Search by Drug Name or NDC
NDC 50804-0584-25 Stool Softener 100 mg/1 Details
Stool Softener 100 mg/1
Stool Softener is a ORAL CAPSULE in the HUMAN OTC DRUG category. It is labeled and distributed by Good Sense (Geiss, Destin & Dunn, Inc.). The primary component is DOCUSATE SODIUM.
MedlinePlus Drug Summary
Stool softeners are used on a short-term basis to relieve constipation by people who should avoid straining during bowel movements because of heart conditions, hemorrhoids, and other problems. They work by softening stools to make them easier to pass.
Related Packages: 50804-0584-25Last Updated: 11/30/2022
MedLinePlus Full Drug Details: Stool Softeners
Product Information
| NDC | 50804-0584 |
|---|---|
| Product ID | 50804-584_2b11970a-ccca-46e7-806e-6563d8996852 |
| Associated GPIs | |
| GCN Sequence Number | n/a |
| GCN Sequence Number Description | n/a |
| HIC3 | n/a |
| HIC3 Description | n/a |
| GCN | n/a |
| HICL Sequence Number | n/a |
| HICL Sequence Number Description | n/a |
| Brand/Generic | n/a |
| Proprietary Name | Stool Softener |
| Proprietary Name Suffix | n/a |
| Non-Proprietary Name | DOCUSATE SODIUM |
| Product Type | HUMAN OTC DRUG |
| Dosage Form | CAPSULE |
| Route | ORAL |
| Active Ingredient Strength | 100 |
| Active Ingredient Units | mg/1 |
| Substance Name | DOCUSATE SODIUM |
| Labeler Name | Good Sense (Geiss, Destin & Dunn, Inc.) |
| Pharmaceutical Class | n/a |
| DEA Schedule | n/a |
| Marketing Category | OTC MONOGRAPH NOT FINAL |
| Application Number | part334 |
| Listing Certified Through | n/a |
Package
Package Images
NDC 50804-0584-25 (50804058425)
| NDC Package Code | 50804-584-25 |
|---|---|
| Billing NDC | 50804058425 |
| Package | 1 BOTTLE, PLASTIC in 1 BOX (50804-584-25) / 25 CAPSULE in 1 BOTTLE, PLASTIC |
| Marketing Start Date | 2018-08-31 |
| NDC Exclude Flag | N |
| Pricing Information | N/A |
Standard Product Labeling (SPL)/Prescribing Information SPL 649b122f-7d12-459b-a281-d712010d1828 Details
Uses
Warnings
Ask a doctor before use if
- stomach pain
- nausea
- vomiting
- a sudden change in bowel habits that lasts more than 2 weeks
Directions
Other information
Inactive ingredients
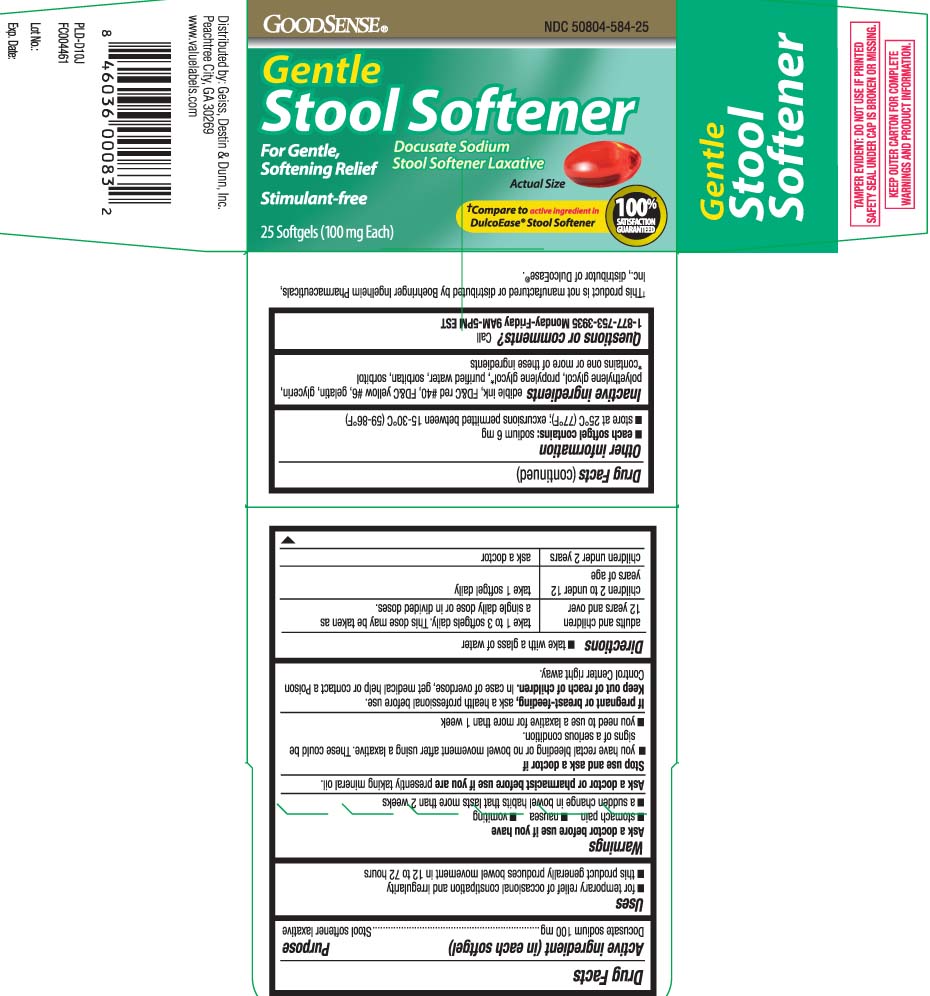
Principal Display Panel
Gentle Stool Softener
Docusate Sodium
Stool Softener Laxative
For Gentle, Softening Relief
Stimulant-Free
†Compare to active ingredient in DulcoEase® Stool Softener
†This product is not manufactured or distributed by Boehringer Ingelheim Pharmaceuticals, distributor of DulcoEase®.
TAMPER EVIDENT: DO NOT USE IF PRINTED SAFETY SEAL UNDER CAP IS BROKEN OR MISSING.
KEEP OUTER CARTON FOR COMPLETE WARNINGS AND PRODUCT INFORMATION.
Distributed by: Geiss, Destin & Dunn, Inc.
Peachtree City, GA 30269
INGREDIENTS AND APPEARANCE
| STOOL SOFTENER
docusate sodium capsule |
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
| Labeler - Good Sense (Geiss, Destin & Dunn, Inc.) (076059836) |