requiring prolonged hospitalization, respiratory support, and tube feeding. Such complications can arise immediately upon delivery. Reported clinical findings have included respiratory distress, cyanosis, apnea, seizures, temperature instability, feeding difficulty, vomiting, hypoglycemia, hypotonia, hypertonia, hyperreflexia, tremors, jitteriness, irritability, and constant crying. These findings are consistent with either a direct toxic effect of SNRIs or possibly a drug discontinuation syndrome. It should be noted that, in some cases, the clinical picture is consistent with serotonin syndrome [see Warnings and Precautions (5.2)]. Monitor neonates who were exposed to venlafaxine hydrochloride extended-release capsules in the third trimester of pregnancy for drug discontinuation syndrome (see Data).
Data
Human Data
Published epidemiological studies of pregnant women exposed to venlafaxine have not established an increased risk of major birth defects, miscarriage or other adverse developmental outcomes. Methodological limitations may both fail to identify true findings and also identify findings that are not true.
Retrospective cohort studies based on claims data have shown an association between venlafaxine use and preeclampsia, compared to depressed women who did not take an antidepressant during pregnancy. One study that assessed venlafaxine exposure in the second trimester or first half of the third trimester and preeclampsia showed an increased risk compared to unexposed depressed women (adjusted [adj] RR 1.57, 95% confidence interval [CI] 1.29 to 1.91). Preeclampsia was observed at venlafaxine doses equal to or greater than 75 mg per day and a duration of treatment >30 days. Another study that assessed venlafaxine exposure in gestational weeks 10 to 20 and preeclampsia showed an increased risk at doses equal to or greater than 150 mg per day. Available data are limited by possible outcome misclassification and possible confounding due to depression severity and other confounders.
Retrospective cohort studies based on claims data have suggested an association between venlafaxine use near the time of delivery or through delivery and postpartum hemorrhage. One study showed an increased risk for postpartum hemorrhage when venlafaxine exposure occurred through delivery, compared to unexposed depressed women (adj RR 2.24 [95% CI 1.69 to 2.97]). There was no increased risk in women who were exposed to venlafaxine earlier in pregnancy. Limitations of this study include possible confounding due to depression severity and other confounders. Another study showed an increased risk for postpartum hemorrhage when SNRI exposure occurred for at least 15 days in the last month of pregnancy or through delivery, compared to unexposed women (adj RR 1.64 to 1.76). The results of this study may be confounded by the effects of depression.
Animal Data
Venlafaxine did not cause malformations in offspring of rats or rabbits given doses up to 2.5 times (rat) or 4 times (rabbit) the maximum recommended human daily dose on a mg/m2 basis. However, in rats, there was a decrease in pup weight, an increase in stillborn pups, and an increase in pup deaths during the first 5 days of lactation, when dosing began during pregnancy and continued until weaning. The cause of these deaths is not known. These effects occurred at 2.5 times (mg/m2) the maximum human daily dose. The no effect dose for rat pup mortality was 0.25 times the human dose on a mg/m2 basis.
When desvenlafaxine succinate, the major metabolite of venlafaxine, was administered orally to pregnant rats and rabbits during the period of organogenesis at doses up to 300 mg/kg/day and 75 mg/kg/day, respectively, no fetal malformations were observed. These doses were associated with a plasma exposure (AUC) 19 times (rats) and 0.5 times (rabbits) the AUC exposure at an adult human dose of 100 mg per day. However, fetal weights were decreased and skeletal ossification was delayed in rats in association with maternal toxicity at the highest dose, with an AUC exposure at the no-effect dose that is 4.5-times the AUC exposure at an adult human dose of 100 mg per day.
8.2 Lactation
Risk Summary
Data from published literature report the presence of venlafaxine and its active metabolite in human milk and have not shown adverse reactions in breastfed infants (see Data). There are no data on the effects of venlafaxine on milk production.
The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for venlafaxine hydrochloride extended-release capsules and any potential adverse effects on the breastfed child from venlafaxine hydrochloride extended-release capsules or from the underlying maternal condition.
Data
In a lactation study conducted in 11 breastfeeding women (at a mean of 20.1 months post-partum) who were taking a mean daily dose of 194.3 mg of venlafaxine and in a lactation study conducted in 6 breastfeeding women who were taking a daily dose of 225 mg to 300 mg of venlafaxine (at a mean of 7 months post-partum), the estimated mean relative infant dose was 8.1 % and 6.4% based on the sum of venlafaxine and its major metabolite, desvenlafaxine. No adverse reactions were seen in the infants.
8.4 Pediatric Use
Safety and effectiveness of venlafaxine hydrochloride extended-release capsules in pediatric patients have not been established.
Two placebo-controlled trials in 766 pediatric patients with MDD and two placebo-controlled trials in 793 pediatric patients with GAD have been conducted with venlafaxine hydrochloride extended-release capsules, and the data were not sufficient to support use in pediatric patients.
In the studies conducted in pediatric patients ages 6 to17 years, the occurrence of blood pressure and cholesterol increases was considered to be clinically relevant in pediatric patients and was similar to that observed in adult patients [see Warnings and Precautions (5.3), Adverse Reactions (6.1)]. The following adverse reactions were also observed in pediatric patients: abdominal pain, agitation, dyspepsia, ecchymosis, epistaxis, and myalgia.
Although no studies have been designed to primarily assess venlafaxine hydrochloride extended-release capsules impact on the growth, development, and maturation of children and adolescents, the studies that have been done suggest that venlafaxine hydrochloride extended-release capsules may adversely affect weight and height [see Warnings and Precautions (5.10, 5.11)]. Decreased appetite and weight loss were observed in placebo-controlled studies of pediatric patients 6 to 17 years.
In pediatric clinical studies, the adverse reaction, suicidal ideation, was observed. Antidepressants increased the risk of suicidal thoughts and behaviors in pediatric patients [see Boxed Warning, Warnings and Precautions (5.1)].
8.5 Geriatric Use
The percentage of patients in clinical studies for venlafaxine hydrochloride extended-release capsules for MDD, GAD, SAD, and PD who were 65 years of age or older are shown in Table 16.
|
Indication |
Venlafaxine Hydrochloride Extended-Release Capsules |
|
MDD |
4 (14/357) |
|
GAD |
6 (77/1,381) |
|
SAD |
1 (10/819) |
|
PD |
2 (16/1,001) |
|
a In addition, in the premarketing assessment of venlafaxine tablets (immediate release), 12% (357/2,897) of patients were ≥ 65 years of age. |
|
No overall differences in effectiveness or safety were observed between geriatric patients and younger patients, and other reported clinical experience generally has not identified differences in response between the elderly and younger patients. However, greater sensitivity of some older individuals cannot be ruled out. SSRIs and SNRIs, including venlafaxine hydrochloride extended-release capsules, have been associated with cases of clinically significant hyponatremia in elderly patients, who may be at greater risk for this adverse event [see Warnings and Precautions (5.9)].
The pharmacokinetics of venlafaxine and ODV are not substantially altered in the elderly [see Clinical Pharmacology (12.3)] (see Figure 1). No dose adjustment is recommended for the elderly on the basis of age alone, although other clinical circumstances, some of which may be more common in the elderly, such as renal or hepatic impairment, may warrant a dose reduction [see Dosage and Administration (2.8, 2.9)].
9 DRUG ABUSE AND DEPENDENCE
9.1 Controlled Substance
Venlafaxine hydrochloride extended-release capsules contain venlafaxine which is not a controlled substance.
9.2 Abuse
Abuse is the intentional, non-therapeutic use of a drug, even once, for its desirable psychological or physiological effects.
While venlafaxine has not been systematically studied in clinical studies for its potential for abuse, there was no indication of drug-seeking behavior in the clinical studies. However, it is not possible to predict on the basis of premarketing experience the extent to which a CNS-active drug will be misused, diverted, and/or abused once marketed. Consequently, providers should carefully evaluate patients for history of drug abuse and follow such patients closely, observing them for signs of misuse or abuse of venlafaxine (e.g., development of tolerance, incrementation of dose, drug-seeking behavior).
9.3 Dependence
Physical dependence is a state that develops as a result of physiological adaptation in response to repeated drug use, manifested by withdrawal signs and symptoms after abrupt discontinuation or a significant dose reduction of a drug.
In vitro studies revealed that venlafaxine has virtually no affinity for opiate, benzodiazepine, phencyclidine (PCP), or N-methyl-D-aspartic acid (NMDA) receptors.
Venlafaxine was not found to have any significant CNS stimulant activity in rodents. In primate drug discrimination studies, venlafaxine showed no significant stimulant or depressant abuse liability. Discontinuation effects have been reported in patients receiving venlafaxine [see Dosage and Administration (2.10) and Warnings and Precautions (5.7)].
10 OVERDOSAGE
Human Experience
During the premarketing evaluations of venlafaxine hydrochloride extended-release capsules (for MDD, GAD, SAD, and PD) and venlafaxine tablets (for MDD), there were twenty reports of acute overdosage with venlafaxine tablets (6 and 14 reports in venlafaxine hydrochloride extended-release capsule and venlafaxine tablet patients, respectively), either alone or in combination with other drugs and/or alcohol.
Somnolence was the most commonly reported symptom. Among the other reported symptoms were paresthesia of all four limbs, moderate dizziness, nausea, numb hands and feet, and hot-cold spells 5 days after the overdose. In most cases, no signs or symptoms were associated with overdose. The majority of the reports involved ingestion in which the total dose of venlafaxine taken was estimated to be no more than several-fold higher than the usual therapeutic dose. One patient who ingested 2.75 g of venlafaxine was observed to have two generalized convulsions and a prolongation of QTc to 500 msec, compared with 405 msec at baseline. Mild sinus tachycardia was reported in two of the other patients.
Actions taken to treat the overdose included no treatment, hospitalization and symptomatic treatment, and hospitalization plus treatment with activated charcoal. All patients recovered.
In postmarketing experience, overdose with venlafaxine has occurred predominantly in combination with alcohol and/or other drugs. The most commonly reported events in overdosage include tachycardia, changes in level of consciousness (ranging from somnolence to coma), mydriasis, seizures, and vomiting. Electrocardiogram changes (e.g., prolongation of QT interval, bundle branch block, QRS prolongation), ventricular tachycardia, bradycardia, hypotension, rhabdomyolysis, vertigo, liver necrosis, serotonin syndrome, and death have been reported.
Published retrospective studies report that venlafaxine overdosage may be associated with an increased risk of fatal outcomes compared to that observed with SSRI antidepressant products, but lower than that for tricyclic antidepressants. Epidemiological studies have shown that venlafaxine-treated patients have a higher preexisting burden of suicide risk factors than SSRI-treated patients. The extent to which the finding of an increased risk of fatal outcomes can be attributed to the toxicity of venlafaxine in overdosage, as opposed to some characteristic(s) of venlafaxine-treated patients, is not clear. Prescriptions for venlafaxine hydrochloride extended-release capsules should be written for the smallest quantity of capsules consistent with good patient management, in order to reduce the risk of overdose.
Management of Overdosage
No specific antidotes for venlafaxine hydrochloride extended-release capsules are known. In managing overdosage, consider the possibility of multiple drug involvement. Consider contacting a Poison Center (1-800-222-1222) or a medical toxicologist for overdosage management recommendations for venlafaxine hydrochloride extended-release capsules.
11 DESCRIPTION
Venlafaxine hydrochloride extended-release capsules, USP are an extended-release capsule for once-a-day oral administration that contains venlafaxine hydrochloride, USP a serotonin and norepinephrine reuptake inhibitor (SNRI).
Venlafaxine is designated (R/S)-1-[2-(dimethylamino)-1-(4-methoxyphenyl)ethyl] cyclohexanol hydrochloride or (±)-1-[α- [(dimethylamino)methyl]-p-methoxybenzyl] cyclohexanol hydrochloride and has the molecular formula of C17H27NO2 HCl. Its molecular weight is 313.86. The structural formula is shown as follows:

Venlafaxine hydrochloride, USP is a white to off-white crystalline solid, with a solubility of 572 mg/mL in water (adjusted to ionic strength of 0.2 M with sodium chloride). Its octanol:water (0.2 M sodium chloride) partition coefficient is 0.43.
Drug release is controlled by diffusion through the coating membrane on the spheroids and is not pH-dependent. Capsules contain venlafaxine hydrochloride, USP equivalent to 37.5 mg, 75 mg, or 150 mg venlafaxine. Inactive ingredients consist of black iron oxide, dibutyl sebacate, ethylcellulose, gelatin, polyethylene glycol, povidone, propylene glycol, shellac, sugar spheres (which contain sucrose and corn starch), FD&C Yellow No. 6, Sunset Yellow FCF, talc, and titanium dioxide. The 37.5 mg capsules also contain D&C Yellow No. 10 and potassium hydroxide, the 75 mg capsules also contain D&C Yellow No. 10 and may contain potassium hydroxide, and the 150 mg capsules also contain potassium hydroxide.
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
The mechanism of action of venlafaxine in the treatment of MDD, GAD, SAD, and PD is unclear, but is thought to be related to the potentiation of serotonin and norepinephrine in the central nervous system, through inhibition of their reuptake.
12.2 Pharmacodynamics
In-vitro studies have demonstrated that venlafaxine and its active metabolite, O-desmethylvenlafaxine (ODV), are potent and selective inhibitors of neuronal serotonin and norepinephrine reuptake and weak inhibitors of dopamine reuptake. Venlafaxine and ODV have no significant affinity for muscarinic-cholinergic, H1-histaminergic, or α1-adrenergic receptors in vitro. Pharmacologic activity at these receptors is hypothesized to be associated with the various anticholinergic, sedative, and cardiovascular effects seen with other psychotropic drugs. Venlafaxine and ODV do not possess monoamine oxidase (MAO) inhibitory activity.
Cardiac Electrophysiology
The effect of venlafaxine on the QT interval was evaluated in a randomized, double-blind, placebo- and positive-controlled three-period crossover thorough QT study in 54 healthy adult subjects. No significant QT prolongation effect of venlafaxine at 450 mg (2 times the maximum recommended dosage) was detected.
12.3 Pharmacokinetics
Venlafaxine and ODV steady-state concentrations are reached within 3 days. Venlafaxine and ODV exhibited linear kinetics over the dosage range of 75 mg to 450 mg per day (0.33 to 2 times the maximum recommended dosage). Time of administration (AM versus PM) did not affect the pharmacokinetics of venlafaxine and ODV from the 75 mg venlafaxine hydrochloride extended-release capsules.
Absorption
Venlafaxine is well absorbed. On the basis of mass balance studies, at least 92% of a single oral dose of venlafaxine is absorbed. The absolute bioavailability of venlafaxine is approximately 45%.
Administration of venlafaxine hydrochloride extended-release capsules (150 mg once daily) generally resulted in lower Cmax and later Tmax values than for venlafaxine tablets (immediate release) administered twice daily (Table 17). When equal daily doses of venlafaxine were administered as either an immediate-release tablet or the extended-release capsule, the exposure to both venlafaxine and ODV was similar for the two treatments, and the fluctuation in plasma concentrations was slightly lower with the venlafaxine hydrochloride extended-release capsules. Therefore, venlafaxine hydrochloride extended-release capsules provides a slower rate of absorption, but the same extent of absorption compared with the immediate-release tablet.
|
Venlafaxine |
ODV |
|||
|
Cmax (ng/mL) |
Tmax (h) |
Cmax (ng/mL) |
Tmax (h) |
|
|
Venlafaxine Hydrochloride Extended-Release Capsules (150 mg once daily) |
150 |
5.5 |
260 |
9 |
|
Venlafaxine Tablets (75 mg twice daily) |
225 |
2 |
290 |
3 |
Effect of Food
Food did not affect the bioavailability of venlafaxine or its active metabolite, ODV.
Distribution
Venlafaxine is 27% and ODV is 30% bound to plasma proteins. The apparent volume of distribution at steady-state is 7.5 ± 3.7 L/kg for venlafaxine and 5.7 ± 1.8 L/kg for ODV.
Elimination
Mean ± SD plasma apparent clearance at steady-state is 1.3 ± 0.6 L/h/kg for venlafaxine and 0.4 ± 0.2 L/h/kg for ODV. The apparent elimination half-life is 5 ± 2 hours for venlafaxine and 11 ± 2 hours for ODV.
Metabolism
Following absorption, venlafaxine undergoes extensive presystemic metabolism in the liver, primarily to ODV, but also to N-desmethylvenlafaxine, N,O-didesmethylvenlafaxine, and other minor metabolites. In vitro studies indicate that the formation of ODV is catalyzed by CYP2D6; this has been confirmed in a clinical study showing that patients with low CYP2D6 levels (poor metabolizers) had increased levels of venlafaxine and reduced levels of ODV compared to people with normal CYP2D6 levels (extensive metabolizers) (see Figure 1).
Excretion
Approximately 87% of a venlafaxine dose is recovered in the urine within 48 hours as unchanged venlafaxine (5%), unconjugated ODV (29%), conjugated ODV (26%), or other minor inactive metabolites (27%).
Specific Populations
The effect of intrinsic patient factors on the pharmacokinetics of venlafaxine and its active metabolite ODV is presented in Figure 1.
Figure 1: Pharmacokinetics of Venlafaxine and Active Metabolite O-desmethylvenlafaxine (ODV) in Special Populations

ODV=O-desmethylvenlafaxine; AUC=area under the curve; Cmax=peak plasma concentrations.
* Similar effect is expected with strong CYP2D6 inhibitors
Drug Interaction Studies
Clinical Studies
Effect of Other Drugs On Venlafaxine Hydrochloride Extended-Release Capsules and Active Metabolite ODV
The effects of other drugs on the exposure of venlafaxine and ODV are summarized in Figure 2.
Figure 2: Effect of Other Drugs on the Pharmacokinetics of Venlafaxine and Active Metabolite O-desmethylvenlafaxine (ODV)
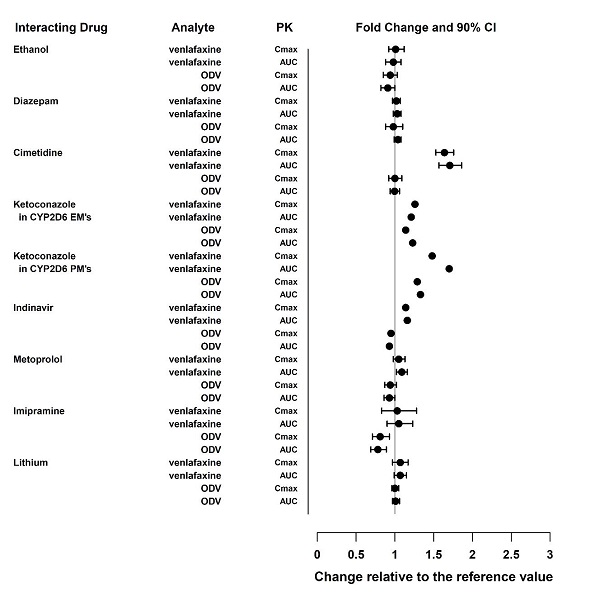
ODV=O-desmethylvenlafaxine; AUC=area under the curve; Cmax=peak plasma concentrations; EM’s=extensive metabolizers; PM’s=poor metabolizers.
Effect of Venlafaxine Hydrochloride Extended-Release Capsules on Other Drugs
The effects of venlafaxine hydrochloride extended-release capsules on the exposure of other drugs are summarized in Figure 3.
Figure 3: Effect of Venlafaxine on the Pharmacokinetics Interacting Drugs and their Active Metabolites

AUC=area under the curve; Cmax=peak plasma concentrations; OH=hydroxyl.
* Data for 2-OH desipramine were not plotted to enhance clarity; the fold change and 90% CI for Cmax and AUC of 2-OH desipramine were 6.6 (5.5, 7.9) and 4.4 (3.8, 5.0), respectively.
Note:*Administration of venlafaxine in a stable regimen did not exaggerate the psychomotor and psychometric effects induced by ethanol in these same subjects when they were not receiving venlafaxine.
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis
Tumors were not increased by venlafaxine treatment in mice or rats. Venlafaxine was given by oral gavage to mice for 18 months at doses up to 120 mg/kg per day, which was 1.7 times the maximum recommended human dose on a mg/m2 basis. Venlafaxine was also given to rats by oral gavage for 24 months at doses up to 120 mg/kg per day. In rats receiving the 120 mg/kg dose, plasma concentrations of venlafaxine at necropsy were 1 times (male rats) and 6 times (female rats) the plasma concentrations of patients receiving the maximum recommended human dose. Plasma levels of the ODV were lower in rats than in patients receiving the maximum recommended dose. ODV, the major human metabolite of venlafaxine, administered by oral gavage to mice and rats for 2 years did not increase the incidence of tumors in either study. Mice received ODV at dosages up to 500/300 mg/kg/day (dosage lowered after 45 weeks of dosing). The exposure at the 300 mg/kg/day dose is 9 times that of a human dose of 225 mg/day. Rats received ODV at dosages up to 300 mg/kg/day (males) or 500 mg/kg/day (females). The exposure at the highest dose is approximately 8 (males) or 11 (females) times that of a human dose of 225 mg/day.
Mutagenesis
Venlafaxine and the major human metabolite, ODV, were not mutagenic in the Ames reverse mutation assay in Salmonella bacteria or the Chinese hamster ovary/HGPRT mammalian cell forward gene mutation assay. Venlafaxine was also not mutagenic or clastogenic in the in vitro BALB/c-3T3 mouse cell transformation assay, the sister chromatid exchange assay in cultured Chinese hamster ovary cells, or in the in vivo chromosomal aberration assay in rat bone marrow. ODV was not clastogenic in the in vitro Chinese hamster ovary cell chromosomal aberration assay or in the in vivo chromosomal aberration assay in rats.
Impairment of Fertility
Reproduction and fertility studies of venlafaxine in rats showed no adverse effects of venlafaxine on male or female fertility at oral doses of up to 2 times the maximum recommended human dose of 225 mg/day on a mg/m2 basis. However, when desvenlafaxine succinate, the major human metabolite of venlafaxine, was administered orally to male and female rats, fertility was reduced at the high dose of 300 mg/kg/day, which is 10 (males) and 19 (females) times the AUC exposure at an adult human dose of 100 mg per day. There was no effect on fertility at 100 mg/kg/day, which is 3 (males) or 5 (females) times the AUC exposure at an adult human dose of 100 mg per day. These studies did not address reversibility of the effect on fertility. The relevance of these findings to humans is not known.
14 CLINICAL STUDIES
14.1 Major Depressive Disorder
The efficacy of venlafaxine hydrochloride extended-release capsules as a treatment for Major Depressive Disorder (MDD) was established in two placebo-controlled, short-term (8 weeks for study 1; 12 weeks for study 2), flexible-dose studies, with doses starting at 75 mg per day and ranging to 225 mg per day in adult outpatients meeting DSM-III-R or DSM-IV criteria for MDD. In moderately depressed outpatients, the initial dose of venlafaxine was 75 mg per day. In both studies, venlafaxine hydrochloride extended-release capsules demonstrated superiority over placebo on the primary efficacy measure defined as change from baseline in the HAM-D-21 total score to the endpoint visit, venlafaxine hydrochloride extended-release capsules also demonstrated superiority over placebo on the key secondary efficacy endpoint, the Clinical Global Impressions (CGI) Severity of Illness scale. Examination of gender subsets of the population studied did not reveal any differential responsiveness on the basis of gender.
A 4-week study of inpatients meeting DSM-III-R criteria for MDD with melancholia utilizing venlafaxine tablets in a range of 150 mg to 375 mg per day (divided in a three-times-a-day schedule) demonstrated superiority of venlafaxine hydrochloride tablets over placebo based on the HAM-D-21 total score. The mean dose in completers was 350 mg per day (study 3).
In a longer-term study, adult outpatients with MDD who had responded during an 8-week open-label study on venlafaxine hydrochloride extended-release capsules (75 mg, 150 mg, or 225 mg, once daily every morning) were randomized to continuation of their same venlafaxine hydrochloride extended-release capsule dose or to placebo, for up to 26 weeks of observation for relapse. Response during the open-label phase was defined as a CGI Severity of Illness item score of ≤ 3 and a HAM-D-21 total score of ≤ 10 at the day 56 evaluation. Relapse during the double-blind phase was defined as follows: (1) a reappearance of major depressive disorder as defined by DSM-IV criteria and a CGI Severity of Illness item score of ≥ 4 (moderately ill), (2) 2 consecutive CGI Severity of Illness item scores of ≥ 4, or (3) a final CGI Severity of Illness item score of ≥ 4 for any patient who withdrew from the study for any reason. Patients receiving continued venlafaxine hydrochloride extended-release capsule treatment experienced statistically significantly lower relapse rates over the subsequent 26 weeks compared with those receiving placebo (study 4).
In a second longer term trial, adult outpatients with MDD, recurrent type, who had responded (HAM-D‑21 total score ≤ 12 at the day 56 evaluation) and continued to be improved [defined as the following criteria being met for days 56 through 180: (1) no HAM-D-21 total score ≥ 20; (2) no more than 2 HAM‑D-21 total scores > 10, and (3) no single CGI Severity of Illness item score ≥ 4 (moderately ill)] during an initial 26 weeks of treatment on venlafaxine tablets [100 mg to 200 mg per day, on a twice daily schedule] were randomized to continuation of their same venlafaxine tablet dose or to placebo. The follow-up period to observe patients for relapse, defined as a CGI Severity of Illness item score ≥ 4, was for up to 52 weeks. Patients receiving continued venlafaxine tablet treatment experienced statistically significantly lower relapse rates over the subsequent 52 weeks compared with those receiving placebo (study 5).
|
Study Number |
Treatment Group |
Primary Efficacy Measure: HAM-D Score |
||
|
Mean Baseline Score (SD) |
LS Mean Change from Baseline |
Placebo Subtracted Differencea (95% CI) |
||
|
Study 1 |
Venlafaxine Hydrochloride Extended-Release Capsules(75 to 225 mg/day)* |
24.5 |
-11.7 |
-4.45 (-6.66, -2.25) |
| Placebo | 23.6 | -7.24 | - | |
|
Study 2 |
Venlafaxine Hydrochloride Extended-Release Capsules (75 to 225 mg/day)* |
24.5 |
-15.11 |
-6.40 (-8.45, -4.34) |
|
Placebo |
24.9 |
-8.71 | ||
|
Study 3 |
Venlafaxine Immediate Release Tablets (150 to 375 mg/day)* |
28.2 (0.5) |
-14.9 |
-10.2 (-14.4, -6.0) |
| Placebo | 28.6 (0.6) | -4.7 | - | |
|
SD=standard deviation; LS Mean=least-squares mean; CI=confidence interval. a Difference (drug minus placebo) in least-squares mean change from baseline * Doses statistically significantly superior to placebo. |
||||
14.2 Generalized Anxiety Disorder
The efficacy of venlafaxine hydrochloride extended-release capsules as a treatment for Generalized Anxiety Disorder (GAD) was established in two 8-week, placebo-controlled, fixed-dose studies (75 mg to 225 mg per day), one 6-month, placebo-controlled, flexible-dose study (75 mg to 225 mg per day), and one 6-month, placebo-controlled, fixed-dose study (37.5 mg, 75 mg, and 150 mg per day) in adult outpatients meeting DSM-IV criteria for GAD.
In one 8-week study, venlafaxine hydrochloride extended-release capsules demonstrated superiority over placebo for the 75 mg, 150 mg, and 225 mg per day doses as measured by the Hamilton Rating Scale for Anxiety (HAM-A) total score, both the HAM-A anxiety and tension items, and the Clinical Global Impressions (CGI) scale. However, the 75 mg and 150 mg per day doses were not as consistently effective as the highest dose (study 1). A second 8-week study evaluating doses of 75 mg and 150 mg per day and placebo showed that both doses were more effective than placebo on some of these same outcomes; however, the 75 mg per day dose was more consistently effective than the 150 mg per day dose (study 2). A dose-response relationship for effectiveness in GAD was not clearly established in the 75 mg to 225 mg per day dose range studied.
Two 6-month studies, one evaluating venlafaxine hydrochloride extended-release capsule doses of 37.5 mg, 75 mg, and 150 mg per day (study 3) and the other evaluating venlafaxine hydrochloride extended-release capsules doses of 75 mg to 225 mg per day (study 4), showed that daily doses of 75 mg or higher were more effective than placebo on the HAM-A total, both the HAM-A anxiety and tension items, and the CGI scale during 6 months of treatment. While there was also evidence for superiority over placebo for the 37.5 mg per day dose, this dose was not as consistently effective as the higher doses.
Examination of gender subsets of the population studied did not reveal any differential responsiveness on the basis of gender.
|
Study Number |
Treatment Group |
Primary Efficacy Measure: HAM-A Score |
||
|
Mean Baseline Score (SD) |
LS Mean Change from Baseline (SE)* |
Placebo Subtracted Differencea (95% CI) |
||
|
Study 1 |
Venlafaxine HCl Extended-Release Capsules 75 mg |
24.7 |
-11.1 (0.95) |
-1.5 (-3.8, 0.8) |
|
Venlafaxine HCl Extended-Release Capsules 150 mg |
24.5 |
-11.7 (0.87) |
-2.2 (-4.5, 0.1) |
|
|
Venlafaxine HCl Extended-Release Capsules 225 mg |
23.6 |
-12.1 (0.81) |
-2.6 (-4.9, -0.3) |
|
|
Placebo |
24.1 |
-9.5 (0.85) | ||
|
Study 2 |
Venlafaxine HCl Extended-Release Capsules 75 mg |
23.7 |
-10.6 (0.82) |
-2.6 (-4.6, -0.5) |
|
Venlafaxine HCl Extended-Release Capsules 150 mg |
23.0 |
-9.8 (0.86) |
-1.7 (-3.8, 0.3) |
|
|
Placebo |
23.7 |
-8.0 (0.73) | ||
|
Study 3 |
Venlafaxine HCl Extended-Release Capsules 37.5 mg |
26.6 (0.4) |
-13.8 |
-2.8 (-5.1, -0.6) |
|
Venlafaxine HCl Extended-Release Capsules 75 mg |
26.3 (0.4) |
-15.5 |
-4.6 (-6.9, -2.3) |
|
|
Venlafaxine HCl Extended-Release Capsules 150 mg |
26.3 (0.4) |
-16.4 |
-5.5 (-7.8, -3.1) |
|
|
Placebo |
26.7 (0.5) |
-11.0 | ||
|
Study 4 |
Venlafaxine HCl Extended-Release Capsules 75 to 225 mg |
25.0 |
-13.4 (0.79) |
-4.7 (-6.6, -2.9) |
|
Placebo |
24.9 |
-8.7 (0.70) | ||
|
SD=standard deviation; SE=standard error; LS Mean=least-squares mean; CI=confidence interval. a Difference (drug minus placebo) in least-squares mean change from baseline * Doses statistically significantly superior to placebo. |
||||
14.3 Social Anxiety Disorder (also known as Social Phobia)
The efficacy of venlafaxine hydrochloride extended-release capsules as a treatment for Social Anxiety Disorder (SAD) was established in four double-blind, parallel-group, 12-week, multicenter, placebo-controlled, flexible-dose studies (studies 1 to 4) and one double-blind, parallel-group, 6-month, placebo-controlled, fixed/flexible-dose study, which included doses in a range of 75 mg to 225 mg per day in adult outpatients meeting DSM-IV criteria for SAD (study 5).
In these five studies, venlafaxine hydrochloride extended-release capsules were statistically significantly more effective than placebo on change from baseline to endpoint on the Liebowitz Social Anxiety Scale (LSAS) total score. There was no evidence for any greater effectiveness of the 150 mg to 225 mg per day group compared to the 75 mg per day group in the 6-month study.
Examination of subsets of the population studied did not reveal any differential responsiveness on the basis of gender. There was insufficient information to determine the effect of age or race on outcome in these studies.
|
Study Number |
Treatment Group |
Primary Efficacy Measure: LSAS Score |
||
|
Mean Baseline Score |
LS Mean Change from Baseline |
Placebo Subtracted Differencea (95% CI) |
||
|
Study 1 |
Venlafaxine HCl Extended-Release Capsules (75 to 225 mg)* |
91.1 |
-31.0 (2.22) |
11.2 (-5.3, -17.1) |
|
Placebo |
86.7 |
-19.9 (2.22) |
- |
|
|
Study 2 |
Venlafaxine HCl Extended-Release Capsules (75 to 225 mg)* |
90.8 |
-32.8 (2.69) |
-10.7 (-3.7, -17.6) |
|
Placebo |
87.4 |
-22.1 (2.66) |
- |
|
|
Study 3 |
Venlafaxine HCl Extended-Release Capsules (75 to 225 mg)* |
83.2 |
-36.0 (2.35) |
-16.9 (-22.6, -11.2) |
|
Placebo |
83.6 |
-19.1 (2.40) |
-12.7 (-6.5, -19.0) |
|
|
Study 4 |
Venlafaxine HCl Extended-Release Capsules (75 to 225 mg)* |
86.2 |
-35.0 (2.64) |
-14.6 (-21.8, -7.4) |
|
Placebo |
86.1 |
-22.2 (2.47) | ||
|
Study 5 |
Venlafaxine HCl Extended-Release Capsules 75 mg |
91.8 |
-38.1 (3.16) |
-14.6 (-21.8, -7.4) |
|
Venlafaxine HCl Extended-Release Capsules (150 to 225 mg)* |
86.2 |
-37.6 (3.05) |
-14.1 (-21.3, -6.9) |
|
|
Placebo |
89.3 |
-23.5 (3.08) | ||
|
SD: standard deviation; SE: standard error; LS Mean: least-squares mean; CI: confidence interval. a Difference (drug minus placebo) in least-squares mean change from baseline * Doses statistically significantly superior to placebo. |
||||
14.4 Panic Disorder
The efficacy of venlafaxine hydrochloride extended-release capsules as a treatment for Panic Disorder (PD) was established in two double-blind, 12-week, multicenter, placebo-controlled studies in adult outpatients meeting DSM-IV criteria for PD, with or without agoraphobia. Patients received fixed doses of 75 mg or 150 mg per day in one study (study 1) and 75 mg or 225 mg per day in the other study (study 2).
Efficacy was assessed on the basis of outcomes in three variables: (1) percentage of patients free of full-symptom panic attacks on the Panic and Anticipatory Anxiety Scale (PAAS); (2) mean change from baseline to endpoint on the Panic Disorder Severity Scale (PDSS) total score; and (3) percentage of patients rated as responders (much improved or very much improved) on the Clinical Global Impressions (CGI) Improvement scale. In these two studies, venlafaxine hydrochloride extended-release capsules were statistically significantly more effective than placebo (for each fixed dose) on all three endpoints, but a dose-response relationship was not clearly established.
Examination of subsets of the population studied did not reveal any differential responsiveness on the basis of gender. There was insufficient information to determine the effect of age or race on outcome in these studies.
In a longer term study (study 3), adult outpatients meeting DSM-IV criteria for PD who had responded during a 12-week open phase with venlafaxine hydrochloride extended-release capsules (75 mg to 225 mg per day) were randomly assigned to continue the same venlafaxine hydrochloride extended-release capsules dose (75 mg, 150 mg, or 225 mg) or switch to placebo for observation for relapse under double-blind conditions. Response during the open phase was defined as ≤ 1 full-symptom panic attack per week during the last 2 weeks of the open phase and a CGI Improvement score of 1 (very much improved) or 2 (much improved). Relapse during the double-blind phase was defined as having 2 or more full-symptom panic attacks per week for 2 consecutive weeks or having discontinued due to loss of effectiveness as determined by the investigators during the study. Randomized patients were in response status for a mean time of 34 days prior to being randomized. In the randomized phase following the 12‑week open-label period, patients receiving continued venlafaxine hydrochloride extended-release capsules experienced a statistically significantly longer time to relapse.
|
Study Number |
Treatment Group |
Primary Efficacy Measure: Whether Free of Full-symptom Panic Attacks |
||
|
Percent of patients Free of Full symptom panic attack |
Adjusted Odds Ratioa to placebo |
Adjusted Odds Ratioa 95% Confidence Interval |
||
|
Study 1 |
Venlafaxine HCl Extended-Release Capsules 75 mg* Venlafaxine HCl Extended-Release Capsules 150 mg* |
54.1% (85/157) 61.4% (97/158) |
2.268 3.035 |
(1.43, 3.59) (1.91, 4.82) |
| Placebo | 34.4% (53/154) | -- | -- | |
|
Study 2 |
Venlafaxine HCl Extended-Release Capsules 75 mg* Venlafaxine HCl Extended-Release Capsules 225 mg* |
64.1% (100/156) 70.0% (112/160) |
2.350 2.890 |
(1.46, 3.78) (1.80, 4.64) |
| Placebo | 46.5% (73/157) | -- | -- | |
|
a Odds ratio (drug to placebo) in terms of probability of free of full-symptom panic attacks based on logistic regression model. 95% CI: 95% confidence interval without adjusting for multiple dose arms. * Doses statistically significantly superior to placebo. |
||||
16 HOW SUPPLIED/STORAGE AND HANDLING
Venlafaxine hydrochloride extended-release capsules, USP are available as follows:
37.5 mg - Light-gray opaque cap/buff opaque body with “93” and “7384” on both body and cap. Capsules are available in bottles of 30 (NDC 0093-7384-56), 90 (NDC 0093-7384-98), and 500 (NDC 0093-7384-05).
75 mg - Buff opaque cap/buff opaque body with “93” and “7385” on both body and cap. Capsules are available in bottles of 30 (NDC 0093-7385-56), 90 (NDC 0093-7385-98), and 500 (NDC 0093-7385-05).
150 mg - Light-orange opaque cap/light-orange opaque body with “93” and “7386” on both body and cap. Capsules are available in bottles of 30 (NDC 0093-7386-56), 90 (NDC 0093-7386-98), and 500 (NDC 0093-7386-05).
Store at 20° to 25°C (68° to 77°F) [see USP Controlled Room Temperature].
Dispense in a tight, light-resistant container as defined in the USP, with a child-resistant closure (as required).
Keep this and all medications out of the reach of children.
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Medication Guide).
Suicidal Thoughts and Behaviors
Advise patients and caregivers to look for the emergence of suicidality, especially early during treatment and when the dose is adjusted up or down, and instruct them to report such symptoms to the healthcare provider [see Boxed Warning and Warnings and Precautions (5.1)].
Concomitant Medication
Instruct patients not to take venlafaxine hydrochloride extended-release capsules with an MAOI or within 14 days of stopping an MAOI [see Contraindications (4)].
Serotonin Syndrome
Caution patients about the risk of serotonin syndrome, particularly with the concomitant use of venlafaxine hydrochloride extended-release capsules with other serotonergic drugs including triptans, tricyclic antidepressants, fentanyl, lithium, tramadol, tryptophan, buspirone, amphetamines, St. John’s Wort, and with drugs that impair metabolism of serotonin (in particular, MAOIs, both those intended to treat psychiatric disorders and also others, such as linezolid). Instruct patients to contact their healthcare provider or report to the emergency room if they experience signs or symptoms of serotonin syndrome [see Warnings and Precautions (5.2) and Drug Interactions (7.1)].
Elevated Blood Pressure
Advise patients that they should have regular monitoring of blood pressure when taking venlafaxine hydrochloride extended-release capsules [see Warnings and Precautions (5.3)].
Increased Risk of Bleeding
Inform patients about the concomitant use of venlafaxine hydrochloride extended-release capsules and NSAIDs, aspirin, other antiplatelet drugs, warfarin, or other drugs that affect coagulation because the combined use has been associated with an increased risk of bleeding. Advise patients to inform their health care providers if they are taking or planning to take any prescription or over-the-counter medications that increase the risk of bleeding [see Warnings and Precautions (5.4)].
Activation of Mania/Hypomania
Advise patients, their families and caregivers to observe for signs of activation of mania/hypomania and instruct them to report such symptoms to the healthcare provider [see Warnings and Precautions (5.6)].
Cardiovascular/Cerebrovascular Disease
Caution is advised in administering venlafaxine hydrochloride extended-release capsules to patients with cardiovascular, cerebrovascular, or lipid metabolism disorders [see Adverse Reactions (6.1)].
Serum Cholesterol and Triglyceride Elevation
Advise patients that elevations in total cholesterol, LDL and triglycerides may occur and that measurement of serum lipids may be considered [see Adverse Reactions (6.1)].
Discontinuation Syndrome
Advise patients not to abruptly stop taking venlafaxine hydrochloride extended-release capsules without talking first with their healthcare provider. Patients should be aware that discontinuation effects may occur when stopping venlafaxine hydrochloride extended-release capsules and they should monitor for discontinuation symptoms [see Warnings and Precautions (5.7) and Adverse Reactions (6.1)].
Sexual Dysfunction
Advise patients that use of venlafaxine hydrochloride extended-release capsules may cause symptoms of sexual dysfunction in both male and female patients. Inform patients that they should discuss any changes in sexual function and potential management strategies with their healthcare provider [see Warnings and Precautions (5.13)].
Interference with Cognitive and Motor Performance
Caution patients about operating hazardous machinery, including automobiles, until they are reasonably certain that venlafaxine hydrochloride extended-release capsules therapy does not adversely affect their ability to engage in such activities.
Alcohol
Advise patients to avoid alcohol while taking venlafaxine hydrochloride extended-release capsules [see Drug Interactions (7.2)].
Allergic Reactions
Advise patients to notify their healthcare provider if they develop allergic phenomena such as rash, hives, swelling, or difficulty breathing [see Contraindications (4) and Adverse Reactions (6.2)].
Pregnancy
Advise patients to notify their healthcare provider if they become pregnant or intend to become pregnant during treatment with venlafaxine hydrochloride extended-release capsules. Advise patients that venlafaxine hydrochloride extended-release capsule use during mid to late pregnancy may lead to an increased risk for preeclampsia; use in late pregnancy may lead to an increased risk for postpartum hemorrhage and may increase the risk for neonatal complications requiring prolonged hospitalization, respiratory support, and tube feeding. Advise patients that there is a pregnancy exposure registry that monitors pregnancy outcomes in women exposed to venlafaxine hydrochloride extended-release capsules during pregnancy [see Use in Specific Populations (8.1)].
Residual Spheroids
Venlafaxine hydrochloride extended-release capsules contain spheroids, which release the drug slowly into the digestive tract. The insoluble portion of these spheroids is eliminated, and patients may notice spheroids passing in the stool or via colostomy. Patients should be informed that the active medication has already been absorbed by the time the patient sees the spheroids.
Dispense with Medication Guide available at: www.tevausa.com/medguides
Manufactured In Israel By:
Teva Pharmaceutical Ind. Ltd.
Kfar Saba, 4410202, Israel
Manufactured For:
Teva Pharmaceuticals
Parsippany, NJ 07054
Rev. Q 9/2022
MEDICATION GUIDE
Dispense with Medication Guide available at: www.tevausa.com/medguides
|
MEDICATION GUIDE
Venlafaxine Hydrochloride (ven′′ la fax′ een hye′′ droe klor′ ide) |
||
| What is the most important information I should know about venlafaxine hydrochloride extended-release capsules? Venlafaxine hydrochloride extended-release capsules may cause serious side effects, including:
How can I watch for and try to prevent suicidal thoughts and actions in myself or a family member?
|
||
|
|
|
| What are venlafaxine hydrochloride extended-release capsules?
Venlafaxine hydrochloride extended-release capsules are a prescription medicine used to treat adults with
It is not known if venlafaxine hydrochloride extended-release capsules are safe and effective for use in children. |
||
Do not take venlafaxine hydrochloride if you:
Ask your healthcare provider or pharmacist if you are not sure if you take an MAOI, including MAOIs such as linezolid or intravenous methylene blue. Do not start taking an MAOI for at least 7 days after you stop treatment with venlafaxine hydrochloride extended-release capsules. |
||
| Before taking venlafaxine hydrochloride extended-release capsules tell your healthcare provider about all your medical conditions, including if you:
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. Venlafaxine hydrochloride extended-release capsules and other medicines may affect each other causing possible serious side effects. Venlafaxine hydrochloride extended-release capsules may affect the way other medicines work and other medicines may affect the way venlafaxine hydrochloride extended-release capsules work.
Especially tell your healthcare provider if you take:
Ask your healthcare provider if you are not sure if you are taking any of these medicines. Your healthcare provider can tell you if it is safe to take venlafaxine hydrochloride extended-release capsules with your other medicines. Do not start or stop any other medicines during treatment with venlafaxine hydrochloride extended-release capsules without first talking to your healthcare provider. Stopping venlafaxine hydrochloride extended-release capsules suddenly may cause you to have serious side effects. See, “What are the possible side effects of venlafaxine hydrochloride extended-release capsules?” Know the medicines you take. Keep a list of them to show to your healthcare provider and pharmacist when you get a new medicine. |
||
How should I take venlafaxine hydrochloride extended-release capsules?
|
||
What should I avoid while taking venlafaxine hydrochloride extended-release capsules?
|
||
| What are the possible side effects of venlafaxine hydrochloride extended-release capsules?
Venlafaxine hydrochloride extended-release capsules may cause serious side effects, including:
|
||
|
|
|
Symptoms may include: |
||
|
|
|
|
||
|
|
|
Symptoms in males may include:
Symptoms in females may include:
Talk to your healthcare provider if you develop any changes in your sexual function or if you have any questions or concerns about sexual problems during treatment with venlafaxine hydrochloride extended-release capsules. There may be treatments your healthcare provider can suggest. Your healthcare provider may tell you to stop taking venlafaxine hydrochloride extended-release capsules if you develop serious side effects during treatment with venlafaxine hydrochloride extended-release capsules.
The most common side effects of venlafaxine hydrochloride extended-release capsules include:
|
||
|
|
|
|
These are not all the possible side effects of venlafaxine hydrochloride extended-release capsules. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088. |
||
How should I store venlafaxine hydrochloride extended-release capsules?
Keep venlafaxine hydrochloride extended-release capsules and all medicines out of the reach of children.
|
||
| General Information about venlafaxine hydrochloride extended-release capsules.
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use venlafaxine hydrochloride extended-release capsules for a condition for which it was not prescribed. Do not give venlafaxine hydrochloride extended-release capsules to other people, even if they have the same symptoms that you have. It may harm them. You can ask your healthcare provider or pharmacist for information about venlafaxine hydrochloride extended-release capsules that is written for healthcare professionals. For more information about venlafaxine hydrochloride extended-release capsules call 1-888-838-2872. |
||
| What are the ingredients in venlafaxine hydrochloride extended-release capsules? Active ingredient: venlafaxine hydrochloride. Inactive ingredients: black iron oxide, dibutyl sebacate, ethylcellulose, gelatin, polyethylene glycol, povidone, propylene glycol, shellac, sugar spheres (which contain sucrose and corn starch), FD&C Yellow No. 6, Sunset Yellow FCF, talc, and titanium dioxide. The 37.5 mg capsules also contain D&C Yellow No.10 and potassium hydroxide, the 75 mg capsules also contain D&C Yellow No. 10 and may contain potassium hydroxide, and the 150 mg capsules also contain potassium hydroxide. Brands listed are trademarks of their respective owners. Manufactured In Israel By: Teva Pharmaceutical Ind. Ltd., Kfar Saba, 4410202, Israel |
||
This Medication Guide has been approved by the U.S. Food and Drug Administration. Rev. I 9/2022
Package/Label Display Panel
Package/Label Display Panel
Package/Label Display Panel
INGREDIENTS AND APPEARANCE
| VENLAFAXINE HYDROCHLORIDE
venlafaxine hydrochloride capsule, extended release |
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
| VENLAFAXINE HYDROCHLORIDE
venlafaxine hydrochloride capsule, extended release |
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
| VENLAFAXINE HYDROCHLORIDE
venlafaxine hydrochloride capsule, extended release |
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
| Labeler - Teva Pharmaceuticals USA, Inc. (001627975) |