Search by Drug Name or NDC
NDC 51206-0302-11 Opalescence Whitening 1.1 mg/g Details
Opalescence Whitening 1.1 mg/g
Opalescence Whitening is a DENTAL GEL, DENTIFRICE in the HUMAN OTC DRUG category. It is labeled and distributed by Ultradent Products, Inc. The primary component is SODIUM FLUORIDE.
MedlinePlus Drug Summary
Fluoride is used to prevent tooth decay. It is taken up by teeth and helps to strengthen teeth, resist acid, and block the cavity-forming action of bacteria. Fluoride usually is prescribed for children and adults whose homes have water that is not fluoridated (already has fluoride added). This medication is sometimes prescribed for other uses; ask your doctor or pharmacist for more information.
Related Packages: 51206-0302-11Last Updated: 11/30/2022
MedLinePlus Full Drug Details: Fluoride
Product Information
| NDC | 51206-0302 |
|---|---|
| Product ID | 51206-302_0eeb825c-4021-469e-bb64-b6fb3126090a |
| Associated GPIs | |
| GCN Sequence Number | n/a |
| GCN Sequence Number Description | n/a |
| HIC3 | n/a |
| HIC3 Description | n/a |
| GCN | n/a |
| HICL Sequence Number | n/a |
| HICL Sequence Number Description | n/a |
| Brand/Generic | n/a |
| Proprietary Name | Opalescence Whitening |
| Proprietary Name Suffix | n/a |
| Non-Proprietary Name | Sodium Fluoride |
| Product Type | HUMAN OTC DRUG |
| Dosage Form | GEL, DENTIFRICE |
| Route | DENTAL |
| Active Ingredient Strength | 1.1 |
| Active Ingredient Units | mg/g |
| Substance Name | SODIUM FLUORIDE |
| Labeler Name | Ultradent Products, Inc |
| Pharmaceutical Class | n/a |
| DEA Schedule | n/a |
| Marketing Category | OTC MONOGRAPH FINAL |
| Application Number | part355 |
| Listing Certified Through | 2024-12-31 |
Package
Package Images
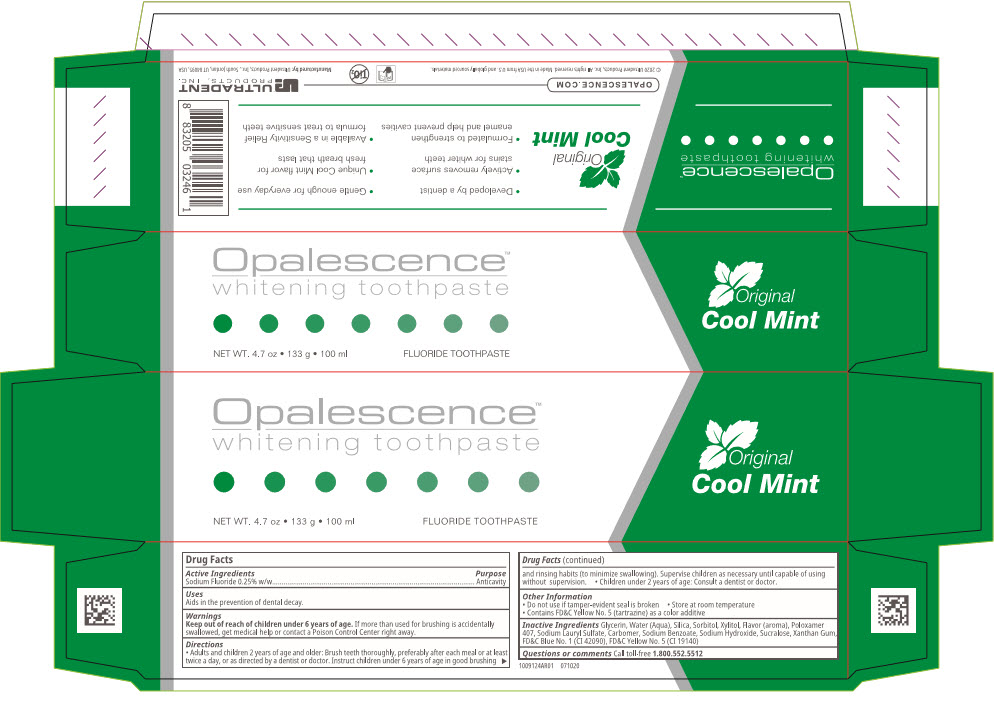
NDC 51206-0302-11 (51206030211)
| NDC Package Code | 51206-302-11 |
|---|---|
| Billing NDC | 51206030211 |
| Package | 6 BOX in 1 PACKAGE, COMBINATION (51206-302-11) / 1 TUBE in 1 BOX (51206-302-06) / 133 g in 1 TUBE |
| Marketing Start Date | 2020-08-24 |
| NDC Exclude Flag | N |
| Pricing Information | N/A |
Standard Product Labeling (SPL)/Prescribing Information SPL d07f842e-b6f1-4ae1-b576-f542ecd02dd4 Details
Warnings
Directions
- Adults and children 2 years of age and older: Brush teeth thoroughly, preferably after each meal or at least twice a day, or as directed by a dentist or doctor. Instruct children under 6 years of age in good brushing and rinsing habits (to minimize swallowing). Supervise children as necessary until capable of using without supervision.
- Children under 2 years of age: Consult a dentist or doctor.
Other Information
Inactive Ingredients
PRINCIPAL DISPLAY PANEL - 100 ml Tube Box
INGREDIENTS AND APPEARANCE
| OPALESCENCE WHITENING
sodium fluoride gel, dentifrice |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Labeler - Ultradent Products, Inc (013369913) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| OraTech, LLC | 827869285 | MANUFACTURE(51206-302) , ANALYSIS(51206-302) , LABEL(51206-302) , PACK(51206-302) | |
Revised: 2/2022
Document Id: 0eeb825c-4021-469e-bb64-b6fb3126090a
Set id: d07f842e-b6f1-4ae1-b576-f542ecd02dd4
Version: 5
Effective Time: 20220202