Search by Drug Name or NDC
NDC 51407-0085-01 Clozapine 25 mg/1 Details
Clozapine 25 mg/1
Clozapine is a ORAL TABLET in the HUMAN PRESCRIPTION DRUG category. It is labeled and distributed by Golden State Medical Supply, Inc.. The primary component is CLOZAPINE.
MedlinePlus Drug Summary
Clozapine is used to treat the symptoms of schizophrenia (a mental illness that causes disturbed or unusual thinking, loss of interest in life, and strong or inappropriate emotions) in people who have not been helped by other medications or who have tried to kill themselves and are likely to try to kill or harm themselves again. Clozapine is in a class of medications called atypical antipsychotics. It works by changing the activity of certain natural substances in the brain.
Related Packages: 51407-0085-01Last Updated: 11/30/2022
MedLinePlus Full Drug Details: Clozapine
Product Information
| NDC | 51407-0085 |
|---|---|
| Product ID | 51407-085_cc23653d-da2f-49a0-e053-2995a90ae72b |
| Associated GPIs | 59152020000320 |
| GCN Sequence Number | 013648 |
| GCN Sequence Number Description | clozapine TABLET 25 MG ORAL |
| HIC3 | H7T |
| HIC3 Description | ANTIPSYCHOTIC,ATYPICAL,DOPAMINE,SEROTONIN ANTAGNST |
| GCN | 18141 |
| HICL Sequence Number | 004834 |
| HICL Sequence Number Description | CLOZAPINE |
| Brand/Generic | Generic |
| Proprietary Name | Clozapine |
| Proprietary Name Suffix | n/a |
| Non-Proprietary Name | Clozapine |
| Product Type | HUMAN PRESCRIPTION DRUG |
| Dosage Form | TABLET |
| Route | ORAL |
| Active Ingredient Strength | 25 |
| Active Ingredient Units | mg/1 |
| Substance Name | CLOZAPINE |
| Labeler Name | Golden State Medical Supply, Inc. |
| Pharmaceutical Class | Atypical Antipsychotic [EPC] |
| DEA Schedule | n/a |
| Marketing Category | ANDA |
| Application Number | ANDA203807 |
| Listing Certified Through | 2022-12-31 |
Package
Package Images
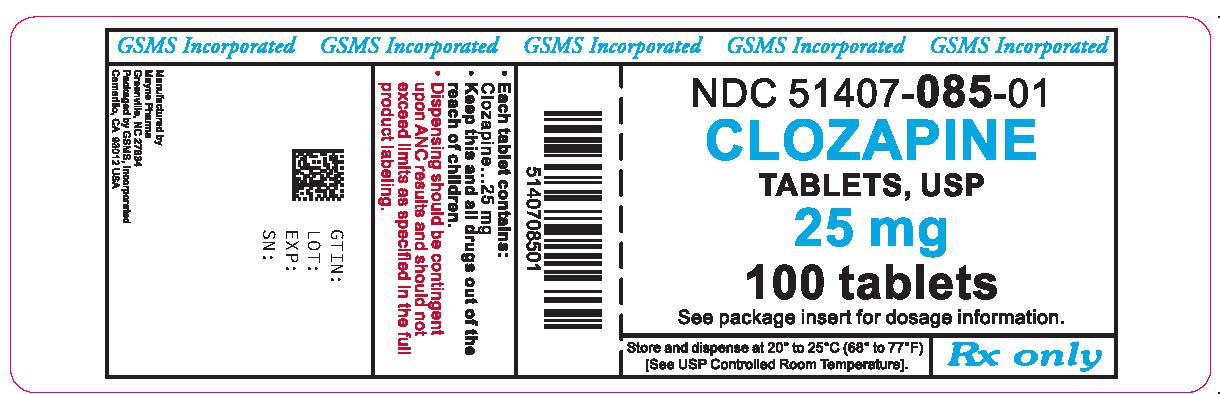

NDC 51407-0085-01 (51407008501)
| NDC Package Code | 51407-085-01 |
|---|---|
| Billing NDC | 51407008501 |
| Package | 100 TABLET in 1 BOTTLE (51407-085-01) |
| Marketing Start Date | 2021-01-21 |
| NDC Exclude Flag | N |
| Pricing Information | N/A |
Standard Product Labeling (SPL)/Prescribing Information SPL baeb67d1-c4a3-533d-e053-2a95a90a51c6 Details
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use CLOZAPINE TABLETS safely and effectively. See full prescribing information for CLOZAPINE TABLETS.
CLOZAPINE tablets, for oral use
Initial U.S. Approval: 1989
WARNING: SEVERE NEUTROPENIA; ORTHOSTATIC HYPOTENSION, BRADYCARDIA, AND SYNCOPE; SEIZURE; MYOCARDITIS AND CARDIOMYOPATHY; INCREASED MORTALITY IN ELDERLY PATIENTS WITH DEMENTIA-RELATED PSYCHOSIS
See full prescribing information for complete boxed warning
- Severe Neutropenia: Clozapine tablets can cause severe neutropenia, which can lead to serious and fatal infections. Patients initiating and continuing treatment with Clozapine tablets must have a baseline blood absolute neutrophil count (ANC) measured before treatment initiation and regular ANC monitoring during treatment ( 2.1, 5.1).
- Clozapine tablets are available only through a restricted program called the Clozapine REMS ( 5.2).
- Orthostatic Hypotension, Bradycardia, and Syncope: Risk is dose-related. Starting dose is 12.5 mg. Titrate gradually and use divided dosages ( 2.2, 2.5, 5.3).
- Seizure: Risk is dose-related. Titrate gradually and use divided doses. Use with caution in patients with history of seizure or risk factors for seizure ( 2.2, 5.5).
- Myocarditis, Cardiomyopathy, and Mitral Valve Incompetence: Can be fatal. Discontinue and obtain cardiac evaluation if findings suggest these cardiac reactions ( 5.6).
- Increased Mortality in Elderly Patients with Dementia-Related Psychosis: Clozapine tablets are not approved for this condition ( 5.7).
INDICATIONS AND USAGE
Clozapine tablets are an atypical antipsychotic indicated for:
DOSAGE AND ADMINISTRATION
- Starting Dose: 12.5 mg once daily or twice daily ( 2.2).
- Use cautious titration and divided dosage schedule ( 2.2, 5.3).
- Titration: increase the total daily dosage in increments of 25 mg to 50 mg per day, if well-tolerated ( 2.2).
- Target dose: 300 mg to 450 mg per day, in divided doses, by the end of 2 weeks ( 2.2).
- Subsequent increases: increase in increments of 100 mg or less, once or twice weekly ( 2.2).
- Maximum daily dose: 900 mg ( 2.2).
DOSAGE FORMS AND STRENGTHS
25 mg, 50 mg, 100 mg and 200 mg tablets with a facilitated score on one side ( 3)
CONTRAINDICATIONS
- Known serious hypersensitivity to clozapine or any other component of clozapine tablets ( 4).
WARNINGS AND PRECAUTIONS
- Gastrointestinal Hypomotility with Severe Complications: Severe gastrointestinal adverse reactions have occurred with the use of clozapine tablets. If constipation is identified close monitoring and prompt treatment is advised. ( 5.8)
- Eosinophilia: Assess for organ involvement (e.g., myocarditis, pancreatitis, hepatitis, colitis, nephritis). Discontinue if these occur. ( 5.9).
- QT Interval Prolongation: Can be fatal. Consider additional risk factors for prolonged QT interval (disorders and drugs) ( 5.10).
-
Metabolic Changes: Atypical antipsychotic drugs have been associated with metabolic changes that may increase cardiovascular/ cerebrovascular risk.
These metabolic changes include:- Hyperglycemia and Diabetes Mellitus: Monitor for symptoms of hyperglycemia including polydipsia, polyuria, polyphagia, and weakness. Monitor glucose regularly in patients with diabetes or at risk for diabetes. ( 5.11).
- Dyslipidemia: Undesirable alterations in lipids have occurred in patients treated with atypical antipsychotics. ( 5.11).
- Weight Gain: Significant weight gain has occurred. Monitor weight gain. ( 5.11).
- Neuroleptic Malignant Syndrome (NMS): Immediately discontinue and monitor closely. Assess for co-morbid conditions. ( 5.12).
- Hepatotoxicity: Can be fatal. Monitor for hepatotoxicity. Discontinue treatment if hepatitis or transaminase elevations combined with other symptoms occur ( 5.13).
- Fever: Evaluate for infection and for neutropenia, NMS ( 5.14).
- Pulmonary Embolism (PE): Consider PE if respiratory distress, chest, pain, or deep-vein thrombosis occur ( 5.15).
- Anticholinergic Toxicity: When possible, avoid use with other anticholinergic drugs and use with caution in patients with a current diagnosis or prior history of constipation, urinary retention, clinically significant prostatic hypertrophy, or other conditions in which anticholinergic effects can lead to significant adverse reactions. ( 5.16, 7.1).
- Interference with Cognitive and Motor Performance: Advise caution when operating machinery, including automobiles ( 5.17).
ADVERSE REACTIONS
Most common adverse reactions (≥ 5%) were: CNS reactions (sedation, dizziness/vertigo, headache, and tremor); cardiovascular reactions (tachycardia, hypotension, and syncope); autonomic nervous system reactions (hypersalivation, sweating, dry mouth, and visual disturbances); gastrointestinal reactions (constipation and nausea); and fever ( 6.1).
To report SUSPECTED ADVERSE REACTIONS, contact Mayne Pharma at 1-844-825-8500 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch
DRUG INTERACTIONS
- Concomitant use of Strong CYP1A2 Inhibitors: Reduce clozapine dose to one-third when coadministered with strong CYP1A2 inhibitors (e.g. fluvoxamine, ciprofloxacin, enoxacin) ( 2.6, 7.1).
- Concomitant use of Strong CYP3A4 Inducers is not recommended. ( 2.6, 7.1).
- Discontinuation of CYP1A2 or CYP3A4 Inducers: Consider reducing clozapine dose when CYP1A2 inducers (e.g., tobacco smoke) or CYP3A4 inducers (e.g., carbamazepine) are discontinued ( 2.6, 7.1).
- Anticholinergic drugs: Concomitant use may increase the risk for anticholinergic toxicity. ( 5.8, 5.16, 7.1)
USE IN SPECIFIC POPULATIONS
- Nursing Mothers: Discontinue drug or discontinue nursing, taking into consideration importance of drug to mother ( 8.3).
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 5/2021
Table Of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
WARNING: SEVERE NEUTROPENIA; ORTHOSTATIC HYPOTENSION, BRADYCARDIA, AND SYNCOPE; SEIZURE; MYOCARDITIS AND CARDIOMYOPATHY; INCREASED MORTALITY IN ELDERLY PATIENTS WITH DEMENTIA-RELATED PSYCHOSIS
1 INDICATIONS AND USAGE
1.1 Treatment-Resistant Schizophrenia
1.2 Reduction in the Risk of Recurrent Suicidal Behavior in Schizophrenia or Schizoaffective Disorder
2 DOSAGE AND ADMINISTRATION
2.1 Required Laboratory Testing Prior to Initiation and During Therapy
2.2 Dosing Information
2.3 Maintenance Treatment
2.4 Discontinuation of Treatment
2.5 Re-Initiation of Treatment
2.6 Dosage Adjustments with Concomitant use of CYP1A2, CYP2D6, CYP3A4 Inhibitors or CYP1A2, CYP3A4 Inducers
2.7 Renal or Hepatic Impairment, or CYP2D6 Poor Metabolizers
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Severe Neutropenia
5.2 Clozapine REMS Program
5.3 Orthostatic Hypotension, Bradycardia, and Syncope
5.4 Falls
5.5 Seizures
5.6 Myocarditis, Cardiomyopathy and Mitral Valve Incompetence
5.7 Increased Mortality in Elderly Patients with Dementia-Related Psychosis
5.8 Gastrointestinal Hypomotility with Severe Complications
5.9 Eosinophilia
5.10 QT Interval Prolongation
5.11 Metabolic Changes
5.12 Neuroleptic Malignant Syndrome
5.13 Hepatotoxicity
5.14 Fever
5.15 Pulmonary Embolism
5.16 Anticholinergic Toxicity
5.17 Interference with Cognitive and Motor Performance
5.18 Tardive Dyskinesia
5.19 Cerebrovascular Adverse Reactions
5.20 Recurrence of Psychosis and Cholinergic Rebound after Abrupt Discontinuation of Clozapine
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Postmarketing Experience
7 DRUG INTERACTIONS
7.1 Potential for Other Drugs to Affect Clozapine
7.2 Potential for Clozapine to Affect Other Drugs
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.3 Nursing Mothers
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Patients with Renal or Hepatic Impairment
8.7 CYP2D6 Poor Metabolizers
8.8 Hospice Patients
10 OVERDOSAGE
10.1 Overdosage Experience
10.2 Management of Overdosage
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
14.1 Treatment-Resistant Schizophrenia
14.2 Recurrent Suicidal Behavior in Schizophrenia or Schizoaffective Disorder
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
16.2 Storage and Handling
17 PATIENT COUNSELING INFORMATION
- *
- Sections or subsections omitted from the full prescribing information are not listed.
WARNING
Severe Neutropenia
Clozapine tablet treatment has caused severe neutropenia, defined as an absolute neutrophil count (ANC) less than 500/µL. Severe neutropenia can lead to serious infection and death. Prior to initiating treatment with Clozapine tablets a baseline ANC must be at least 1500/ µL for the general population; and must be at least 1000/µL for patients with documented Benign Ethnic Neutropenia (BEN). During treatment, patients must have regular ANC monitoring. Advise patients to immediately report symptoms consistent with severe neutropenia or infection (e.g., fever, weakness, lethargy, or sore throat) [see Dosage and Administration (2.1) and Warnings and Precautions (5.1)].
Because of the risk of severe neutropenia, Clozapine tablets are available only through a restricted program under a Risk Evaluation Mitigation Strategy (REMS) called the Clozapine REMS Program. [see Warnings and Precautions (5.2)].
Orthostatic Hypotension, Bradycardia, Syncope
Orthostatic hypotension, bradycardia, syncope, and cardiac arrest have occurred with Clozapine tablet treatment. The risk is highest during the initial titration period, particularly with rapid dose escalation. These reactions can occur with the first dose, with doses as low as 12.5 mg per day. Initiate treatment at 12.5 mg once or twice daily; titrate s lowly; and use divided dosages. Use Clozapine tablets cautiously in patients with cardiovascular or cerebrovascular disease or conditions predisposing to hypotension (e.g., dehydration, use of antihypertensive medications) [see Dosage and Administration (2.2, 2.5) and Warnings and Precautions (5.3)].
Seizures
Seizures have occurred with Clozapine tablet treatment. The risk is dose-related. Initiate treatment at 12.5 mg, titrate gradually, and use divided dosing. Use caution when administering Clozapine tablets to patients with a history of seizures or other predisposing risk factors for seizure (CNS pathology, medications that lower the seizure threshold, alcohol abuse). Caution patients about engaging in any activity where sudden loss of consciousness could cause serious risk to themselves or others [see Dosage and Administration (2.2), Warnings and Precautions (5.5)].
Myocarditis, Cardiomyopathy, and Mitral Valve Incompetence
Fatal myocarditis and cardiomyopathy have occurred with Clozapine tablet treatment. Discontinue Clozapine tablets and obtain a cardiac evaluation upon suspicion of these reactions. Generally, patients with Clozapine tablet-related myocarditis or cardiomyopathy should not be rechallenged with Clozapine tablets. Consider the possibility of myocarditis or cardiomyopathy if chest pain, tachycardia, palpitations, dyspnea, fever, flu-like symptoms, hypotension, or ECG changes occur [see Warnings and Precautions (5.6)].
Increased Mortality in Elderly Patients with Dementia-Related Psychosis
Elderly patients with dementia-related psychosis treated with antipsychotic drugs are at an increased risk of death. Clozapine tablets are not approved for use in patients with dementia-related psychosis [see Warnings and Precautions (5.7)].
1 INDICATIONS AND USAGE
1.1 Treatment-Resistant Schizophrenia
Clozapine tablets are indicated for the treatment of severely ill patients with schizophrenia who fail to respond adequately to standard antipsychotic treatment. Because of the risks of severe neutropenia and of seizure associated with its use, clozapine tablets should be used only in patients who have failed to respond adequately to standard antipsychotic treatment [see Warnings and Precautions (5.1, 5.5)].
The effectiveness of clozapine tablets in treatment-resistant schizophrenia was demonstrated in a 6-week, randomized, double-blind, active-controlled study comparing clozapine and chlorpromazine in patients who had failed other antipsychotics [see Clinical Studies (14.1)].
1.2 Reduction in the Risk of Recurrent Suicidal Behavior in Schizophrenia or Schizoaffective Disorder
Clozapine tablets are indicated for reducing the risk of recurrent suicidal behavior in patients with schizophrenia or schizoaffective disorder who are judged to be at chronic risk for re-experiencing suicidal behavior, based on history and recent clinical state. Suicidal behavior refers to actions by a patient that put him/herself at risk for death.
The effectiveness of clozapine tablets in reducing the risk of recurrent suicidal behavior was demonstrated over a two-year treatment period in the InterSePT™ trial [see Clinical Studies (14.2)] .
2 DOSAGE AND ADMINISTRATION
2.1 Required Laboratory Testing Prior to Initiation and During Therapy
Prior to initiating treatment with clozapine tablets, a baseline ANC must be obtained. The baseline ANC must be at least 1500/µL for the general population, and at least 1000/µL for patients with documented Benign Ethnic Neutropenia (BEN). To continue treatment, the ANC must be monitored regularly [see Warnings and Precautions (5.1)].
2.2 Dosing Information
The starting dose is 12.5 mg once daily or twice daily. The total daily dose can be increased in increments of 25 mg to 50 mg per day, if well-tolerated, to achieve a target dose of 300 mg to 450 mg per day (administered in divided doses) by the end of 2 weeks. Subsequently, the dose can be increased once weekly or twice weekly, in increments of up to 100 mg. The maximum dose is 900 mg per day. To minimize the risk of orthostatic hypotension, bradycardia, and syncope, it is necessary to use this low starting dose, gradual titration schedule, and divided dosages [see Warnings and Precautions (5.3)].
Clozapine tablets can be taken with or without food [see Pharmacokinetics (12.3)].
2.3 Maintenance Treatment
Generally, patients responding to clozapine tablets should continue maintenance treatment on their effective dose beyond the acute episode.
2.4 Discontinuation of Treatment
Method of treatment discontinuation will vary depending on the patient's last ANC:
- See Tables 2 or 3 for appropriate ANC monitoring based on the level of neutropenia if abrupt treatment discontinuation is necessary because of moderate to severe neutropenia.
- Reduce the dose gradually over a period of 1 to 2 weeks if termination of clozapine tablets therapy is planned and there is no evidence of moderate to severe neutropenia.
- For abrupt clozapine discontinuation for a reason unrelated to neutropenia, continuation of the existing ANC monitoring is recommended for general population patients until their ANC is ≥1500/µL and for BEN patients until their ANC is ≥1000/µL or above their baseline.
- Additional ANC monitoring is required for any patient reporting onset of fever (temperature of 38.5°C or 101.3°F, or greater) during the 2 weeks after discontinuation [see Warnings and Precautions (5.1)].
- Monitor all patients carefully for the recurrence of psychotic symptoms and symptoms related to cholinergic rebound such as profuse sweating, headache, nausea, vomiting, and diarrhea.
2.5 Re-Initiation of Treatment
When restarting clozapine tablets in patients who have discontinued clozapine tablets (i.e., 2 days or more since the last dose), re-initiate with 12.5 mg once daily or twice daily. This is necessary to minimize the risk of hypotension, bradycardia, and syncope [see Warnings and Precautions (5.3)]. If that dose is well-tolerated, the dose may be increased to the previously therapeutic dose more quickly than recommended for initial treatment.
2.6 Dosage Adjustments with Concomitant use of CYP1A2, CYP2D6, CYP3A4 Inhibitors or CYP1A2, CYP3A4 Inducers
Dose adjustments may be necessary in patients with concomitant use of: strong CYP1A2 inhibitors (e.g., fluvoxamine, ciprofloxacin, or enoxacin); moderate or weak CYP1A2 inhibitors (e.g., oral contraceptives, or caffeine); CYP2D6 or CYP3A4 inhibitors (e.g., cimetidine, escitalopram, erythromycin, paroxetine, bupropion, fluoxetine, quinidine, duloxetine, terbinafine, or sertraline); CYP3A4 inducers (e.g., phenytoin, carbamazepine, St. John's wort, and rifampin); or CYP1A2 inducers (e.g., tobacco smoking) (Table 1) [see Drug Interactions (7)].
| Co- medications | Scenarios | ||
|---|---|---|---|
| Initiating clozapine tablets while taking a co-medication | Adding a co- medication while taking clozapine tablets | Discontinuing a co- medication while continuing clozapine tablets | |
| Strong CYP1A2 Inhibitors | Use one-third of the clozapine tablet dose. | Increase clozapine tablet dose based on clinical response. | |
| Moderate or Weak CYP1A2 Inhibitors | Monitor for adverse reactions. Consider reducing the clozapine tablet dose if necessary. | Monitor for lack of effectiveness.
Consider increasing clozapine tablet dose if necessary. |
|
| CYP2D6 or CYP3A4 Inhibitors | |||
| Strong CYP3A4 Inducers | Concomitant use is not recommended. However, if the inducer is necessary, it may be necessary to increase the clozapine tablet dose. Monitor for decreased effectiveness. | Reduce clozapine tablet dose based on clinical response. | |
| Moderate or weak CYP1A2 or CYP3A4 Inducers | Monitor for decreased effectiveness. Consider increasing the clozapine tablet dose if necessary. | Monitor for adverse reactions. Consider reducing the clozapine tablet dose if necessary. | |
2.7 Renal or Hepatic Impairment, or CYP2D6 Poor Metabolizers
It may be necessary to reduce the clozapine tablet dose in patients with significant renal or hepatic impairment, or in CYP2D6 poor metabolizers [see Use in Specific Populations (8.6, 8.7)].
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
Clozapine tablets are contraindicated in patients with a history of serious hypersensitivity to clozapine (e.g., photosensitivity, vasculitis, erythema multiforme, or Stevens-Johnson Syndrome) or any other component of clozapine tablets [see Adverse Reactions (6.2)] .
5 WARNINGS AND PRECAUTIONS
5.1 Severe Neutropenia
Background
Clozapine can cause neutropenia (a low absolute neutrophil count (ANC)), defined as a reduction below pre-treatment normal levels of blood neutrophils. The ANC is usually available as a component of the complete blood count (CBC), including differential, and is more relevant to drug-induced neutropenia than is the white blood cell (WBC) count. The ANC may also be calculated using the following formula: ANC equals the Total WBC count multiplied by the total percentage of neutrophils obtained from the differential (neutrophil "segs" plus neutrophil "bands"). Other granulocytes (basophils and eosinophils) contribute minimally to neutropenia and their measurement is not necessary [see Adverse Reactions (6.2)] . Neutropenia may be mild, moderate, or severe (see Tables 2 and 3). To improve and standardize understanding, "severe neutropenia" replaces the previous terms severe leukopenia, severe granulocytopenia, or agranulocytosis.
Severe neutropenia, ANC less than ( <) 500/µL, occurs in a small percentage of patients taking clozapine and is associated with an increase in the risk of serious and potentially fatal infections. Risk of neutropenia appears greatest during the first 18 weeks on treatment and then declines. The mechanism by which clozapine causes neutropenia is unknown and is not dose-dependent.
Two separate management algorithms are provided below, the first for patients in the general population, and the second for patients identified to have baseline neutropenia.
Clozapine Treatment and Monitoring in the General Patient Population (see Table 2)
Obtain a CBC, including the ANC value, prior to initiating treatment with clozapine to ensure the presence of a normal baseline neutrophil count (equal to or greater than 1500/µL) and to permit later comparisons. Patients in the general population with an ANC equal to or greater than (≥) 1500/µL are considered within normal range (Table 2) and are eligible to initiate treatment. Weekly ANC monitoring is required for all patients during the first 6 months of treatment. If a patient's ANC remains equal to or greater than 1500/µL for the first 6 months of treatment, monitoring frequency may be reduced to every 2 weeks for the next 6 months. If the ANC remains equal to or greater than 1500/µL for the second 6 months of continuous therapy, ANC monitoring frequency may be reduced to once every 4 weeks thereafter.
| ANC Level | Clozapine
Treatment Recommendations | ANC Monitoring |
|---|---|---|
| Normal range
(≥1500/ µL ) |
|
|
|
|
|
| Mild Neutropenia
(1000 to 1499/µL) * |
|
|
| Moderate Neutropenia
(500 to 999/µL) * |
|
|
| Severe Neutropenia
(less than 500/µL) * |
|
|
Clozapine Treatment and Monitoring in Patients with Benign Ethnic Neutropenia (see Table 3)
Benign ethnic neutropenia (BEN) is a condition observed in certain ethnic groups whose average ANC values are lower than "standard" laboratory ranges for neutrophils. It is most commonly observed in individuals of African descent (approximate prevalence of 25 - 50%), some Middle Eastern ethnic groups, and in other non-Caucasian ethnic groups with darker skin. BEN is more common in men. Patients with BEN have normal hematopoietic stem-cell number and myeloid maturation, are healthy, and do not suffer from repeated or severe infections. They are not at increased risk for developing clozapine-induced neutropenia. Additional evaluation may be needed to determine if baseline neutropenia is due to BEN. Consider hematology consultation before initiating or during clozapine treatment as necessary.
Patients with BEN require a different ANC algorithm for clozapine management due to their lower baseline ANC levels. Table 3 provides guidelines for managing clozapine treatment and ANC monitoring in patients with BEN.
| ANC Level | Treatment Recommendations | ANC Monitoring |
|---|---|---|
| Normal BEN Range (Established ANC baseline ≥1000/µL) |
|
|
|
|
|
| BEN Neutropenia 500 to 999/µL * |
|
|
| BEN Severe Neutropenia less than 500/µL * |
|
|
General Guidelines for Management of All Patients with Fever or with Neutropenia
- Fever: Interrupt clozapine as a precautionary measure in any patient who develops fever, defined as a temperature of 38.5°C [101.3°F] or greater, and obtain an ANC level. Fever is often the first sign of neutropenic infection.
- ANC less than 1000/µL: If fever occurs in any patient with an ANC less than 1000/µL, initiate appropriate workup and treatment for infection and refer to Tables 2 or 3 for management.
- Consider hematology consultation.
- See Neuroleptic Malignant Syndrome [NMS] and Fever under WARNINGS and PRECAUTIONS (5) and Instructions for Patients, under PATIENT COUNSELING INFORMATION (17).
Rechallenge after an ANC less than 500/µL (severe neutropenia)
For some patients who experience severe clozapine-related neutropenia, the risk of serious psychiatric illness from discontinuing clozapine treatment may be greater than the risk of rechallenge (e.g. patients with severe schizophrenic illness who have no treatment options other than clozapine). A hematology consultation may be useful in deciding to rechallenge a patient. In general, however, do not rechallenge patients who develop severe neutropenia with clozapine or a clozapine product.
If a patient will be rechallenged, the clinician should consider thresholds provided in Tables 2 and 3, the patient's medical and psychiatric history, a discussion with the patient and his/her caregiver about the benefits and risks of clozapine rechallenge, and the severity and characteristics of the neutropenic episode.
Using Clozapine Tablets with Other Drugs Associated with Neutropenia
It is unclear if concurrent use of other drugs known to cause neutropenia increases the risk or severity of clozapine-induced neutropenia. There is no strong scientific rationale to avoid clozapine treatment in patients concurrently treated with these drugs. If clozapine is used concurrently with an agent known to cause neutropenia (e.g., some chemotherapeutic agents), consider monitoring patients more closely than the treatment the guidelines provided in Tables 2 and 3. Consult with the treating oncologist in patients receiving concomitant chemotherapy.
5.2 Clozapine REMS Program
Clozapine is only available through a restricted program under a REMS called the Clozapine REMS Program because of the risk of severe neutropenia.
Notable requirements of the Clozapine REMS Program include:
- Healthcare professionals who prescribe clozapine must be certified with the program by enrolling and completing training
- Patients who receive clozapine must be enrolled in the program and comply with the ANC testing and monitoring requirements
- Pharmacies dispensing clozapine must be certified with the program by enrolling and completing training and must only dispense to patients who are eligible to receive clozapine.
Further information is available at www.clozapinerems.com or 1-844-267-8678.
5.3 Orthostatic Hypotension, Bradycardia, and Syncope
Hypotension, bradycardia, syncope, and cardiac arrest have occurred with clozapine treatment. The risk is highest during the initial titration period, particularly with rapid dose-escalation. These reactions can occur with the first dose, at doses as low as 12.5 mg. These reactions can be fatal. The syndrome is consistent with neurally mediated reflex bradycardia (NMRB).
Treatment must begin at a maximum dose of 12.5 mg once daily or twice daily. The total daily dose can be increased in increments of 25 mg to 50 mg per day, if well-tolerated, to a target dose of 300 mg to 450 mg per day (administered in divided doses) by the end of 2 weeks. Subsequently, the dose can be increased weekly or twice weekly, in increments of up to 100 mg. The maximum dose is 900 mg per day. Use cautious titration and a divided dosage schedule to minimize the risk of serious cardiovascular reactions [see Dosage and Administration (2.2)] . Consider reducing the dose if hypotension occurs.
When restarting patients who have had even a brief interval off clozapine (i.e., 2 days or more since the last dose), re-initiate treatment at 12.5 mg once daily or twice daily [see Dosage and Administration (2.5)].
Use clozapine cautiously in patients with cardiovascular disease (history of myocardial infarction or ischemia, heart failure, or conduction abnormalities), cerebrovascular disease, and conditions which would predispose patients to hypotension (e.g., concomitant use of antihypertensives, dehydration and hypovolemia).
5.4 Falls
Clozapine may cause somnolence, postural hypotension, motor and sensory instability, which may lead to falls and, consequently, fractures or other injuries. For patients with diseases, conditions, or medications that could exacerbate these effects, complete fall risk assessments when initiating antipsychotic treatment and recurrently for patients on long term antipsychotic therapy.
5.5 Seizures
Seizure has been estimated to occur in association with clozapine use at a cumulative incidence at one year of approximately 5%, based on the occurrence of one or more seizures in 61 of 1743 patients exposed to clozapine during its clinical testing prior to domestic marketing (i.e., a crude rate of 3.5%). The risk of seizure is dose-related. Initiate treatment with a low dose (12.5 mg), titrate slowly, and use divided dosing.
Use caution when administering clozapine to patients with a history of seizures or other predisposing risk factors for seizure (e.g., head trauma or other CNS pathology, use of medications that lower the seizure threshold, or alcohol abuse). Because of the substantial risk of seizure associated with clozapine use, caution patients about engaging in any activity where sudden loss of consciousness could cause serious risk to themselves or others (e.g., driving an automobile, operating complex machinery, swimming, climbing).
5.6 Myocarditis, Cardiomyopathy and Mitral Valve Incompetence
Myocarditis and cardiomyopathy have occurred with the use of clozapine. These reactions can be fatal. Discontinue clozapine and obtain a cardiac evaluation upon suspicion of myocarditis or cardiomyopathy. Generally, patients with a history of clozapine-associated myocarditis or cardiomyopathy should not be rechallenged with clozapine. However, if the benefit of clozapine treatment is judged to outweigh the potential risks of recurrent myocarditis or cardiomyopathy, the clinician may consider rechallenge with clozapine in consultation with a cardiologist, after a complete cardiac evaluation, and under close monitoring.
Consider the possibility of myocarditis or cardiomyopathy in patients receiving clozapine who present with chest pain, dyspnea, persistent tachycardia at rest, palpitations, fever, flu-like symptoms, hypotension, other signs or symptoms of heart failure, or electrocardiographic findings (low voltages, ST-T abnormalities, arrhythmias, right axis deviation, and poor R wave progression). Myocarditis most frequently presents within the first two months of clozapine treatment. Symptoms of cardiomyopathy generally occur later than clozapine-associated myocarditis and usually after 8 weeks of treatment. However, myocarditis and cardiomyopathy can occur at any period during treatment with clozapine. It is common for nonspecific flu-like symptoms such as malaise, myalgia, pleuritic chest pain, and low-grade fevers to precede more overt signs of heart failure. Typical laboratory findings include elevated troponin I or T, elevated creatinine kinase-MB, peripheral eosinophilia, and elevated C-reactive protein (CRP). Chest roentgenogram may demonstrate cardiac silhouette enlargement, and cardiac imaging (echocardiogram, radionucleotide studies, or cardiac catheterization) may reveal evidence of left ventricular dysfunction. In patients who are diagnosed with cardiomyopathy while taking clozapine mitral valve incompetence has been reported. These cases reported either mild or moderate mitral regurgitation on two-dimensional echocardiography. In patients with suspected cardiomyopathy, consider a 2D-echo Doppler examination to identify mitral valve incompetence.
5.7 Increased Mortality in Elderly Patients with Dementia-Related Psychosis
Elderly patients with dementia-related psychosis treated with antipsychotic drugs are at an increased risk of death. Analyses of 17 placebo-controlled trials (modal duration of 10 weeks), largely in patients taking atypical antipsychotic drugs, revealed a risk of death in drug-treated patients of between 1.6 to 1.7 times the risk of death in placebo-treated patients. Over the course of a typical 10-week controlled trial, the rate of death in drug-treated patients was about 4.5%, compared to a rate of about 2.6% in the placebo group. Although the causes of death were varied, most of the deaths appeared to be either cardiovascular (e.g., heart failure, sudden death) or infectious (e.g., pneumonia) in nature. Observational studies suggest that, similar to atypical antipsychotic drugs, treatment with conventional antipsychotic drugs may increase mortality in this population. The extent to which the findings of increased mortality in observational studies may be attributed to the antipsychotic drug as opposed to some characteristic(s) of the patients is not clear. Clozapine is not approved for the treatment of patients with dementia-related psychosis [see Boxed Warning].
5.8 Gastrointestinal Hypomotility with Severe Complications
Severe gastrointestinal adverse reactions have occurred with the use of clozapine tablets, primarily due to its potent anticholinergic effects and resulting gastrointestinal hypomotility. In post marketing experience, reported effects range from constipation to paralytic ileus. Increased frequency of constipation and delayed diagnosis and treatment increased the risk of severe complications of gastrointestinal hypomotility, resulting in intestinal obstruction, fecal impaction, megacolon and intestinal ischemia or infarction [see Adverse Reaction (6.2)] . These reactions have resulted in hospitalization, surgery, and death. The risk of severe adverse reactions is further increased with anticholinergic medications (and other medications that decrease gastrointestinal peristalsis); therefore, concomitant use should be avoided when possible [see Warnings and Precautions (5.16), Drug Interactions (7.1)].
Prior to initiating clozapine tablets, screen for constipation and treat as necessary. Subjective symptoms of constipation may not accurately reflect the degree of gastrointestinal hypomotility in clozapine treated patients. Therefore, reassess bowel function frequently with careful attention to any changes in the frequency or character of bowel movements, as well as signs and symptoms of complications of hypomotility (e.g., nausea, vomiting, abdominal distension, abdominal pain). If constipation or gastrointestinal hypomotility are identified, monitor closely and treat promptly with appropriate laxatives, as necessary, to prevent severe complications. Consider prophylactic laxatives in high risk patients.
5.9 Eosinophilia
Eosinophilia, defined as a blood eosinophil count of greater than 700/µL, has occurred with clozapine treatment. In clinical trials, approximately 1% of patients developed eosinophilia. Clozapine-related eosinophilia usually occurs during the first month of treatment. In some patients, it has been associated with myocarditis, pancreatitis, hepatitis, colitis, and nephritis. Such organ involvement could be consistent with a drug reaction with eosinophilia and systemic symptoms syndrome (DRESS), also known as drug induced hypersensitivity syndrome (DIHS). If eosinophilia develops during clozapine treatment, evaluate promptly for signs and symptoms of systemic reactions, such as rash or other allergic symptoms, myocarditis, or other organ-specific disease associated with eosinophilia. If clozapine-related systemic disease is suspected, discontinue clozapine immediately.
If a cause of eosinophilia unrelated to clozapine is identified (e.g., asthma, allergies, collagen vascular disease, parasitic infections, and specific neoplasms), treat the underlying cause and continue clozapine.
Clozapine-related eosinophilia has also occurred in the absence of organ involvement and can resolve without intervention. There are reports of successful rechallenge after discontinuation of clozapine, without recurrence of eosinophilia. In the absence of organ involvement, continue clozapine under careful monitoring. If the total eosinophil count continues to increase over several weeks in the absence of systemic disease, the decision to interrupt clozapine therapy and rechallenge after the eosinophil count decreases should be based on the overall clinical assessment, in consultation with an internist or hematologist.
5.10 QT Interval Prolongation
QT prolongation, Torsade de Pointes and other life-threatening ventricular arrhythmias, cardiac arrest, and sudden death have occurred with clozapine treatment. When prescribing clozapine, consider the presence of additional risk factors for QT prolongation and serious cardiovascular reactions. Conditions that increase these risks include the following: history of QT prolongation, long QT syndrome, family history of long QT syndrome or sudden cardiac death, significant cardiac arrhythmia, recent myocardial infarction, uncompensated heart failure, treatment with other medications that cause QT prolongation, treatment with medications that inhibit the metabolism of clozapine, and electrolyte abnormalities.
Prior to initiating treatment with clozapine, perform a careful physical examination, medical history, and concomitant medication history. Consider obtaining a baseline ECG and serum chemistry panel. Correct electrolyte abnormalities. Discontinue clozapine if the QTc interval exceeds 500 msec. If patients experience symptoms consistent with Torsades de Pointes or other arrhythmias, (e.g., syncope, presyncope, dizziness, or palpitations), obtain a cardiac evaluation and discontinue clozapine.
Use caution when administering concomitant medications that prolong the QT interval or inhibit the metabolism of clozapine. Drugs that cause QT prolongation include: specific antipsychotics (e.g., ziprasidone, iloperidone, chlorpromazine, thioridazine, mesoridazine, droperidol, pimozide), specific antibiotics (e.g., erythromycin, gatifloxacin, moxifloxacin, sparfloxacin), Class 1A antiarrhythmic medications (e.g., quinidine, procainamide) or Class III antiarrhythmics (e.g., amiodarone, sotalol), and others (e.g., pentamidine, levomethadyl acetate, methadone, halofantrine, mefloquine, dolasetron mesylate, probucol or tacrolimus). Clozapine is primarily metabolized by CYP isoenzymes 1A2, 2D6, and 3A4. Concomitant treatment with inhibitors of these enzymes can increase the concentration of clozapine [see Drug Interactions (7.1) and Clinical Pharmacology (12.3)] .
Hypokalemia and hypomagnesemia increase the risk of QT prolongation. Hypokalemia can result from diuretic therapy, diarrhea, and other causes. Use caution when treating patients at risk for significant electrolyte disturbance, particularly hypokalemia. Obtain baseline measurements of serum potassium and magnesium levels, and periodically monitor electrolytes. Correct electrolyte abnormalities before initiating treatment with clozapine.
5.11 Metabolic Changes
Atypical antipsychotic drugs, including clozapine have been associated with metabolic changes that can increase cardiovascular and cerebrovascular risk. These metabolic changes include hyperglycemia, dyslipidemia, and body weight gain. While atypical antipsychotic drugs may produce some metabolic changes, each drug in the class has its own specific risk profile.
Hyperglycemia and Diabetes Mellitus
Hyperglycemia, in some cases extreme and associated with ketoacidosis or hyperosmolar coma or death, has been reported in patients treated with atypical antipsychotics including clozapine. Assessment of the relationship between atypical antipsychotic use and glucose abnormalities is complicated by the possibility of an increased background risk of diabetes mellitus in patients with schizophrenia and the increasing incidence of diabetes mellitus in the general population. Given these confounders, the relationship between atypical antipsychotic use and hyperglycemia-related adverse reactions is not completely understood. However, epidemiological studies suggest an increased risk of treatment- emergent, hyperglycemia-related adverse reactions in patients treated with the atypical antipsychotics. Precise risk estimates for hyperglycemia-related adverse reactions in patients treated with atypical antipsychotics are not available.
Patients with an established diagnosis of diabetes mellitus who are started on clozapine should be monitored regularly for worsening of glucose control. Patients with risk factors for diabetes mellitus (e.g., obesity, family history of diabetes) who are starting treatment with atypical antipsychotics should undergo fasting blood glucose testing at the beginning of treatment and periodically during treatment. Any patient treated with atypical antipsychotics should be monitored for symptoms of hyperglycemia including polydipsia, polyuria, polyphagia, and weakness. Patients who develop symptoms of hyperglycemia during treatment with atypical antipsychotics should undergo fasting blood glucose testing. In some cases, hyperglycemia has resolved when the atypical antipsychotic was discontinued; however, some patients required continuation of antidiabetic treatment despite discontinuation of the suspect drug.
In a pooled data analysis of 8 studies in adult subjects with schizophrenia, the mean changes in fasting glucose concentration in the clozapine and chlorpromazine groups were +11 mg/dL and +4 mg/dL respectively. A higher proportion of the clozapine group demonstrated categorical increases from baseline in fasting glucose concentrations, compared to the chlorpromazine group (Table 4). The clozapine doses were 100-900 mg per day (mean modal dose: 512 mg per day). The maximum chlorpromazine dose was 1800 mg per day (mean modal dose: 1029 mg per day). The median duration of exposure was 42 days for clozapine and chlorpromazine.
| Laboratory Parameter | Category Change (at least once) from baseline | Treatment Arm | N | n (%) |
|---|---|---|---|---|
| Fasting Glucose | Normal (< 100 mg/dL) to High (≥ 126 mg/dL) | Clozapine | 198 | 53 (27) |
| Chlorpromazine | 135 | 14 (10) | ||
| Borderline (100 to 125 mg/dL) to High (≥ 126 mg/dL) | Clozapine | 57 | 24 (42) | |
| Chlorpromazine | 43 | 12 (28) |
Dyslipidemia
Undesirable alterations in lipids have occurred in patients treated with atypical antipsychotics, including clozapine. Clinical monitoring, including baseline and periodic follow-up lipid evaluations in patients using clozapine, is recommended.
In a pooled data analysis of 10 studies in adult subjects with schizophrenia, clozapine treatment was associated with increases in serum total cholesterol. No data were collected on LDL and HDL cholesterol. The mean increase in total cholesterol was 13 mg/dL in the clozapine group and 15 mg/dL in the chlorpromazine group. In a pooled data analysis of 2 studies in adult subjects with schizophrenia, clozapine treatment was associated with increases in fasting serum triglyceride. The mean increase in fasting triglyceride was 71 mg/dL (54%) in the clozapine group and 39 mg/dL (35%) in the chlorpromazine group (Table 5). In addition, clozapine treatment was associated with categorical increases in serum total cholesterol and triglyceride, as illustrated in Table 6. The proportion of patients with categorical increases in total cholesterol or fasting triglyceride increased with the duration of exposure. The median duration of clozapine and chlorpromazine exposure was 45 days and 38 days, respectively. The clozapine dose range was 100 mg to 900 mg daily; the maximum chlorpromazine dose was 1800 mg daily.
| Treatment Arm | Baseline total cholesterol concentration (mg/dL) | Change from baseline mg/dL (%) |
| Clozapine (N=334) | 184 | +13 (7) |
| Chlorpromazine (N=185) | 182 | +15 (8) |
| Baseline triglyceride concentration (mg/dL) | Change from baseline mg/dL (%) | |
| Clozapine (N=6) | 130 | +71 (54) |
| Chlorpromazine (N=7) | 110 | +39 (35) |
| Laboratory Parameter | Category Change (at least once) from baseline | Treatment Arm | N | n (%) |
|---|---|---|---|---|
| Total Cholesterol
(random or fasting) | Increase by ≥ 40 mg/dL | Clozapine | 334 | 111(33) |
| Chlorpromazine | 185 | 46 (25) | ||
| Normal (< 200 mg/dL) to High (≥ 240 mg/dL) | Clozapine | 222 | 18 (8) | |
| Chlorpromazine | 132 | 3 (2) | ||
| Borderline (200 to 239 mg/dL) to High (≥ 240 mg/dL) | Clozapine | 79 | 30 (38) | |
| Chlorpromazine | 34 | 14 (41) | ||
| Triglycerides
(fasting) | Increase by ≥ 50 mg/dL | Clozapine | 6 | 3 (50) |
| Chlorpromazine | 7 | 3 (43) | ||
| Normal (<150 mg/dL) to High (≥ 200 mg/dL) | Clozapine | 4 | 0 (0) | |
| Chlorpromazine | 6 | 2 (33) | ||
| Borderline (≥150 mg/dL and < 200 mg/dL) to High (≥ 200 mg/dL) | Clozapine | 1 | 1 (100) | |
| Chlorpromazine | 1 | 0 (0) |
Weight Gain
Weight gain has occurred with the use of antipsychotics, including clozapine. Monitor weight during treatment with clozapine. Table 7 summarizes the data on weight gain by the duration of exposure pooled from 11 studies with clozapine and active comparators. The median duration of exposure was 609, 728, and 42 days, in the clozapine, olanzapine, and chlorpromazine group, respectively.
| Metabolic parameter | Exposure duration | Clozapine
(N = 669) | Olanzapine
(N = 442) | Chlorpromazine
(N = 155) |
|||
|---|---|---|---|---|---|---|---|
| n | Mean | n | Mean | n | Mean | ||
| Weight change from baseline | 2 weeks (Day 11 to 17) | 6 | +0.9 | 3 | +0.7 | 2 | -0.5 |
| 4 weeks (Day 21 to 35) | 23 | +0.7 | 8 | +0.8 | 17 | +0.6 | |
| 8 weeks (Day 49 to 63) | 12 | +1.9 | 13 | +1.8 | 16 | +0.9 | |
| 12 weeks (Day 70 to 98) | 17 | +2.8 | 5 | +3.1 | 0 | 0 | |
| 24 weeks (Day 154 to 182) | 42 | - 0.6 | 12 | +5.7 | 0 | 0 | |
| 48 weeks (Day 322 to 350) | 3 | +3.7 | 3 | +13.7 | 0 | 0 | |
Table 8 summarizes pooled data from 11 studies in adult subjects with schizophrenia demonstrating weight gain ≥7% of body weight relative to baseline. The median duration of exposure was 609, 728, and 42 days, in the clozapine, olanzapine, and chlorpromazine group, respectively.
| Weight change | Clozapine | Olanzapine | Chlorpromazine |
|---|---|---|---|
| N | 669 | 442 | 155 |
| ≥ 7% (inclusive) | 236 (35%) | 203 (46%) | 13 (8%) |
5.12 Neuroleptic Malignant Syndrome
Antipsychotic drugs including clozapine can cause a potentially fatal symptom complex referred to as Neuroleptic Malignant Syndrome (NMS). Clinical manifestations of NMS include hyperpyrexia, muscle rigidity, altered mental status, and autonomic instability (irregular pulse or blood pressure, tachycardia, diaphoresis, and cardiac dysrhythmias). Associated findings can include elevated creatine phosphokinase (CPK), myoglobinuria, rhabdomyolysis, and acute renal failure.
The diagnostic evaluation of patients with this syndrome is complicated. It is important to consider the presence of other serious medical conditions (e.g., severe neutropenia, infection, heat stroke, primary CNS pathology, central anticholinergic toxicity, extrapyramidal symptoms, and drug fever).
The management of NMS should include (1) immediate discontinuation of antipsychotic drugs and other drugs not essential to concurrent therapy, (2) intensive symptomatic treatment and medical monitoring, and (3) treatment of comorbid medical conditions. There is no general agreement about specific pharmacological treatments for NMS.
If a patient requires antipsychotic drug treatment after recovery from NMS, the potential reintroduction of drug therapy should be carefully considered. NMS can recur. Monitor closely if restarting treatment with antipsychotics.
NMS has occurred with clozapine monotherapy and with concomitant CNS-active medications, including lithium.
5.13 Hepatotoxicity
Severe, life threatening, and in some cases fatal hepatotoxicity including hepatic failure, hepatic necrosis, and hepatitis have been reported in post marketing studies in patients treated with clozapine [see Adverse Reactions (6.2)]. Monitor for the appearance of signs and symptoms of hepatotoxicity such as fatigue, malaise, anorexia, nausea, jaundice, bilirubinemia, coagulopathy, and hepatic encephalopathy. Perform serum tests for liver injury and consider permanently discontinuing treatment if hepatitis or transaminase elevations combined with other systemic symptoms are due to clozapine.
5.14 Fever
During clozapine therapy, patients have experienced transient, clozapine-related fever. The peak incidence is within the first 3 weeks of treatment. While this fever is generally benign and self-limited, it may necessitate discontinuing treatment. The fever can be associated with an increase or decrease in WBC count. Carefully evaluate patients with fever to rule out severe neutropenia or infection. Consider the possibility of NMS [see Warnings and Precautions (5.11)].
5.15 Pulmonary Embolism
Pulmonary embolism and deep-vein thrombosis have occurred in patients treated with clozapine. Consider the possibility of pulmonary embolism in patients who present with deep-vein thrombosis, acute dyspnea, chest pain, or with other respiratory signs and symptoms. Whether pulmonary embolus and deep-vein thrombosis can be attributed to clozapine or some characteristic(s) of patients is not clear.
5.16 Anticholinergic Toxicity
Clozapine has potent anticholinergic effects. Treatment with clozapine can result in CNS and peripheral anticholinergic toxicity, especially at higher dosages, or in overdose situations [see Overdosage (10)] . Use with caution in patients with a current diagnosis or prior history of constipation, urinary retention, clinically significant prostatic hypertrophy, or other conditions in which anticholinergic effects can lead to significant adverse reactions. When possible, avoid concomitant use, with other anticholinergic medications because the risk for anticholinergic toxicity or severe gastrointestinal adverse reactions is increased [see Warnings and Precautions (5.8), Drug Interactions (7.1)].
5.17 Interference with Cognitive and Motor Performance
Clozapine can cause sedation and impairment of cognitive and motor performance. Caution patients about operating hazardous machinery, including automobiles, until they are reasonably certain that clozapine does not affect them adversely. These reactions may be dose-related. Consider reducing the dose if they occur.
5.18 Tardive Dyskinesia
Tardive dyskinesia (TD) has occurred in patients treated with antipsychotic drugs, including clozapine. The syndrome consists of potentially irreversible, involuntary, dyskinetic movements. The risk of TD and the likelihood that it will become irreversible are believed to increase with greater durations of treatment and higher total cumulative doses. However, the syndrome can develop after relatively brief treatment periods at low doses. Prescribe clozapine in a manner that is most likely to minimize the risk of developing TD. Use the lowest effective dose and the shortest duration necessary to control symptoms. Periodically assess the need for continued treatment. Consider discontinuing treatment if TD occurs. However, some patients may require treatment with clozapine despite the presence of the syndrome.
There is no known treatment for TD. However, the syndrome may remit partially or completely if treatment is discontinued. Antipsychotic treatment, itself, may suppress (or partially suppress) the signs and symptoms, and it has the potential to mask the underlying process. The effect of symptom suppression on the long-term course of TD is unknown.
5.19 Cerebrovascular Adverse Reactions
In controlled trials, elderly patients with dementia-related psychosis treated with some atypical antipsychotics had an increased risk (compared to placebo) of cerebrovascular adverse reactions (e.g., stroke, transient ischemic attack), including fatalities. The mechanism for this increased risk is not known. An increased risk cannot be excluded for clozapine or other antipsychotics or other patient populations. Clozapine should be used with caution in patients with risk factors for cerebrovascular adverse reactions.
5.20 Recurrence of Psychosis and Cholinergic Rebound after Abrupt Discontinuation of Clozapine
If abrupt discontinuation of clozapine is necessary (because of severe neutropenia or another medical condition, for example) [see Dosage and Administration (2.4), Warnings and Precautions (5.1)] , monitor carefully for the recurrence of psychotic symptoms and adverse reactions related to cholinergic rebound, such as profuse sweating, headache, nausea, vomiting and diarrhea.
6 ADVERSE REACTIONS
The following adverse reactions are discussed in more detail in other sections of the labeling:
- Severe Neutropenia [see Warnings and Precautions (5.1)].
- Orthostatic Hypotension, Bradycardia, and Syncope [see Warnings and Precautions (5.3)].
- Falls [see Warnings and Precautions (5.4)].
- Seizures [see Warnings and Precautions (5.5)].
- Myocarditis, Cardiomyopathy, and Mitral Valve Incompetence [see Warnings and Precautions (5.6)].
- Increased Mortality in Elderly Patients with Dementia-Related Psychosis [see Warnings and Precautions (5.7)].
- Gastrointestinal Hypomotility with Severe Complications [See Warnings and Precautions (5.8)].
- Eosinophilia [see Warnings and Precautions (5.9)].
- QT Interval Prolongation [see Warnings and Precautions (5.10)].
- Metabolic Changes (Hyperglycemia and Diabetes Mellitus, Dyslipidemia, and Weight Gain) [see Warnings and Precautions (5.11)].
- Neuroleptic Malignant Syndrome [see Warnings and Precautions (5.12)].
- Hepatotoxicity [see Warnings and Precautions (5.13)].
- Fever [see Warnings and Precautions (5.14)].
- Pulmonary Embolism [see Warnings and Precautions (5.15)].
- Anticholinergic Toxicity [see Warnings and Precautions (5.16)].
- Interference with Cognitive and Motor Performance [see Warnings and Precautions (5.17)].
- Tardive Dyskinesia [see Warnings and Precautions (5.18)].
- Cerebrovascular Adverse Reactions [see Warnings and Precautions (5.19)].
- Recurrence of Psychosis and Cholinergic Rebound after Abrupt Discontinuation [see Warnings and Precautions (5.20)].
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in clinical practice.
The most commonly reported adverse reactions (≥ 5%) across clozapine clinical trials were: CNS reactions, including sedation, dizziness/vertigo, headache, and tremor; cardiovascular reactions, including tachycardia, hypotension, and syncope; autonomic nervous system reactions, including hypersalivation, sweating, dry mouth, and visual disturbances; gastrointestinal reactions, including constipation and nausea; and fever. Table 9 summarizes the most commonly reported adverse reactions (≥ 5%) in clozapine-treated patients (compared to chlorpromazine-treated patients) in the pivotal, 6- week, controlled trial in treatment-resistant schizophrenia.
| Adverse Reaction | Clozapine
(N = 126) (%) | Chlorpromazine
(N = 142) (%) |
|---|---|---|
| Sedation | 21 | 13 |
| Tachycardia | 17 | 11 |
| Constipation | 16 | 12 |
| Dizziness | 14 | 16 |
| Hypotension | 13 | 38 |
| Fever (hyperthermia) | 13 | 4 |
| Hypersalivation | 13 | 1 |
| Hypertension | 12 | 5 |
| Headache | 10 | 10 |
| Nausea/vomiting | 10 | 12 |
| Dry mouth | 5 | 20 |
Table 10 summarizes the adverse reactions reported in clozapine-treated patients at a frequency of 2% or greater across all clozapine studies (excluding the 2-year InterSePT™ Study). These rates are not adjusted for duration of exposure.
| Body System
Adverse Reaction * | Clozapine
N = 842 Percentage of Patients |
|---|---|
|
|
| Central Nervous System | |
| Drowsiness/Sedation | 39 |
| Dizziness/Vertigo | 19 |
| Headache | 7 |
| Tremor | 6 |
| Syncope | 6 |
| Disturbed Sleep/Nightmares | 4 |
| Restlessness | 4 |
| Hypokinesia/Akinesia | 4 |
| Agitation | 4 |
| Seizures (convulsions) | 3 * |
| Rigidity | 3 |
| Akathisia | 3 |
| Confusion | 3 |
| Fatigue | 2 |
| Insomnia | 2 |
| Cardiovascular | |
| Tachycardia | 25 * |
| Hypotension | 9 |
| Hypertension | 4 |
| Gastrointestinal | |
| Constipation | 14 |
| Nausea | 5 |
| Abdominal Discomfort/Heartburn | 4 |
| Nausea/Vomiting | 3 |
| Vomiting | 3 |
| Diarrhea | 2 |
| Urogenital | |
| Urinary Abnormalities | 2 |
| Autonomic Nervous System | |
| Salivation | 31 |
| Sweating | 6 |
| Dry Mouth | 6 |
| Visual Disturbances | 5 |
| Skin | |
| Rash | 2 |
| Hemic/Lymphatic | |
| Leukopenia/Decreased WBC/Neutropenia | 3 |
| Miscellaneous | |
| Fever | 5 |
| Weight Gain | 4 |
Table 11 summarizes the most commonly reported adverse reactions (≥ 10% of the clozapine or olanzapine group) in the InterSePT™ Study. This was an adequate and well-controlled, two-year study evaluating the efficacy of clozapine relative to olanzapine in reducing the risk of suicidal behavior in patients with schizophrenia or schizoaffective disorder. The rates are not adjusted for duration of exposure.
| Adverse Reactions | Clozapine
N = 479 % Reporting | Olanzapine
N = 477 % Reporting |
|---|---|---|
| Salivary hypersecretion | 48 | 6 |
| Somnolence | 46 | 25 |
| Weight increased | 31 | 56 |
| Dizziness (excluding vertigo) | 27 | 12 |
| Constipation | 25 | 10 |
| Insomnia | 20 | 33 |
| Nausea | 17 | 10 |
| Vomiting | 17 | 9 |
| Dyspepsia | 14 | 8 |
Dystonia
Class effect: Symptoms of dystonia, prolonged abnormal contractions of muscle groups, may occur in susceptible individuals during the first few days of treatment. Dystonic symptoms include: spasm of the neck muscles, sometimes progressing to tightness of the throat, swallowing difficulty, difficulty breathing, and/or protrusion of the tongue. While these symptoms can occur at low doses, they occur more frequently and with greater severity with high potency and at higher doses of first generation antipsychotic drugs. An elevated risk of acute dystonia is observed in males and younger age groups.
6.2 Postmarketing Experience
The following adverse reactions have been identified during post-approval use of clozapine. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Central Nervous System
Delirium, EEG abnormal, myoclonus, paresthesia, possible cataplexy, status epilepticus, obsessive compulsive symptoms, restless leg syndrome and post-discontinuation cholinergic rebound adverse reactions.
Cardiovascular System
Atrial or ventricular fibrillation, ventricular tachycardia, palpitations, QT interval prolongation, Torsades de Pointes, mitral valve incompetence associated with clozapine-related cardiomyopathy, myocardial infarction, cardiac arrest, myocarditis and periorbital edema.
Gastrointestinal System
Acute pancreatitis, dysphagia, salivary gland swelling, colitis, megacolon, intestinal ischemia or infarction.
Hepatobiliary System
Cholestasis, hepatitis, jaundice, hepatotoxicity, hepatic steatosis, hepatic necrosis, hepatic fibrosis, hepatic cirrhosis, liver injury (hepatic, cholestatic, and mixed), and liver failure.
Urogenital System
Acute interstitial nephritis, nocturnal enuresis, priapism, renal failure, and retrograde ejaculation.
Skin and Subcutaneous Tissue Disorders
Hypersensitivity reactions: photosensitivity, vasculitis, erythema multiforme, skin pigmentation disorder, and Stevens-Johnson Syndrome.
Musculoskeletal System and Connective Tissue Disorders
Myasthenic syndrome, rhabdomyolysis, and systemic lupus erythematosus.
Respiratory System
Aspiration, pleural effusion, pneumonia, lower respiratory tract infection, sleep apnea.
7 DRUG INTERACTIONS
7.1 Potential for Other Drugs to Affect Clozapine
Clozapine is a substrate for many cytochrome P450 isozymes, in particular CYP1A2, CYP3A4, and CYP2D6. Use caution when administering clozapine concomitantly with drugs that are inducers or inhibitors of these enzymes.
CYP1A2 Inhibitors
Concomitant use of clozapine and CYP1A2 inhibitors can increase plasma levels of clozapine, potentially resulting in adverse reactions. Reduce the clozapine dose to one-third of the original dose when clozapine is coadministered with strong CYP1A2 inhibitors (e.g., fluvoxamine, ciprofloxacin, or enoxacin). The clozapine dose should be increased to the original dose when coadministration of strong CYP1A2 inhibitors is discontinued [see Dosage and Administration (2.6), Clinical Pharmacology (12.3)].
Moderate or weak CYP1A2 inhibitors include oral contraceptives and caffeine. Monitor patients closely when clozapine is coadministered with these inhibitors. Consider reducing the clozapine dosage if necessary [see Dosage and Administration (2.6)] .
CYP2D6 and CYP3A4 Inhibitors
Concomitant treatment with clozapine and CYP2D6 or CYP3A4 inhibitors (e.g., cimetidine, escitalopram, erythromycin, paroxetine, bupropion, fluoxetine, quinidine, duloxetine, terbinafine, or sertraline) can increase clozapine levels and lead to adverse reactions [see Clinical Pharmacology (12.3)] . Use caution and monitor patients closely when using such inhibitors. Consider reducing the clozapine dose [see Dosage and Administration (2.6)] .
CYP1A2 and CYP3A4 Inducers
Concomitant treatment with drugs that induce CYP1A2 or CYP3A4 can decrease the plasma concentration of clozapine, resulting in decreased effectiveness of clozapine. Tobacco smoke is a moderate inducer of CYP1A2. Strong CYP3A4 inducers include carbamazepine, phenytoin, St. John's wort, and rifampin. It may be necessary to increase the clozapine dose if used concomitantly with inducers of these enzymes. However, concomitant use of clozapine and strong CYP3A4 inducers is not recommended [see Dosage and Administration (2.6)].
Consider reducing the clozapine dosage when discontinuing coadministered enzyme inducers; because discontinuation of inducers can result in increased clozapine plasma levels and an increased risk of adverse reactions [see Dosage and Administration (2.6)].
Anticholinergic Drugs
Concomitant treatment with clozapine and other drugs with anticholinergic activity (e.g., benztropine, cyclobenzaprine, diphenhydramine) can increase the risk for anticholinergic toxicity and severe gastrointestinal adverse reactions related to hypomotility. Avoid concomitant use of clozapine with anticholinergic drugs when possible [see Warnings and Precautions (5.8, 5.16)] .
Drugs that Cause QT Interval Prolongation
Use caution when administering concomitant medications that prolong the QT interval or inhibit the metabolism of clozapine. Drugs that cause QT prolongation include: specific antipsychotics (e.g., ziprasidone, iloperidone, chlorpromazine, thioridazine, mesoridazine, droperidol, and pimozide), specific antibiotics (e.g., erythromycin, gatifloxacin, moxifloxacin, sparfloxacin), Class 1A antiarrhythmics (e.g., quinidine, procainamide) or Class III antiarrhythmics (e.g., amiodarone, sotalol), and others (e.g., pentamidine, levomethadyl acetate, methadone, halofantrine, mefloquine, dolasetron mesylate, probucol or tacrolimus) [see Warnings and Precautions (5.10)] .
7.2 Potential for Clozapine to Affect Other Drugs
Concomitant use of clozapine with other drugs metabolized by CYP2D6 can increase levels of these CYPD26 substrates. Use caution when coadministering clozapine with other drugs that are metabolized by CYP2D6. It may be necessary to use lower doses of such drugs than usually prescribed. Such drugs include specific antidepressants, phenothiazines, carbamazepine, and Type 1C antiarrhythmics (e.g., propafenone, flecainide, and encainide).
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
There are no adequate or well-controlled studies of clozapine in pregnant women.
Reproduction studies have been performed in rats and rabbits at doses up to 0.4 and 0.9 times, respectively, the maximum recommended human dose (MRHD) of 900 mg/day on a mg/m 2 body surface area basis. The studies revealed no evidence of impaired fertility or harm to the fetus due to clozapine. Because animal reproduction studies are not always predictive of human response, clozapine should be used during pregnancy only if clearly needed.
Clinical Considerations
Consider the risk of exacerbation of psychosis when discontinuing or changing treatment with antipsychotic medications during pregnancy and postpartum. Consider early screening for gestational diabetes for patients treated with antipsychotic medications [see Warnings and Precautions (5.11)] .
Neonates exposed to antipsychotic drugs during the third trimester of pregnancy are at risk for extrapyramidal and/or withdrawal symptoms following delivery. Monitor neonates for symptoms of agitation, hypertonia, hypotonia, tremor, somnolence, respiratory distress, and feeding difficulties. The severity of complications can vary from self-limited symptoms to some neonates requiring intensive care unit support and prolonged hospitalization.
Animal Data
In embryofetal developmental studies, clozapine had no effects on maternal parameters, litter sizes, or fetal parameters when administered orally to pregnant rats and rabbits during the period of organogenesis at doses up to 0.4 and 0.9 times, respectively, the MRHD of 900 mg/day on a mg/m 2 body surface area basis.
In peri/postnatal developmental studies, pregnant female rats were administered clozapine over the last third of pregnancy and until day 21 postpartum. Observations were made on fetuses at birth and during the postnatal period; the offspring were allowed to reach sexual maturity and mated. Clozapine caused a decrease in maternal body weight but had no effects on litter size or body weights of either F1or F2 generations at doses up to 0.4 times the MRHD of 900 mg/day on a mg/m 2 body surface area basis.
8.3 Nursing Mothers
Clozapine is present in human milk. Because of the potential for serious adverse reactions in nursing infants from clozapine, a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother.
8.5 Geriatric Use
There have not been sufficient numbers of geriatric patients in clinical studies utilizing clozapine to determine whether those over 65 years of age differ from younger subjects in their response to clozapine.
Orthostatic hypotension and tachycardia can occur with clozapine treatment [see Boxed Warning and Warnings and Precautions (5.3)]. Elderly patients, particularly those with compromised cardiovascular functioning, may be more susceptible to these effects.
Elderly patients may be particularly susceptible to the anticholinergic effects of clozapine, such as urinary retention and constipation [see Warnings and Precautions (5.16)].
Carefully select clozapine doses in elderly patients, taking into consideration their greater frequency of decreased hepatic, renal, or cardiac function, as well as other concomitant disease and other drug therapy. Clinical experience suggests that the prevalence of tardive dyskinesia appears to be highest among the elderly; especially elderly women [see Warnings and Precautions (5.18)].
8.6 Patients with Renal or Hepatic Impairment
Dose reduction may be necessary in patients with significant impairment of renal or hepatic function. Clozapine concentrations may be increased in these patients, because clozapine is almost completely metabolized and then excreted [see Dosage and Administration (2.7), Clinical Pharmacology (12.3)] .
8.7 CYP2D6 Poor Metabolizers
Dose reduction may be necessary in patients who are CYP2D6 poor metabolizers. Clozapine concentrations may be increased in these patients, because clozapine is almost completely metabolized and then excreted [see Dosage and Administration (2.7), Clinical Pharmacology (12.3)] .
8.8 Hospice Patients
For hospice patients (i.e., terminally ill patients with an estimated life expectancy of six months or less), the prescriber may reduce the ANC monitoring frequency to once every 6 months, after a discussion with the patient and his/her caregiver. Individual treatment decisions should weigh the importance of monitoring ANC in the context of the need to control psychiatric symptoms and the patient's terminal illness.
10 OVERDOSAGE
10.1 Overdosage Experience
The most commonly reported signs and symptoms associated with clozapine overdose are: sedation, delirium, coma, tachycardia, hypotension, respiratory depression or failure; and hypersalivation. There are reports of aspiration pneumonia, cardiac arrhythmias, and seizure. Fatal overdoses have been reported with clozapine, generally at doses above 2500 mg. There have also been reports of patients recovering from overdoses well in excess of 4 g.
10.2 Management of Overdosage
There is no available specific antidote to an overdose of clozapine. Establish and maintain an airway; ensure adequate oxygenation and ventilation. Monitor cardiac status and vital signs. Use general symptomatic and supportive measures. Consider the possibility of multiple-drug involvement.
Contact a Certified Poison Control Center for the most up to date information on the management of overdosage (1-800-222-1222).
11 DESCRIPTION
Clozapine, an atypical antipsychotic drug, is a tricyclic dibenzodiazepine derivative, 8-chloro-11-(4- methyl-1-piperazinyl)-5 H-dibenzo [ b,e] [1,4] diazepine. The structural formula is:

C 18 H 19 ClN 4 Mol. wt. 326.83
Clozapine tablets are available in pale yellow tablets of 25 mg, 50 mg, 100 mg, and 200 mg for oral administration.
Active Ingredient: clozapine
Inactive Ingredients are colloidal silicon dioxide, lactose, magnesium stearate, povidone, starch (corn), sodium starch glycolate, and talc.
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
The mechanism of action of clozapine is unknown. However, it has been proposed that the therapeutic efficacy of clozapine in schizophrenia is mediated through antagonism of the dopamine type 2 (D 2) and the serotonin type 2A (5-HT2A) receptors. Clozapine also acts as an antagonist at adrenergic, cholinergic, histaminergic and other dopaminergic and serotonergic receptors.
12.2 Pharmacodynamics
Clozapine demonstrated binding affinity to the following receptors: histamine H 1 (K i 1.1 nM), adrenergic α 1A (K i 1.6 nM), serotonin 5-HT 6 (K i 4 nM), serotonin 5-HT 2A (K i 5.4 nM), muscarinic M 1 (K i 6.2 nM), serotonin 5-HT 7 (K i 6.3 nM), serotonin 5-HT 2C (K i 9.4 nM), dopamine D 4 (K i 24 nM), adrenergic α 2A (K i 90 nM), serotonin 5-HT 3 (K i 95 nM), serotonin 5-HT 1A (K i 120 nM), dopamine D 2 (K i 160 nM), dopamine D 1 (K i 270 nM), dopamine D 5 (K i 454 nM), and dopamine D 3 (K i 555 nM).
Clozapine causes little or no prolactin elevation.
Clinical electroencephalogram (EEG) studies demonstrated that clozapine increases delta and theta activity and slows dominant alpha frequencies. Enhanced synchronization occurs. Sharp wave activity and spike and wave complexes may also develop. Patients have reported an intensification of dream activity during clozapine therapy. REM sleep was found to be increased to 85% of the total sleep time. In these patients, the onset of REM sleep occurred almost immediately after falling asleep.
12.3 Pharmacokinetics
Absorption
In humans, clozapine tablets (25 mg and 100 mg) are equally bioavailable relative to a clozapine solution. Following oral administration of clozapine 100 mg twice daily, the average steady-state peak plasma concentration was 319 ng/mL (range: 102 to 771 ng/mL), occurring at the average of 2.5 hours (range: 1 to 6 hours) after dosing. The average minimum concentration at steady state was 122 ng/mL (range: 41 to 343 ng/mL), after 100 mg twice daily dosing. Food does not appear to affect the systemic bioavailability of clozapine. Thus, clozapine may be administered with or without food.
Distribution
Clozapine is approximately 97% bound to serum proteins. The interaction between clozapine and other highly protein-bound drugs has not been fully evaluated but may be important [see Drug Interactions (7)].
Metabolism and Excretion
Clozapine is almost completely metabolized prior to excretion, and only trace amounts of unchanged drug are detected in the urine and feces. Clozapine is a substrate for many cytochrome P450 isozymes, in particular CYP1A2, CYP2D6, and CYP3A4. Approximately 50% of the administered dose is excreted in the urine and 30% in the feces. The demethylated, hydroxylated, and N-oxide derivatives are components in both urine and feces. Pharmacological testing has shown the desmethyl metabolite (norclozapine) to have only limited activity, while the hydroxylated and N-oxide derivatives were inactive. The mean elimination half-life of clozapine after a single 75 mg dose was 8 hours (range: 4 to 12 hours), compared to a mean elimination half-life of 12 hours (range: 4 to 66 hours), after achieving steady state with 100 mg twice daily dosing.
A comparison of single-dose and multiple-dose administration of clozapine demonstrated that the elimination half-life increased significantly after multiple dosing relative to that after single-dose administration, suggesting the possibility of concentration-dependent pharmacokinetics. However, at steady state, approximately dose-proportional changes with respect to AUC (area under the curve), peak, and minimum clozapine plasma concentrations were observed after administration of 37.5, 75, and 150 mg twice daily.
Drug-Drug Interaction Studies
Fluvoxamine
A pharmacokinetic study was conducted in 16 schizophrenic patients who received clozapine under steady-state conditions. After coadministration of fluvoxamine for 14 days, mean trough concentrations of clozapine and its metabolites, N-desmethylclozapine and clozapine N-oxide, were elevated about three-fold compared to baseline steady-state concentrations.
Paroxetine, Fluoxetine, and Sertraline
In a study of schizophrenic patients (n=14) who received clozapine under steady-state conditions, coadministration of paroxetine produced only minor changes in the levels of clozapine and its metabolites. However, other published reports describe modest elevations (less than two-fold) of clozapine and metabolite concentrations when clozapine was taken with paroxetine, fluoxetine, and sertraline.
Specific Population Studies
Renal or Hepatic Impairment
No specific pharmacokinetic studies were conducted to investigate the effects of renal or hepatic impairment on the pharmacokinetics of clozapine. Higher clozapine plasma concentrations are likely in patients with significant renal or hepatic impairment when given usual doses.
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis
No carcinogenic potential was demonstrated in long-term studies in mice and rats at doses up to 0.3 times and 0.4 times, respectively, the maximum recommended human dose (MRHD) of 900 mg/day on a mg/m 2 body surface area basis.
Mutagenesis
Clozapine was not genotoxic when tested in the following gene mutation and chromosomal aberration tests: the bacterial Ames test, the in vitro mammalian V79 in Chinese hamster cells, the in vitro unscheduled DNA synthesis in rat hepatocytes or the in vivo micronucleus assay in mice.
Impairment of Fertility
Clozapine had no effect on any parameters of fertility, pregnancy, fetal weight or postnatal development when administered orally to male rats 70 days before mating and to female rats for 14 days before mating at doses up to 0.4 times the MRHD of 900 mg/day on a mg/m 2 body surface area basis.
14 CLINICAL STUDIES
14.1 Treatment-Resistant Schizophrenia
The efficacy of clozapine in treatment-resistant schizophrenia was established in a multicenter, randomized, double-blind, active-controlled (chlorpromazine) study in patients with a DSM-III diagnosis of schizophrenia who had inadequate responses to at least 3 different antipsychotics (from at least 2 different chemical classes) during the preceding 5 years. The antipsychotic trials must have been judged adequate; the antipsychotic dosages must have been equivalent to or greater than 1000 mg per day of chlorpromazine for a period of at least 6 weeks, each without significant reduction of symptoms. There must have been no period of good functioning within the preceding 5 years. Patients must have had a baseline score of at least 45 on the investigator-rated Brief Psychiatric Rating Scale (BPRS). On the 18-item BPRS, 1 indicates the absence of symptoms, and 7 indicates severe symptoms; the maximum potential total BPRS score is 126. At baseline, the mean BPRS score was 61. In addition, patients must have had a score of at least 4 on at least two of the following four individual BPRS items: conceptual disorganization, suspiciousness, hallucinatory behavior, and unusual thought content. Patients must have had a Clinical Global Impressions – Severity Scale score of at least 4 (moderately ill).
In the prospective, lead-in phase of the trial, all patients (N=305) initially received single-blind treatment with haloperidol (the mean dose was 61 mg per day) for 6 weeks. More than 80% of patients completed the 6-week trial. Patients with an inadequate response to haloperidol (n=268) were randomized to double-blind treatment with clozapine (N=126) or chlorpromazine (N=142). The maximum daily clozapine dose was 900 mg; the mean daily dose was >600 mg). The maximum daily chlorpromazine dose was 1800 mg; the mean daily dose was >1200 mg.
The primary endpoint was treatment response, predefined as a decrease in BPRS score of at least 20% and either (1) a CGI-S score of ≤3 (mildly ill), or (2) a BPRS score of ≤ 35, at the end of 6 weeks of treatment. Approximately 88% of patients from the clozapine and chlorpromazine groups completed the 6-week trial. At the end of 6 weeks, 30% of the clozapine group responded to treatment, and 4% of the chlorpromazine group responded to treatment. The difference was statistically significant (p < 0.001). The mean change in total BPRS score was - 16 and -5 in the clozapine and chlorpromazine group, respectively; the mean change in the 4 key BPRS item scores was -5 and -2 in the clozapine and chlorpromazine group, respectively; and the mean change in CGI-S score was -1.2 and -0.4, in the clozapine and chlorpromazine group, respectively. These changes in the clozapine group were statistically significantly greater than in the chlorpromazine group (p < 0.001 in each analysis).
14.2 Recurrent Suicidal Behavior in Schizophrenia or Schizoaffective Disorder
The effectiveness of clozapine in reducing the risk of recurrent suicidal behavior was assessed in the International Suicide Prevention Trial (InterSePT™, a trademark of Novartis Pharmaceuticals Corporation). This was a prospective, randomized, open-label, active-controlled, multicenter, international, parallel-group comparison of clozapine versus olanzapine (Zyprexa®, a registered trademark of Eli Lilly and Company) in 956 patients with schizophrenia or schizoaffective disorder (DSM-IV) who were judged to be at risk for recurrent suicidal behavior. Only about one-fourth of these patients (27%) were considered resistant to standard antipsychotic drug treatment. To enter the trial, patients must have met 1 of the following criteria:
- They had attempted suicide within the three years prior to their baseline evaluation.
- They had been hospitalized to prevent a suicide attempt within the three years prior to their baseline evaluation.
- They demonstrated moderate-to-severe suicidal ideation with a depressive component within one week prior to their baseline evaluation.
- They demonstrated moderate-to-severe suicidal ideation accompanied by command hallucinations to do self-harm within one week prior to their baseline evaluation.
Dosing regimens for each treatment group were determined by individual investigators and were individualized by patient. Dosing was flexible, with a dose range of 200 to 900 mg/day for clozapine and 5 to 20 mg/day for olanzapine. For the 956 patients who received clozapine or olanzapine in this study, there was extensive use of concomitant psychotropics: 84% with antipsychotics, 65% with anxiolytics, 53% with antidepressants, and 28% with mood stabilizers. There was significantly greater use of concomitant psychotropic medications among the patients in the olanzapine group.
The primary efficacy measure was time to (1) a significant suicide attempt, including a completed suicide; (2) hospitalization due to imminent suicide risk, including increased level of surveillance for suicidality for patients already hospitalized; or (3) worsening of suicidality severity as demonstrated by "much worsening" or "very much worsening" from baseline in the Clinical Global Impression of Severity of Suicidality as assessed by the Blinded Psychiatrist (CGI-SS-BP) scale. A determination of whether or not a reported event met criterion 1 or 2 above was made by the Suicide Monitoring Board (SMB), a group of experts blinded to patient data.
A total of 980 patients were randomized to the study and 956 received study medication. Sixty-two percent of the patients were diagnosed with schizophrenia, and the remainder (38%) were diagnosed with schizoaffective disorder. Only about one-fourth of the total patient population (27%) was identified as "treatment-resistant" at baseline. There were more males than females in the study (61% of all patients were male). The mean age of patients entering the study was 37 years of age (range 18 to 69). Most patients were Caucasian (71%), 15% were Black, 1% were Asian, and 13% were classified as being of "other" races.
Patients treated with clozapine had a statistically significant longer delay in the time to recurrent suicidal behavior in comparison with olanzapine. This result should be interpreted only as evidence of the effectiveness of clozapine in delaying time to recurrent suicidal behavior and not a demonstration of the superior efficacy of clozapine over olanzapine.
The probability of experiencing (1) a significant suicide attempt, including a completed suicide, or (2) hospitalization because of imminent suicide risk, including increased level of surveillance for suicidality for patients already hospitalized, was lower for clozapine patients than for olanzapine patients at Week 104: clozapine 24% versus olanzapine 32%; 95% CI of the difference: 2%, 14% (Figure 1).
Figure 1. Cumulative Probability of a Significant Suicide Attempt or Hospitalization to Prevent Suicide in Patients with Schizophrenia or Schizoaffective Disorder at High Risk of Suicidality

16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
Discuss the following issues with patients and caregivers:
-
Severe Neutropenia:
- Instruct patients (and caregivers) beginning treatment with clozapine tablets about the risk of developing severe neutropenia and infection.
- Instruct patients to immediately report to their physician any symptom or sign of infection (e.g., flu- like illness; fever; lethargy; general weakness or malaise; mucus membrane ulceration; skin, pharyngeal, vaginal, urinary, or pulmonary infection; or extreme weakness or lethargy) occurring at any time during clozapine tablet therapy, to aid in evaluation for neutropenia and to institute prompt and appropriate management. [see Warnings and Precautions (5.1), (5.12), and (5.14)] .
- Inform patients and caregivers clozapine tablets are available only through a restricted program called the Clozapine REMS Program designed to ensure the required blood monitoring, in order to reduce the risk of developing severe neutropenia. Advise patients and caregivers of the importance of having blood tested as follows:
- Weekly blood tests are required for the first 6 months.
- An ANC is required every 2 weeks for the next 6 months if an acceptable ANC is maintained during the first 6 months of continuous therapy,
- An ANC is required once every 4 weeks thereafter if an acceptable ANC is maintained during the second 6 months of continuous therapy.
- Clozapine tablets are available only from certified pharmacies participating in the program.
Provide patients (and caregivers) with website information and the telephone number on how to obtain the product (www.clozapinerems.com or 1-844-267-8678) [ see Warnings and Precautions (5.2)].
- Orthostatic Hypotension, Bradycardia, and Syncope: Inform patients and caregivers about the risk of orthostatic hypotension and syncope, especially during the period of initial dose titration. Instruct them to strictly follow the clinician's instructions for dosage and administration. Advise patients to consult their clinician immediately if they feel faint, lose consciousness or have signs or symptoms suggestive of bradycardia or arrhythmia [see Dosage and Administration (2.2) and Warnings and Precautions (5.3)].
- Seizures: Inform patients and caregivers about the significant risk of seizure during clozapine tablet treatment. Caution them about driving and any other potentially hazardous activity while taking clozapine tablets [see Warnings and Precautions (5.5)].
- Gastrointestinal Hypomotility with Severe Complications: Educate patients and caregivers on the risks, prevention and treatment of clozapine-induced constipation, including medications to avoid when possible (e.g., drugs with anticholinergic activity). Encourage appropriate hydration, physical activity, and fiber intake and emphasize that prompt attention and treatment to the development of constipation or other gastrointestinal symptoms is critical in preventing severe complications. Advise patients and caregivers to contact their health care provider if they experience symptoms of constipation (e.g., difficulty passing stools, incomplete passage of stool, decreased bowel movement frequency) or other symptoms associated with gastrointestinal hypomotility (e.g., nausea, abdominal distension or pain, vomiting) [see Warnings and Precautions (5.8), Drug Interactions (7.1)] .
- QT Interval Prolongation: Advise patients to consult their clinician immediately if they feel faint, lose consciousness or have signs or symptoms suggestive of arrhythmia. Instruct patients to not take clozapine tablets with other drugs that cause QT interval prolongation. Instruct patients to inform their clinicians that they are taking clozapine tablets before any new drug [see Warnings and Precautions (5.10.) and Drug Interactions (7.1)].
- Metabolic Changes (hyperglycemia and diabetes mellitus, dyslipidemia, weight gain): Educate patients and caregivers about the risk of metabolic changes and the need for specific monitoring. The risks include hyperglycemia and diabetes mellitus, dyslipidemia, weight gain, and cardiovascular reactions. Educate patients and caregivers about the symptoms of hyperglycemia (high blood sugar) and diabetes mellitus (e.g., polydipsia, polyuria, polyphagia, and weakness). Monitor all patients for these symptoms. Patients who are diagnosed with diabetes or have risk factors for diabetes (obesity, family history of diabetes) should have their fasting blood glucose monitored before beginning treatment and periodically during treatment. Patients who develop symptoms of hyperglycemia should have assessments of fasting glucose. Clinical monitoring of weight is recommended [see Warnings and Precautions (5.11)] .
- Interference with Cognitive and Motor Performance: Because clozapine tablets may have the potential to impair judgment, thinking, or motor skills, patients should be cautioned about operating hazardous machinery, including automobiles, until they are reasonably certain that clozapine tablets therapy does not affect them adversely [see Warnings and Precautions (5.17)] . Missed Doses and Re-initiating Treatment: Inform patients and caregivers that if the patient misses taking clozapine tablets for more than 2 days, they should not restart their medication at the same dosage but should contact their physician for dosing instructions [see Dosage and Administration (2.5) and Warnings and Precautions (5.1, 5.3)].
- Pregnancy: Patients and caregivers should notify the clinician if the patient becomes pregnant or intends to become pregnant during therapy [see Use in Specific Populations (8.1)].
- Nursing: Advise patients and caregivers that the patient should not breastfeed an infant if they are taking clozapine tablets [see Use in Specific Populations (8.3)].
- Concomitant Medication: Advise patients to inform their healthcare provider if they are taking, or plan to take, any prescription or over-the-counter drugs; there is a potential for significant drug-drug interactions [see Dosage and Administration (2.6), Drug Interactions (7.1).
SPL UNCLASSIFIED SECTION
PRINCIPAL DISPLAY PANEL - 25 mg Tablet Bottle Label
PRINCIPAL DISPLAY PANEL - 100 mg Tablet Bottle Label
INGREDIENTS AND APPEARANCE
| CLOZAPINE
clozapine tablet |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| CLOZAPINE
clozapine tablet |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Golden State Medical Supply, Inc. (603184490) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Golden State Medical Supply, Inc. | 603184490 | relabel(51407-085, 51407-086) , repack(51407-085, 51407-086) | |