Search by Drug Name or NDC
NDC 00597-0075-47 Spiriva 18 ug/1 Details
Spiriva 18 ug/1
Spiriva is a ORAL; RESPIRATORY (INHALATION) CAPSULE in the HUMAN PRESCRIPTION DRUG category. It is labeled and distributed by Boehringer Ingelheim Pharmaceuticals, Inc.. The primary component is TIOTROPIUM BROMIDE MONOHYDRATE.
MedlinePlus Drug Summary
Tiotropium is used to prevent wheezing, shortness of breath, coughing, and chest tightness in patients with chronic obstructive pulmonary disease (COPD, a group of diseases that affect the lungs and airways) such as chronic bronchitis (swelling of the air passages that lead to the lungs) and emphysema (damage to air sacs in the lungs). Tiotropium is in a class of medications called bronchodilators. It works by relaxing and opening the air passages to the lungs to make breathing easier.
Related Packages: 00597-0075-47Last Updated: 11/30/2022
MedLinePlus Full Drug Details: Tiotropium Oral Inhalation
Product Information
| NDC | 00597-0075 |
|---|---|
| Product ID | 0597-0075_74c01ed6-9299-467e-bda9-8043c8d887fb |
| Associated GPIs | 44100080100120 |
| GCN Sequence Number | 050714 |
| GCN Sequence Number Description | tiotropium bromide CAP W/DEV 18 MCG INHALATION |
| HIC3 | B61 |
| HIC3 Description | ANTICHOLINERGICS, ORALLY INHALED LONG ACTING |
| GCN | 17853 |
| HICL Sequence Number | 024024 |
| HICL Sequence Number Description | TIOTROPIUM BROMIDE |
| Brand/Generic | Brand |
| Proprietary Name | Spiriva |
| Proprietary Name Suffix | HandiHaler |
| Non-Proprietary Name | TIOTROPIUM BROMIDE |
| Product Type | HUMAN PRESCRIPTION DRUG |
| Dosage Form | CAPSULE |
| Route | ORAL; RESPIRATORY (INHALATION) |
| Active Ingredient Strength | 18 |
| Active Ingredient Units | ug/1 |
| Substance Name | TIOTROPIUM BROMIDE MONOHYDRATE |
| Labeler Name | Boehringer Ingelheim Pharmaceuticals, Inc. |
| Pharmaceutical Class | Anticholinergic [EPC], Cholinergic Antagonists [MoA] |
| DEA Schedule | n/a |
| Marketing Category | NDA |
| Application Number | NDA021395 |
| Listing Certified Through | 2024-12-31 |
Package
Package Images

NDC 00597-0075-47 (00597007547)
| NDC Package Code | 0597-0075-47 |
|---|---|
| Billing NDC | 00597007547 |
| Package | 9 BLISTER PACK in 1 CARTON (0597-0075-47) / 10 CAPSULE in 1 BLISTER PACK |
| Marketing Start Date | 2005-10-11 |
| NDC Exclude Flag | N |
| Pricing Information | |
| Price Per Unit | 16.3401 |
| Pricing Unit | EA |
| Effective Date | 2023-01-01 |
| NDC Description | SPIRIVA HANDIHALER 18 MCG CAP |
| Pharmacy Type Indicator | C/I |
| OTC | N |
| Explanation Code | 4, 5 |
| Classification for Rate Setting | B |
| As of Date | 2024-02-21 |
Standard Product Labeling (SPL)/Prescribing Information SPL 820839ef-e53d-47e8-a3b9-d911ff92e6a9 Details
HIGHLIGHTS OF PRESCRIBING INFORMATION
SPIRIVA® HANDIHALER® (tiotropium bromide inhalation powder), for oral inhalation use
Initial U.S. Approval: 2004
INDICATIONS AND USAGE
SPIRIVA HANDIHALER is an anticholinergic indicated for the long-term, once-daily, maintenance treatment of bronchospasm associated with chronic obstructive pulmonary disease (COPD), and for reducing COPD exacerbations (1)
DOSAGE AND ADMINISTRATION
DOSAGE FORMS AND STRENGTHS
Inhalation powder: SPIRIVA capsules contain 18 mcg tiotropium powder for use with HANDIHALER device (3)
CONTRAINDICATIONS
Hypersensitivity to tiotropium, ipratropium, or any components of SPIRIVA capsules (4)
WARNINGS AND PRECAUTIONS
- Not for acute use: Not a rescue medication (5.1)
- Immediate hypersensitivity reactions: Discontinue SPIRIVA HANDIHALER at once and consider alternatives if immediate hypersensitivity reactions, including angioedema, urticaria, rash, bronchospasm, or anaphylaxis, occur. Use with caution in patients with severe hypersensitivity to milk proteins. (5.2)
- Paradoxical bronchospasm: Discontinue SPIRIVA HANDIHALER and consider other treatments if paradoxical bronchospasm occurs (5.3)
- Worsening of narrow-angle glaucoma may occur. Use with caution in patients with narrow-angle glaucoma and instruct patients to consult a physician immediately if this occurs. (5.4)
- Worsening of urinary retention may occur. Use with caution in patients with prostatic hyperplasia or bladder-neck obstruction and instruct patients to consult a physician immediately if this occurs. (5.5)
ADVERSE REACTIONS
The most common adverse reactions (>5% incidence in the 1-year placebo-controlled trials) were upper respiratory tract infection, dry mouth, sinusitis, pharyngitis, non-specific chest pain, urinary tract infection, dyspepsia, and rhinitis (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Boehringer Ingelheim Pharmaceuticals, Inc. at (800) 542-6257 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS
Anticholinergics: May interact additively with concomitantly used anticholinergic medications. Avoid administration of SPIRIVA HANDIHALER with other anticholinergic-containing drugs. (7.2)
USE IN SPECIFIC POPULATIONS
Patients with moderate to severe renal impairment should be monitored closely for potential anticholinergic side effects (2, 8.6)
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 11/2021
Table Of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Not for Acute Use
5.2 Immediate Hypersensitivity Reactions
5.3 Paradoxical Bronchospasm
5.4 Worsening of Narrow-Angle Glaucoma
5.5 Worsening of Urinary Retention
5.6 Renal Impairment
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Postmarketing Experience
7 DRUG INTERACTIONS
7.1 Sympathomimetics, Methylxanthines, Steroids
7.2 Anticholinergics
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Renal Impairment
8.7 Hepatic Impairment
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- *
- Sections or subsections omitted from the full prescribing information are not listed.
1 INDICATIONS AND USAGE
SPIRIVA HANDIHALER (tiotropium bromide inhalation powder) is indicated for the long-term, once-daily, maintenance treatment of bronchospasm associated with chronic obstructive pulmonary disease (COPD), including chronic bronchitis and emphysema. SPIRIVA HANDIHALER is indicated to reduce exacerbations in COPD patients.
2 DOSAGE AND ADMINISTRATION
For oral inhalation only. Do not swallow SPIRIVA capsules, as the intended effects on the lungs will not be obtained. The contents of the SPIRIVA capsules should only be used with the HANDIHALER device [see Overdosage (10)].
The recommended dose of SPIRIVA HANDIHALER is two inhalations of the powder contents of one SPIRIVA capsule, once-daily, with the HANDIHALER device [see Patient Counseling Information (17)]. Do not take more than one dose in 24 hours.
For administration of SPIRIVA HANDIHALER, a SPIRIVA capsule is placed into the center chamber of the HANDIHALER device. The SPIRIVA capsule is pierced by pressing and releasing the green piercing button on the side of the HANDIHALER device. The tiotropium formulation is dispersed into the air stream when the patient inhales through the mouthpiece [see Patient Counseling Information (17)].
No dosage adjustment is required for geriatric, hepatically-impaired, or renally-impaired patients. However, patients with moderate to severe renal impairment given SPIRIVA HANDIHALER should be monitored closely for anticholinergic effects [see Warnings and Precautions (5.6), Use in Specific Populations (8.5, 8.6, 8.7), and Clinical Pharmacology (12.3)].
3 DOSAGE FORMS AND STRENGTHS
Inhalation Powder: SPIRIVA HANDIHALER consists of SPIRIVA capsules containing tiotropium powder for oral inhalation and a HANDIHALER device. SPIRIVA capsules contain 18 mcg of tiotropium in a light green, hard gelatin capsule with "TI 01" printed on one side and Boehringer Ingelheim company logo on the other side. The HANDIHALER device is only intended for use with the SPIRIVA capsules.
4 CONTRAINDICATIONS
SPIRIVA HANDIHALER is contraindicated in patients with a hypersensitivity to tiotropium, ipratropium, or any components of this product [see Warnings and Precautions (5.2)]. In clinical trials and postmarketing experience with SPIRIVA HANDIHALER, immediate hypersensitivity reactions, including angioedema (including swelling of the lips, tongue, or throat), itching, or rash have been reported [see Warnings and Precautions (5.2)].
5 WARNINGS AND PRECAUTIONS
5.1 Not for Acute Use
SPIRIVA HANDIHALER is intended as a once-daily maintenance treatment for COPD and should not be used for relief of acute symptoms, i.e., as rescue therapy for the treatment of acute episodes of bronchospasm.
5.2 Immediate Hypersensitivity Reactions
Immediate hypersensitivity reactions, including urticaria, angioedema (including swelling of the lips, tongue, or throat), rash, bronchospasm, anaphylaxis, or itching, may occur after administration of SPIRIVA HANDIHALER. If such a reaction occurs, therapy with SPIRIVA HANDIHALER should be stopped at once and alternative treatments should be considered. Given the similar structural formula of atropine to tiotropium, patients with a history of hypersensitivity reactions to atropine or its derivatives should be closely monitored for similar hypersensitivity reactions to SPIRIVA HANDIHALER. In addition, SPIRIVA HANDIHALER should be used with caution in patients with severe hypersensitivity to milk proteins.
5.3 Paradoxical Bronchospasm
Inhaled medicines, including SPIRIVA HANDIHALER, may cause paradoxical bronchospasm. If this occurs, it should be treated immediately with an inhaled short-acting beta2-agonist such as albuterol. Treatment with SPIRIVA HANDIHALER should be stopped and other treatments considered.
5.4 Worsening of Narrow-Angle Glaucoma
SPIRIVA HANDIHALER should be used with caution in patients with narrow-angle glaucoma. Prescribers and patients should be alert for signs and symptoms of acute narrow-angle glaucoma (e.g., eye pain or discomfort, blurred vision, visual halos or colored images in association with red eyes from conjunctival congestion and corneal edema). Instruct patients to consult a physician immediately should any of these signs or symptoms develop.
5.5 Worsening of Urinary Retention
SPIRIVA HANDIHALER should be used with caution in patients with urinary retention. Prescribers and patients should be alert for signs and symptoms of urinary retention (e.g., difficulty passing urine, painful urination), especially in patients with prostatic hyperplasia or bladder-neck obstruction. Instruct patients to consult a physician immediately should any of these signs or symptoms develop.
5.6 Renal Impairment
As a predominantly renally excreted drug, patients with moderate to severe renal impairment (creatinine clearance of <60 mL/min) treated with SPIRIVA HANDIHALER should be monitored closely for anticholinergic side effects [see Clinical Pharmacology (12.3)].
6 ADVERSE REACTIONS
The following adverse reactions are described, or described in greater detail, in other sections:
- Immediate hypersensitivity reactions [see Warnings and Precautions (5.2)]
- Paradoxical bronchospasm [see Warnings and Precautions (5.3)]
- Worsening of narrow-angle glaucoma [see Warnings and Precautions (5.4)]
- Worsening of urinary retention [see Warnings and Precautions (5.5)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, the incidence of adverse reactions observed in the clinical trials of a drug cannot be directly compared to the incidences in the clinical trials of another drug and may not reflect the incidences observed in practice.
6-Month to 1-Year Trials
The data described below reflect exposure to SPIRIVA HANDIHALER in 2663 patients. SPIRIVA HANDIHALER was studied in two 1-year placebo-controlled trials, two 1-year active-controlled trials, and two 6-month placebo-controlled trials in patients with COPD. In these trials, 1308 patients were treated with SPIRIVA HANDIHALER at the recommended dose of 18 mcg once a day. The population had an age ranging from 39 to 87 years with 65% to 85% males, 95% Caucasian, and had COPD with a mean pre-bronchodilator forced expiratory volume in one second (FEV1) percent predicted of 39% to 43%. Patients with narrow-angle glaucoma, or symptomatic prostatic hypertrophy or bladder outlet obstruction were excluded from these trials. An additional 6-month trial conducted in a Veteran's Affairs setting is not included in this safety database because only serious adverse events were collected.
The most commonly reported adverse drug reaction was dry mouth. Dry mouth was usually mild and often resolved during continued treatment. Other reactions reported in individual patients and consistent with possible anticholinergic effects included constipation, tachycardia, blurred vision, glaucoma (new onset or worsening), dysuria, and urinary retention.
Four multicenter, 1-year, placebo-controlled and active-controlled trials evaluated SPIRIVA HANDIHALER in patients with COPD. Table 1 shows all adverse reactions that occurred with a frequency of ≥3% in the SPIRIVA HANDIHALER group in the 1-year placebo-controlled trials where the rates in the SPIRIVA HANDIHALER group exceeded placebo by ≥1%. The frequency of corresponding reactions in the ipratropium-controlled trials is included for comparison.
| Body System (Event) | Placebo-Controlled Trials | Ipratropium-Controlled Trials | ||
|---|---|---|---|---|
| SPIRIVA (n = 550) | Placebo (n = 371) | SPIRIVA (n = 356) | Ipratropium (n = 179) |
|
| Body as a Whole | ||||
| Chest Pain (non-specific) | 7 | 5 | 5 | 2 |
| Edema, Dependent | 5 | 4 | 3 | 5 |
| Gastrointestinal System Disorders | ||||
| Dry Mouth | 16 | 3 | 12 | 6 |
| Dyspepsia | 6 | 5 | 1 | 1 |
| Abdominal Pain | 5 | 3 | 6 | 6 |
| Constipation | 4 | 2 | 1 | 1 |
| Vomiting | 4 | 2 | 1 | 2 |
| Musculoskeletal System | ||||
| Myalgia | 4 | 3 | 4 | 3 |
| Resistance Mechanism Disorders | ||||
| Infection | 4 | 3 | 1 | 3 |
| Moniliasis | 4 | 2 | 3 | 2 |
| Respiratory System (Upper) | ||||
| Upper Respiratory Tract Infection | 41 | 37 | 43 | 35 |
| Sinusitis | 11 | 9 | 3 | 2 |
| Pharyngitis | 9 | 7 | 7 | 3 |
| Rhinitis | 6 | 5 | 3 | 2 |
| Epistaxis | 4 | 2 | 1 | 1 |
| Skin and Appendage Disorders | ||||
| Rash | 4 | 2 | 2 | 2 |
| Urinary System | ||||
| Urinary Tract Infection | 7 | 5 | 4 | 2 |
Arthritis, coughing, and influenza-like symptoms occurred at a rate of ≥3% in the SPIRIVA HANDIHALER treatment group, but were <1% in excess of the placebo group.
Other reactions that occurred in the SPIRIVA HANDIHALER group at a frequency of 1% to 3% in the placebo-controlled trials where the rates exceeded that in the placebo group include: Body as a Whole: allergic reaction, leg pain; Central and Peripheral Nervous System: dysphonia, paresthesia; Gastrointestinal System Disorders: gastrointestinal disorder not otherwise specified (NOS), gastroesophageal reflux, stomatitis (including ulcerative stomatitis); Metabolic and Nutritional Disorders: hypercholesterolemia, hyperglycemia; Musculoskeletal System Disorders: skeletal pain; Cardiac Events: angina pectoris (including aggravated angina pectoris); Psychiatric Disorder: depression; Infections: herpes zoster; Respiratory System Disorder (Upper): laryngitis; Vision Disorder: cataract. In addition, among the adverse reactions observed in the clinical trials with an incidence of <1% were atrial fibrillation, supraventricular tachycardia, angioedema, and urinary retention.
In the 1-year trials, the incidence of dry mouth, constipation, and urinary tract infection increased with age [see Use in Specific Populations (8.5)].
Two multicenter, 6-month, controlled studies evaluated SPIRIVA HANDIHALER in patients with COPD. The adverse reactions and the incidence rates were similar to those seen in the 1-year controlled trials.
4-Year Trial
The data described below reflect exposure to SPIRIVA HANDIHALER in 5992 COPD patients in a 4-year placebo-controlled trial. In this trial, 2986 patients were treated with SPIRIVA HANDIHALER at the recommended dose of 18 mcg once a day. The population had an age range from 40 to 88 years, was 75% male, 90% Caucasian, and had COPD with a mean pre-bronchodilator FEV1 percent predicted of 40%. Patients with narrow-angle glaucoma, or symptomatic prostatic hypertrophy or bladder outlet obstruction were excluded from these trials. When the adverse reactions were analyzed with a frequency of ≥3% in the SPIRIVA HANDIHALER group where the rates in the SPIRIVA HANDIHALER group exceeded placebo by ≥1%, adverse reactions included (SPIRIVA HANDIHALER, placebo): pharyngitis (12.5%, 10.8%), sinusitis (6.5%, 5.3%), headache (5.7%, 4.5%), constipation (5.1%, 3.7%), dry mouth (5.1%, 2.7%), depression (4.4%, 3.3%), insomnia (4.4%, 3.0%), and arthralgia (4.2%, 3.1%).
Additional Adverse Reactions
Other adverse reactions not previously listed that were reported more frequently in COPD patients treated with SPIRIVA HANDIHALER than placebo include: dehydration, skin ulcer, stomatitis, gingivitis, oropharyngeal candidiasis, dry skin, skin infection, and joint swelling.
6.2 Postmarketing Experience
Adverse reactions have been identified during worldwide post-approval use of SPIRIVA HANDIHALER. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure. These adverse reactions are: application site irritation (glossitis, mouth ulceration, and pharyngolaryngeal pain), dizziness, dysphagia, hoarseness, intestinal obstruction including ileus paralytic, intraocular pressure increased, oral candidiasis, palpitations, pruritus, tachycardia, throat irritation, and urticaria.
7 DRUG INTERACTIONS
7.1 Sympathomimetics, Methylxanthines, Steroids
SPIRIVA HANDIHALER has been used concomitantly with short-acting and long-acting sympathomimetic (beta-agonists) bronchodilators, methylxanthines, and oral and inhaled steroids without increases in adverse reactions.
7.2 Anticholinergics
There is potential for an additive interaction with concomitantly used anticholinergic medications. Therefore, avoid coadministration of SPIRIVA HANDIHALER with other anticholinergic-containing drugs as this may lead to an increase in anticholinergic adverse effects [see Warnings and Precautions (5.4, 5.5) and Adverse Reactions (6)].
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
The limited human data with SPIRIVA HANDIHALER use during pregnancy are insufficient to inform a drug-associated risk of adverse pregnancy-related outcomes. Based on animal reproduction studies, no structural abnormalities were observed when tiotropium was administered by inhalation to pregnant rats and rabbits during the period of organogenesis at doses 790 and 8 times, respectively, the maximum recommended human daily inhalation dose (MRHDID). Increased post-implantation loss was observed in rats and rabbits administered tiotropium at maternally toxic doses 430 times and 40 times the MRHDID, respectively [see Data].
The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a background risk of birth defect, loss or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively.
Data
Animal Data
In 2 separate embryo-fetal development studies, pregnant rats and rabbits received tiotropium during the period of organogenesis at doses up to approximately 790 and 8 times the MRHDID, respectively (on a mcg/m2 basis at inhalation doses of 1471 and 7 mcg/kg/day in rats and rabbits, respectively). No evidence of structural abnormalities was observed in rats or rabbits. However, in rats, tiotropium caused fetal resorption, litter loss, decreases in the number of live pups at birth and the mean pup weights, and a delay in pup sexual maturation at tiotropium doses of approximately 40 times the MRHDID (on a mcg/m2 basis at a maternal inhalation dose of 78 mcg/kg/day). In rabbits, tiotropium caused an increase in post-implantation loss at a tiotropium dose of approximately 430 times the MRHDID (on a mcg/m2 basis at a maternal inhalation dose of 400 mcg/kg/day). Such effects were not observed at approximately 5 and 95 times the MRHDID, respectively (on a mcg/m2 basis at inhalation doses of 9 and 88 mcg/kg/day in rats and rabbits, respectively).
8.2 Lactation
Risk Summary
There are no data on the presence of tiotropium in human milk, the effects on the breastfed infant, or the effects on milk production. Tiotropium is present in milk of lactating rats; however, due to species-specific differences in lactation physiology, the clinical relevance of these data are not clear [see Data]. The developmental and health benefits of breastfeeding should be considered along with the mother's clinical need for SPIRIVA HANDIHALER and any potential adverse effects on the breastfed child from SPIRIVA HANDIHALER or from the underlying maternal condition.
8.4 Pediatric Use
SPIRIVA HANDIHALER is not indicated for use in children. The safety and effectiveness of SPIRIVA HANDIHALER in pediatric patients have not been established.
8.5 Geriatric Use
Based on available data, no adjustment of SPIRIVA HANDIHALER dosage in geriatric patients is warranted [see Clinical Pharmacology (12.3)].
Of the total number of patients who received SPIRIVA HANDIHALER in the 1-year clinical trials, 426 were <65 years, 375 were 65 to 74 years, and 105 were ≥75 years of age. Within each age subgroup, there were no differences between the proportion of patients with adverse events in the SPIRIVA HANDIHALER and the comparator groups for most events. Dry mouth increased with age in the SPIRIVA HANDIHALER group (differences from placebo were 9.0%, 17.1%, and 16.2% in the aforementioned age subgroups). A higher frequency of constipation and urinary tract infections with increasing age was observed in the SPIRIVA HANDIHALER group in the placebo-controlled studies. The differences from placebo for constipation were 0%, 1.8%, and 7.8% for each of the age groups. The differences from placebo for urinary tract infections were –0.6%, 4.6%, and 4.5%. No overall differences in effectiveness were observed among these groups.
8.6 Renal Impairment
Patients with moderate to severe renal impairment (creatinine clearance of <60 mL/min) treated with SPIRIVA HANDIHALER should be monitored closely for anticholinergic side effects [see Dosage and Administration (2), Warnings and Precautions (5.6), and Clinical Pharmacology (12.3)].
10 OVERDOSAGE
High doses of tiotropium may lead to anticholinergic signs and symptoms. However, there were no systemic anticholinergic adverse effects following a single inhaled dose of up to 282 mcg tiotropium in 6 healthy volunteers. In a study of 12 healthy volunteers, bilateral conjunctivitis and dry mouth were seen following repeated once-daily inhalation of 141 mcg of tiotropium.
Treatment of overdosage consists of discontinuation of SPIRIVA HANDIHALER together with institution of appropriate symptomatic and/or supportive therapy.
Accidental Ingestion
Acute intoxication by inadvertent oral ingestion of SPIRIVA capsules is unlikely since it is not well-absorbed systemically.
A case of overdose has been reported from postmarketing experience. A female patient was reported to have inhaled 30 capsules over a 2.5 day period, and developed altered mental status, tremors, abdominal pain, and severe constipation. The patient was hospitalized, SPIRIVA HANDIHALER was discontinued, and the constipation was treated with an enema. The patient recovered and was discharged on the same day.
11 DESCRIPTION
SPIRIVA HANDIHALER consists of SPIRIVA capsules and a HANDIHALER device. Each light green, hard gelatin SPIRIVA capsule contains a dry powder consisting of 18 mcg tiotropium (equivalent to 22.5 mcg tiotropium bromide monohydrate) blended with lactose monohydrate (which may contain milk proteins).
The contents of SPIRIVA capsules are intended for oral inhalation only, and are intended for administration only with the HANDIHALER device.
The active component of SPIRIVA HANDIHALER is tiotropium. The drug substance, tiotropium bromide monohydrate, is an anticholinergic with specificity for muscarinic receptors. It is chemically described as (1α, 2β, 4β, 5α, 7β)-7-[(Hydroxydi-2-thienylacetyl)oxy]-9,9-dimethyl-3-oxa-9-azoniatricyclo[3.3.1.02,4]nonane bromide monohydrate. It is a synthetic, non-chiral, quaternary ammonium compound. Tiotropium bromide is a white or yellowish white powder. It is sparingly soluble in water and soluble in methanol.
The structural formula is:

Tiotropium bromide (monohydrate) has a molecular mass of 490.4 and a molecular formula of C19H22NO4S2Br ∙ H2O.
The HANDIHALER device is an inhalation device used to inhale the dry powder contained in the SPIRIVA capsule. The dry powder is delivered from the HANDIHALER device at flow rates as low as 20 L/min. Under standardized in vitro testing, the HANDIHALER device delivers a mean of 10.4 mcg tiotropium when tested at a flow rate of 39 L/min for 3.1 seconds (2 L total). In a study of 26 adult patients with COPD and severely compromised lung function [mean FEV1 1.02 L (range 0.45 to 2.24 L); 37.6% of predicted (range 16% to 65%)], the median peak inspiratory flow (PIF) through the HANDIHALER device was 30.0 L/min (range 20.4 to 45.6 L/min). The amount of drug delivered to the lungs will vary depending on patient factors such as inspiratory flow and peak inspiratory flow through the HANDIHALER device, which may vary from patient to patient, and may vary with the exposure time of the SPIRIVA capsule outside the blister pack.
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Tiotropium is a long-acting, antimuscarinic agent, which is often referred to as an anticholinergic. It has similar affinity to the subtypes of muscarinic receptors, M1 to M5. In the airways, it exhibits pharmacological effects through inhibition of M3-receptors at the smooth muscle leading to bronchodilation. The competitive and reversible nature of antagonism was shown with human and animal origin receptors and isolated organ preparations. In preclinical in vitro as well as in vivo studies, prevention of methacholine-induced bronchoconstriction effects was dose-dependent and lasted longer than 24 hours. The bronchodilation following inhalation of tiotropium is predominantly a site-specific effect.
12.2 Pharmacodynamics
Cardiac Electrophysiology
In a multicenter, randomized, double-blind trial using tiotropium dry powder for inhalation that enrolled 198 patients with COPD, the number of subjects with changes from baseline-corrected QT interval of 30 to 60 msec was higher in the SPIRIVA HANDIHALER group as compared with placebo. This difference was apparent using both the Bazett (QTcB) [20 (20%) patients vs. 12 (12%) patients] and Fredericia (QTcF) [16 (16%) patients vs. 1 (1%) patient] corrections of QT for heart rate. No patients in either group had either QTcB or QTcF of >500 msec. Other clinical studies with SPIRIVA HANDIHALER did not detect an effect of the drug on QTc intervals.
The effect of tiotropium dry powder for inhalation on QT interval was also evaluated in a randomized, placebo- and positive-controlled crossover study in 53 healthy volunteers. Subjects received tiotropium dry powder for inhalation 18 mcg, 54 mcg (3 times the recommended dose), or placebo for 12 days. ECG assessments were performed at baseline and throughout the dosing interval following the first and last dose of study medication. Relative to placebo, the maximum mean change from baseline in study-specific QTc interval was 3.2 msec and 0.8 msec for tiotropium dry powder for inhalation 18 mcg and 54 mcg, respectively. No subject showed a new onset of QTc >500 msec or QTc changes from baseline of ≥60 msec.
12.3 Pharmacokinetics
Tiotropium is administered by dry powder inhalation. Some of the pharmacokinetic data described below were obtained with higher doses than recommended for therapy. A dedicated pharmacokinetic study in patients with COPD evaluating once-daily tiotropium delivered from the RESPIMAT inhaler (5 mcg) and as inhalation powder (18 mcg) from the HANDIHALER device resulted in a similar systemic exposure between the two products.
Absorption
Following dry powder inhalation by young healthy volunteers, the absolute bioavailability of 19.5% suggests that the fraction reaching the lung is highly bioavailable. Oral solutions of tiotropium have an absolute bioavailability of 2-3%. Food is not expected to influence the absorption of tiotropium. Maximum tiotropium plasma concentrations were observed 7 minutes after inhalation.
Distribution
Tiotropium is 72% bound to plasma protein and had a volume of distribution of 32 L/kg after intravenous administration to young healthy volunteers. Local concentrations in the lung are not known, but the mode of administration suggests substantially higher concentrations in the lung. Studies in rats have shown that tiotropium does not readily penetrate the blood-brain barrier.
Elimination
The terminal half-life of tiotropium in COPD patients following once daily inhalation of 5 mcg tiotropium was approximately 25 hours. Total clearance was 880 mL/min after intravenous administration in young healthy volunteers. After chronic once-daily dry powder inhalation by COPD patients, pharmacokinetic steady state was reached by day 7 with no accumulation thereafter.
Metabolism
The extent of metabolism is small. This is evident from a urinary excretion of 74% of unchanged substance after an intravenous dose to young healthy volunteers. Tiotropium, an ester, is nonenzymatically cleaved to the alcohol N-methylscopine and dithienylglycolic acid, neither of which binds to muscarinic receptors.
In vitro experiments with human liver microsomes and human hepatocytes suggest that a fraction of the administered dose (74% of an intravenous dose is excreted unchanged in the urine, leaving 25% for metabolism) is metabolized by cytochrome P450-dependent oxidation and subsequent glutathione conjugation to a variety of Phase II metabolites. This enzymatic pathway can be inhibited by CYP450 2D6 and 3A4 inhibitors, such as quinidine, ketoconazole, and gestodene. Thus, CYP450 2D6 and 3A4 are involved in the metabolic pathway that is responsible for the elimination of a small part of the administered dose. In vitro studies using human liver microsomes showed that tiotropium in supra-therapeutic concentrations did not inhibit CYP450 1A1, 1A2, 2B6, 2C9, 2C19, 2D6, 2E1, or 3A4.
Excretion
Intravenously administered tiotropium bromide is mainly excreted unchanged in urine (74%). After dry powder inhalation to COPD patients at steady state, urinary excretion was 7% (1.3mcg) of the unchanged dose over 24 hours. The renal clearance of tiotropium exceeds the creatinine clearance, indicating secretion into the urine.
Specific Populations
Geriatric Patients
As expected for all predominantly renally excreted drugs, advancing age was associated with a decrease of tiotropium renal clearance (365 mL/min in COPD patients <65 years to 271 mL/min in COPD patients ≥65 years). This did not result in a corresponding increase in AUC0-6,ss and Cmax,ss values following administration via HANDIHALER device.
Renal Impairment
Following 4-week SPIRIVA HANDIHALER or SPIRIVA RESPIMAT once daily dosing in patients with COPD, mild renal impairment (creatinine clearance 60-<90 mL/min) resulted in 6-23% higher AUC0-6,ss and 6-17% higher Cmax,ss values; moderate renal impairment (creatinine clearance 30-<60 mL/min) resulted in 54-57% higher AUC0-6,ss and 15-31% higher Cmax,ss values compared to COPD patients with normal renal function (creatinine clearance ≥90 mL/min). There is insufficient data for tiotropium exposure in patients with severe renal impairment (creatinine clearance <30 mL/min) following inhalation of SPIRIVA HANDIHALER or SPIRIVA RESPIMAT. However AUC0-4 and Cmax were 94% and 52% higher, respectively, in patients with severe renal impairment following intravenous infusion of tiotropium bromide.
Drug Interactions
An interaction study with tiotropium (14.4 mcg intravenous infusion over 15 minutes) and cimetidine 400 mg three times daily or ranitidine 300 mg once daily was conducted. Concomitant administration of cimetidine with tiotropium resulted in a 20% increase in the AUC0-4h, a 28% decrease in the renal clearance of tiotropium and no significant change in the Cmax and amount excreted in urine over 96 hours. Co-administration of tiotropium with ranitidine did not affect the pharmacokinetics of tiotropium.
Common concomitant medications (long-acting beta2-adrenergic agonists (LABA), inhaled corticosteroids (ICS)) used by patients with COPD were not found to alter the exposure to tiotropium.
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
No evidence of tumorigenicity was observed in a 104-week inhalation study in rats at tiotropium doses up to 59 mcg/kg/day, in an 83-week inhalation study in female mice at doses up to 145 mcg/kg/day, and in a 101-week inhalation study in male mice at doses up to 2 mcg/kg/day. These doses correspond to approximately 30, 40, and 0.5 times the recommended human daily inhalation dose (MRHDID) on a mcg/m2 basis, respectively.
Tiotropium bromide demonstrated no evidence of mutagenicity or clastogenicity in the following assays: the bacterial gene mutation assay, the V79 Chinese hamster cell mutagenesis assay, the chromosomal aberration assays in human lymphocytes in vitro and mouse micronucleus formation in vivo, and the unscheduled DNA synthesis in primary rat hepatocytes in vitro assay.
In rats, decreases in the number of corpora lutea and the percentage of implants were noted at inhalation tiotropium doses of 78 mcg/kg/day or greater (approximately 40 times the MRHDID on a mcg/m2 basis). No such effects were observed at 9 mcg/kg/day (approximately 5 times the MRHDID on a mcg/m2 basis). The fertility index, however, was not affected at inhalation doses up to 1689 mcg/kg/day (approximately 910 times the MRHDID on a mcg/m2 basis).
14 CLINICAL STUDIES
The SPIRIVA HANDIHALER (tiotropium bromide inhalation powder) clinical development program consisted of six Phase 3 studies in 2663 patients with COPD (1308 receiving SPIRIVA HANDIHALER): two 1-year, placebo-controlled studies, two 6-month, placebo-controlled studies and two 1-year, ipratropium-controlled studies. These studies enrolled patients who had a clinical diagnosis of COPD, were 40 years of age or older, had a history of smoking greater than 10 pack-years, had a forced expiratory volume in one second (FEV1) less than or equal to 60% or 65% of predicted, and a ratio of FEV1/FVC of less than or equal to 0.7.
In these studies, SPIRIVA HANDIHALER, administered once-daily in the morning, provided improvement in lung function (FEV1), with peak effect occurring within 3 hours following the first dose.
Two additional trials evaluated exacerbations: a 6-month, randomized, double-blind, placebo-controlled, multicenter clinical trial of 1829 COPD patients in a US Veterans Affairs setting and a 4-year, randomized, double-blind, placebo-controlled, multicenter, clinical trial of 5992 COPD patients. Long-term effects on lung function and other outcomes, were also evaluated in the 4-year multicenter trial.
6-Month to 1-Year Effects on Lung Function
In the 1-year, placebo-controlled trials, the mean improvement in FEV1 at 30 minutes was 0.13 liters (13%) with a peak improvement of 0.24 liters (24%) relative to baseline after the first dose (Day 1). Further improvements in FEV1 and forced vital capacity (FVC) were observed with pharmacodynamic steady state reached by Day 8 with once-daily treatment. The mean peak improvement in FEV1, relative to baseline, was 0.28 to 0.31 liters (28% to 31%), after 1 week (Day 8) of once-daily treatment. Improvement of lung function was maintained for 24 hours after a single dose and consistently maintained over the 1-year treatment period with no evidence of tolerance.
In the two 6-month, placebo-controlled trials, serial spirometric evaluations were performed throughout daytime hours in Trial A (12 hours) and limited to 3 hours in Trial B. The serial FEV1 values over 12 hours (Trial A) are displayed in Figure 1. These trials further support the improvement in pulmonary function (FEV1) with SPIRIVA HANDIHALER, which persisted over the spirometric observational period. Effectiveness was maintained for 24 hours after administration over the 6-month treatment period.
|
|
| Figure 1 Mean FEV1 Over Time (prior to and after administration of study drug) on Days 1 and 169 for Trial A (a Six-Month Placebo-Controlled Study)* | |
| Day 1 | Day 169 |
|
|
|
Results of each of the 1-year ipratropium-controlled trials were similar to the results of the 1-year placebo-controlled trials. The results of one of these trials are shown in Figure 2.
|
|
| Figure 2 Mean FEV1 Over Time (0 to 6 hours post-dose) on Days 1 and 92, Respectively for One of the Two Ipratropium-Controlled Studies* | |
| Day 1 | Day 92 |
|
|
|
A randomized, placebo-controlled clinical study in 105 patients with COPD demonstrated that bronchodilation was maintained throughout the 24-hour dosing interval in comparison to placebo, regardless of whether SPIRIVA HANDIHALER was administered in the morning or in the evening.
Throughout each week of the 1-year treatment period in the two placebo-controlled trials, patients taking SPIRIVA HANDIHALER had a reduced requirement for the use of rescue short-acting beta2-agonists. Reduction in the use of rescue short-acting beta2-agonists, as compared to placebo, was demonstrated in one of the two 6-month studies.
4-Year Effects on Lung Function
A 4-year, randomized, double-blind, placebo-controlled, multicenter clinical trial involving 5992 COPD patients was conducted to evaluate the long-term effects of SPIRIVA HANDIHALER on disease progression (rate of decline in FEV1). Patients were permitted to use all respiratory medications (including short-acting and long-acting beta-agonists, inhaled and systemic steroids, and theophyllines) other than inhaled anticholinergics. The patients were 40 to 88 years of age, 75% male, and 90% Caucasian with a diagnosis of COPD and a mean pre-bronchodilator FEV1 of 39% predicted (range = 9% to 76%) at study entry. There was no difference between the groups in either of the co-primary efficacy endpoints, yearly rate of decline in pre- and post-bronchodilator FEV1, as demonstrated by similar slopes of FEV1 decline over time (Figure 3).
SPIRIVA HANDIHALER maintained improvements in trough (pre-dose) FEV1 (adjusted means over time: 87 to 103 mL) throughout the 4 years of the study (Figure 3).
Figure 3 Trough (pre-dose) FEV1 Mean Values at Each Time Point

Repeated measure ANOVA was used to estimate means. Means are adjusted for baseline measurements. Baseline trough FEV1 (observed mean) = 1.12. Patients with ≥3 acceptable pulmonary function tests after Day 30 and non-missing baseline value were included in the analysis.
Exacerbations
The effect of SPIRIVA HANDIHALER on COPD exacerbations was evaluated in two clinical trials: a 4-year clinical trial described above and a 6-month clinical trial of 1829 COPD patients in a Veterans Affairs setting. In the 6-month trial, COPD exacerbations were defined as a complex of respiratory symptoms (increase or new onset) of more than one of the following: cough, sputum, wheezing, dyspnea, or chest tightness with a duration of at least 3 days requiring treatment with antibiotics, systemic steroids, or hospitalization. The population had an age ranging from 40 to 90 years with 99% males, 91% Caucasian, and had COPD with a mean pre-bronchodilator FEV1 percent predicted of 36% (range = 8% to 93%). Patients were permitted to use respiratory medications (including short-acting and long-acting beta-agonists, inhaled and systemic steroids, and theophyllines) other than inhaled anticholinergics. In the 6-month trial, the co-primary endpoints were the proportion of patients with COPD exacerbation and the proportion of patients with hospitalization due to COPD exacerbation. SPIRIVA HANDIHALER significantly reduced the proportion of COPD patients who experienced exacerbations compared to placebo (27.9% vs. 32.3%, respectively; Odds Ratio (OR) (tiotropium/placebo) = 0.81; 95% CI = 0.66, 0.99; p = 0.037). The proportion of patients with hospitalization due to COPD exacerbations in patients who used SPIRIVA HANDIHALER compared to placebo was 7.0% vs. 9.5%, respectively; OR = 0.72; 95% CI = 0.51, 1.01; p = 0.056.
Exacerbations were evaluated as a secondary outcome in the 4-year multicenter trial. In this trial, COPD exacerbations were defined as an increase or new onset of more than one of the following respiratory symptoms (cough, sputum, sputum purulence, wheezing, dyspnea) with a duration of three or more days requiring treatment with antibiotics and/or systemic (oral, intramuscular, or intravenous) steroids. SPIRIVA HANDIHALER significantly reduced the risk of an exacerbation by 14% (Hazard Ratio (HR) = 0.86; 95% CI = 0.81, 0.91; p<0.001) and reduced the risk of exacerbation-related hospitalization by 14% (HR = 0.86; 95% CI = 0.78, 0.95; p<0.002) compared to placebo. The median time to first exacerbation was delayed from 12.5 months (95% CI = 11.5, 13.8) in the placebo group to 16.7 months (95% CI = 14.9, 17.9) in the SPIRIVA HANDIHALER group.
All-Cause Mortality
In the 4-year placebo-controlled lung-function trial described above, all-cause mortality compared to placebo was assessed. There were no significant differences in all-cause mortality rates between SPIRIVA HANDIHALER and placebo.
The all-cause mortality of SPIRIVA HANDIHALER was also compared to tiotropium inhalation spray 5 mcg (SPIRIVA RESPIMAT 5 mcg) in an additional long-term, randomized, double-blind, double-dummy active-controlled study with an observation period up to 3 years. All-cause mortality was similar between SPIRIVA HANDIHALER and SPIRIVA RESPIMAT.
16 HOW SUPPLIED/STORAGE AND HANDLING
SPIRIVA HANDIHALER consists of SPIRIVA capsules and the HANDIHALER device. SPIRIVA capsules contain 18 mcg of tiotropium and are light green, with the Boehringer Ingelheim company logo on the SPIRIVA capsule cap and "TI 01" on the SPIRIVA capsule body, or vice versa.
The HANDIHALER device is gray colored with a green piercing button. It is imprinted with SPIRIVA HANDIHALER (tiotropium bromide inhalation powder), the Boehringer Ingelheim company logo. It is also imprinted to indicate that SPIRIVA capsules should not be stored in the HANDIHALER device and that the HANDIHALER device is only to be used with SPIRIVA capsules.
SPIRIVA capsules are packaged in an aluminum/aluminum blister card and joined along a perforated-cut line. SPIRIVA capsules should always be stored in the blister and only removed immediately before use. The drug should be used immediately after the packaging over an individual SPIRIVA capsule is opened.
The following packages are available:
- carton containing 5 SPIRIVA capsules (1 unit-dose blister card) and 1 HANDIHALER inhalation device (NDC 0597-0075-75) (institutional pack)
- carton containing 30 SPIRIVA capsules (3 unit-dose blister cards) and 1 HANDIHALER inhalation device (NDC 0597-0075-41)
- carton containing 90 SPIRIVA capsules (9 unit-dose blister cards) and 1 HANDIHALER inhalation device (NDC 0597-0075-47)
Keep out of reach of children. Do not get powder into eyes.
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Patient Information and Instructions for Use).
Not for Acute Use:
Instruct patients that SPIRIVA HANDIHALER is a once-daily maintenance bronchodilator and should not be used for immediate relief of breathing problems (i.e., as a rescue medication).
Immediate Hypersensitivity Reactions:
Inform patients that anaphylaxis, angioedema (including swelling of the lips, tongue, or throat), urticaria, rash, bronchospasm, or itching, may occur after administration of SPIRIVA HANDIHALER. Advise patient to immediately discontinue treatment and consult a physician should any of these signs or symptoms develop.
Paradoxical Bronchospasm:
Inform patients that SPIRIVA HANDIHALER can produce paradoxical bronchospasm. Advise patients that if paradoxical bronchospasm occurs, patients should discontinue SPIRIVA HANDIHALER.
Worsening of Narrow-Angle Glaucoma:
Instruct patients to be alert for signs and symptoms of narrow-angle glaucoma (e.g., eye pain or discomfort, blurred vision, visual halos or colored images in association with red eyes from conjunctival congestion and corneal edema). Instruct patients to consult a physician immediately should any of these signs and symptoms develop.
Inform patients that care must be taken not to allow the powder to enter into the eyes as this may cause blurring of vision and pupil dilation.
Since dizziness and blurred vision may occur with the use of SPIRIVA HANDIHALER, caution patients about engaging in activities such as driving a vehicle or operating appliances or machinery.
Worsening of Urinary Retention:
Instruct patients to be alert for signs and symptoms of urinary retention (e.g., difficulty passing urine, painful urination). Instruct patients to consult a physician immediately should any of these signs or symptoms develop.
Instructions for Administering SPIRIVA HANDIHALER:
Instruct patients on how to correctly administer SPIRIVA capsules using the HANDIHALER device [see Patient Counseling Information (17)]. Instruct patients that SPIRIVA capsules should only be administered via the HANDIHALER device and the HANDIHALER device should not be used for administering other medications. Remind patients that the contents of SPIRIVA capsules are for oral inhalation only and must not be swallowed.
Instruct patients always to store SPIRIVA capsules in sealed blisters and to remove only one SPIRIVA capsule immediately before use or its effectiveness may be reduced. Instruct patients to discard unused additional SPIRIVA capsules that are exposed to air (i.e., not intended for immediate use).
SPL UNCLASSIFIED SECTION
Distributed by:
Boehringer Ingelheim Pharmaceuticals, Inc.
Ridgefield, CT 06877 USA
Licensed from:
Boehringer Ingelheim International GmbH
Address medical inquiries to: (800) 542-6257.
SPIRIVA® and HANDIHALER® are registered trademarks of and are used under license from Boehringer Ingelheim International GmbH.
Copyright © 2021 Boehringer Ingelheim International GmbH
ALL RIGHTS RESERVED
COL10494AK082021
Patient Information
SPIRIVA® (speh REE vah) HANDIHALER®
(tiotropium bromide inhalation powder)
|
| Do NOT swallow SPIRIVA capsules. |
Important Information: Do not swallow SPIRIVA capsules. SPIRIVA capsules should only be used with the HANDIHALER device and inhaled through your mouth (oral inhalation).
Read the information that comes with your SPIRIVA HANDIHALER before you start using it and each time you refill your prescription. There may be new information. This leaflet does not take the place of talking with your doctor about your medical condition or your treatment.
What is SPIRIVA HANDIHALER?
- SPIRIVA HANDIHALER is a prescription medicine used each day (a maintenance medicine) to control symptoms of chronic obstructive pulmonary disease (COPD), including chronic bronchitis and emphysema.
- SPIRIVA HANDIHALER helps make your lungs work better for 24 hours. SPIRIVA HANDIHALER relaxes your airways and helps keep them open. You may start to feel like it is easier to breathe on the first day, but it may take longer for you to feel the full effects of the medicine. SPIRIVA HANDIHALER works best and may help make it easier to breathe when you use it every day.
- SPIRIVA HANDIHALER reduces the likelihood of flare-ups and worsening of COPD symptoms (COPD exacerbations). A COPD exacerbation is defined as an increase or new onset of more than one COPD symptom such as cough, mucus, shortness of breath, and wheezing that requires medicine beyond your rescue medicine.
SPIRIVA HANDIHALER is not a rescue medicine and should not be used for treating sudden breathing problems. Your doctor may give you other medicine to use for sudden breathing problems.
It is not known if SPIRIVA HANDIHALER is safe and effective in children.
Who should not take SPIRIVA HANDIHALER?
Do not use SPIRIVA HANDIHALER if you:
- are allergic to tiotropium, ipratropium (Atrovent®), or any of the ingredients in SPIRIVA HANDIHALER. See the end of this leaflet for a complete list of ingredients in SPIRIVA HANDIHALER.
Symptoms of a serious allergic reaction to SPIRIVA HANDIHALER may include:
- raised red patches on your skin (hives)
- itching
- rash
- swelling of the face, lips, tongue, and throat that may cause difficulty in breathing or swallowing
If you have these symptoms of an allergic reaction, stop taking SPIRIVA HANDIHALER and call your doctor right away or go to the nearest hospital emergency room.
What should I tell my doctor before using SPIRIVA HANDIHALER?
Before taking SPIRIVA HANDIHALER, tell your doctor about all your medical conditions, including if you:
- have kidney problems.
- have glaucoma. SPIRIVA HANDIHALER may make your glaucoma worse.
- have an enlarged prostate, problems passing urine, or a blockage in your bladder. SPIRIVA HANDIHALER may make these problems worse.
- are pregnant or plan to become pregnant. It is not known if SPIRIVA HANDIHALER could harm your unborn baby.
- are breast-feeding or plan to breast-feed. It is not known if SPIRIVA HANDIHALER passes into breast milk. You and your doctor will decide if SPIRIVA HANDIHALER is right for you while you breast-feed.
- have a severe allergy to milk proteins. Ask your doctor if you are not sure.
Tell your doctor about all the medicines you take, including prescription and non-prescription medicines and eye drops, vitamins, and herbal supplements. Some of your other medicines or supplements may affect the way SPIRIVA HANDIHALER works. SPIRIVA HANDIHALER is an anticholinergic medicine. You should not take other anticholinergic medicines while using SPIRIVA HANDIHALER, including ipratropium. Ask your doctor or pharmacist if you are not sure if one of your medicines is an anticholinergic.
Know the medicines you take. Keep a list of your medicines with you to show your doctor and pharmacist when you get a new medicine.
How should I take SPIRIVA HANDIHALER?
- Use SPIRIVA HANDIHALER exactly as prescribed. Use SPIRIVA HANDIHALER one time every day.
- Read the "Instructions for Use" at the end of this leaflet before you use SPIRIVA HANDIHALER. Talk with your doctor if you do not understand the instructions.
- Do not swallow SPIRIVA capsules.
- Only use SPIRIVA capsules with the HANDIHALER device.
- Do not use the HANDIHALER device to take any other medicine.
- SPIRIVA HANDIHALER comes as a powder in a SPIRIVA capsule that fits the HANDIHALER device. Each SPIRIVA capsule, containing only a small amount of SPIRIVA powder, is one full dose of medicine.
- Separate one blister from the blister card. Then take out one of the SPIRIVA capsules from the blister package right before you use it.
- After the capsule is pierced, take a complete dose of SPIRIVA HANDIHALER by breathing in the powder by mouth two times, using the HANDIHALER device (take 2 inhalations from one SPIRIVA capsule). See the "Instructions for Use" at the end of this leaflet.
- Throw away any SPIRIVA capsule that is not used right away after it is taken out of the blister package. Do not leave the SPIRIVA capsules open to air; they may not work as well.
- If you miss a dose, take it as soon as you remember. Do not use SPIRIVA HANDIHALER more than one time every 24 hours.
- If you use more than your prescribed dose of SPIRIVA HANDIHALER, call your doctor or a poison control center.
What should I avoid while using SPIRIVA HANDIHALER?
- Do not let the powder from the SPIRIVA capsule get into your eyes. Your vision may get blurry and the pupil in your eye may get larger (dilate). If this happens, call your doctor.
- SPIRIVA HANDIHALER can cause dizziness and blurred vision. Should you experience these symptoms you should use caution when engaging in activities such as driving a car or operating appliances or other machines.
What are the possible side effects of SPIRIVA HANDIHALER?
SPIRIVA HANDIHALER can cause serious side effects, including: Allergic reaction. Symptoms may include:
- raised red patches on your skin (hives)
- itching
- rash
- swelling of the lips, tongue, or throat that may cause difficulty in breathing or swallowing
If you have these symptoms of an allergic reaction, stop taking SPIRIVA HANDIHALER and call your doctor right away or go to the nearest hospital emergency room.
- Sudden narrowing and blockage of the airways into the lungs (bronchospasm). Your breathing suddenly gets worse.
If you have these symptoms of bronchospasm, stop taking SPIRIVA HANDIHALER and call your doctor right away or go to the nearest hospital emergency room.
-
New or worsened increased pressure in the eyes (acute narrow-angle glaucoma). Symptoms of acute narrow-angle glaucoma may include:
- eye pain
- blurred vision
- seeing halos (visual halos) or colored images along with red eyes
Using only eye drops to treat these symptoms may not work. If you have these symptoms, stop taking SPIRIVA HANDIHALER and call your doctor right away.
- New or worsened urinary retention. Symptoms of blockage in your bladder and/or enlarged prostate may include: difficulty passing urine, painful urination.
If you have these symptoms of urinary retention, stop taking SPIRIVA HANDIHALER and call your doctor right away.
Other side effects with SPIRIVA HANDIHALER include:
- upper respiratory tract infection
- dry mouth
- sinus infection
- sore throat
- non-specific chest pain
- urinary tract infection
- indigestion
- runny nose
- constipation
- increased heart rate
- blurred vision
These are not all the possible side effects with SPIRIVA HANDIHALER. Tell your doctor if you have any side effect that bothers you or that does not go away.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
How do I store SPIRIVA HANDIHALER?
- Do not store SPIRIVA capsules in the HANDIHALER device.
- Store SPIRIVA capsules in the sealed blister package at room temperature 68°F to 77°F (20°C to 25°C).
- Keep SPIRIVA capsules away from heat and cold (do not freeze).
- Store SPIRIVA capsules in a dry place. Throw away any unused SPIRIVA capsules that have been open to air.
Ask your doctor or pharmacist if you have any questions about storing your SPIRIVA capsules.
Keep SPIRIVA HANDIHALER, SPIRIVA capsules, and all medicines out of the reach of children.
General information about SPIRIVA HANDIHALER
Medicines are sometimes prescribed for purposes other than those listed in Patient Information leaflets. Do not use SPIRIVA HANDIHALER for a purpose for which it has not been prescribed. Do not give SPIRIVA HANDIHALER to other people even if they have the same symptoms that you have. It may harm them.
For more information about SPIRIVA HANDIHALER, talk with your doctor. You can ask your doctor or pharmacist for information about SPIRIVA HANDIHALER that is written for health professionals.
For current prescribing information for SPIRIVA HANDIHALER, scan the code or for additional information you may also call Boehringer Ingelheim Pharmaceuticals, Inc., at 1-800-542-6257.

What are the ingredients in SPIRIVA HANDIHALER?
Active ingredient: tiotropium
Inactive ingredient: lactose monohydrate
What is COPD (Chronic Obstructive Pulmonary Disease)?
COPD is a serious lung disease that includes chronic bronchitis, emphysema, or both. Most COPD is caused by smoking. When you have COPD, your airways become narrow. So, air moves out of your lungs more slowly. This makes it hard to breathe.
This Patient Information has been approved by the U.S. Food and Drug Administration.
Distributed by:
Boehringer Ingelheim Pharmaceuticals, Inc.
Ridgefield, CT 06877 USA
Licensed from:
Boehringer Ingelheim International GmbH
SPIRIVA® and HANDIHALER® are registered trademarks of and are used under license from Boehringer Ingelheim International GmbH.
Copyright © 2021 Boehringer Ingelheim International GmbH
ALL RIGHTS RESERVED
Revised: November 2021
COL10494AK082021
Instructions for Use
SPIRIVA® (speh REE vah) HANDIHALER®
(tiotropium bromide inhalation powder)
|
| Do not swallow SPIRIVA capsules. |
Important Information about using your SPIRIVA HANDIHALER
- Do not swallow SPIRIVA capsules.
- SPIRIVA capsules should only be used with the HANDIHALER device and inhaled through your mouth (oral inhalation).
- Do not use your HANDIHALER device to take any other medicine.
First read the Patient Information, then read these Instructions for Use before you start to use SPIRIVA HANDIHALER and each time you refill your prescription. There may be new information.
Becoming familiar with your HANDIHALER device and SPIRIVA capsules:
Your SPIRIVA HANDIHALER comes with SPIRIVA capsules in blister packaging and a HANDIHALER device. Use the new HANDIHALER device provided with your medicine.
| The parts of your HANDIHALER device include:
(See Figure A)
|
|
Each SPIRIVA capsule is packaged in a blister. (See Figure B) |
|
Taking your full daily dose of medicine requires 4 main steps.
Step 1. Opening your HANDIHALER device:
|
After removing your HANDIHALER device from the pouch:
|
|
|
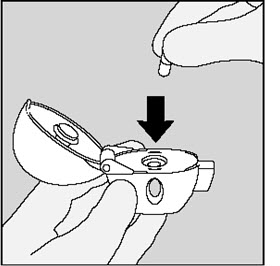
Step 2. Inserting the SPIRIVA capsule into your HANDIHALER device:
|
Each day, separate only 1 of the blisters from the blister card by tearing along the perforated line. (See Figure G) |
Remove the SPIRIVA capsule from the blister:
|
|
Place the SPIRIVA capsule in the center chamber of your HANDIHALER device. (See Figure I) |
|
Close the mouthpiece firmly against the gray base until you hear a click. Leave the dust cap (lid) open. (See Figure J) |
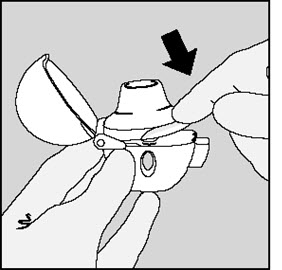
Step 3. Piercing the SPIRIVA capsule:
|
Step 4. Taking your full daily dose (2 inhalations from the same SPIRIVA capsule):
|
Breathe out completely in 1 breath, emptying your lungs of any air. (See Figure L) Important: Do not breathe into your HANDIHALER device. |
With your next breath, take your medicine:
|
|
To get your full daily dose, you must again, breathe out completely (See Figure N) and for a second time, breathe in (See Figure O) from the same SPIRIVA capsule. Important: Do not press the green piercing button again. |
| Remember: To get your full medicine dose each day, you must breathe in 2 times from the same SPIRIVA capsule. Make sure you breathe out completely each time before you breathe in from your HANDIHALER device. |
Caring for and storing your SPIRIVA HANDIHALER:
|
If you do not hear or feel the SPIRIVA capsule rattle as you breathe in your medicine:
| Do not press the green piercing button again. Hold your HANDIHALER device with the mouthpiece pointed up and tap your HANDIHALER device gently on a table. (See Figure Q) Check to see that the mouthpiece is completely closed. Breathe out completely before deeply breathing in again with the mouthpiece in your mouth. (See Figure O) If you still do not hear or feel the SPIRIVA capsule rattle after repeating the above steps:
|
Cleaning your HANDIHALER device:
Clean your HANDIHALER device as needed. (See Figure R)
|
Helpful Hints to help ensure that you are properly taking your full daily dose of SPIRIVA HANDIHALER:
|
For current prescribing information for SPIRIVA HANDIHALER, scan the code or for additional information you may also call Boehringer Ingelheim Pharmaceuticals, Inc., at 1-800-542-6257.

This Instructions for Use has been approved by the U.S. Food and Drug Administration.
Distributed by:
Boehringer Ingelheim Pharmaceuticals, Inc.
Ridgefield, CT 06877 USA
Licensed from:
Boehringer Ingelheim International GmbH
SPIRIVA® and HANDIHALER® are registered trademarks of and are used under license from Boehringer Ingelheim International GmbH.
Copyright © 2021 Boehringer Ingelheim International GmbH
ALL RIGHTS RESERVED
Revised: November 2021
COL10494AK082021
PRINCIPAL DISPLAY PANEL - 30 Capsule Blister Pack Carton
NDC 0597-0075-41
SPIRIVA® HandiHaler®
(tiotropium bromide inhalation powder)
18 mcg/Capsule*
DO NOT SWALLOW SPIRIVA CAPSULES.
For Use With HandiHaler Device Only.
FOR ORAL INHALATION ONLY
Rx only
30 capsules
3 blister cards. Each card contains 10 capsules.
SPIRIVA capsules should only
be used with the HandiHaler
device and inhaled through
your mouth (oral inhalation).
Boehringer
Ingelheim

INGREDIENTS AND APPEARANCE
| SPIRIVA
HANDIHALER
tiotropium bromide capsule |
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
| Labeler - Boehringer Ingelheim Pharmaceuticals, Inc. (603175944) |
| Registrant - Boehringer Ingelheim Pharmaceuticals, Inc. (603175944) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Boehringer Ingelheim Pharma GmbH and Co. KG | 551147440 | ANALYSIS(0597-0075) , API MANUFACTURE(0597-0075) , LABEL(0597-0075) , MANUFACTURE(0597-0075) , PACK(0597-0075) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| West-Ward Columbus Inc. | 058839929 | LABEL(0597-0075) , PACK(0597-0075) | |